Morphological, Anatomical, Physiological and Biochemical Changes during Adventitious Roots Formation of Bougainvillea buttiana ‘Miss Manila’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Morphological and Histological Observations
2.3. Enzyme Assays
2.4. Extraction, Purification, and Quantification of Hormones
2.5. Statistical Analysis
3. Results
3.1. Morphological and Histological Observations
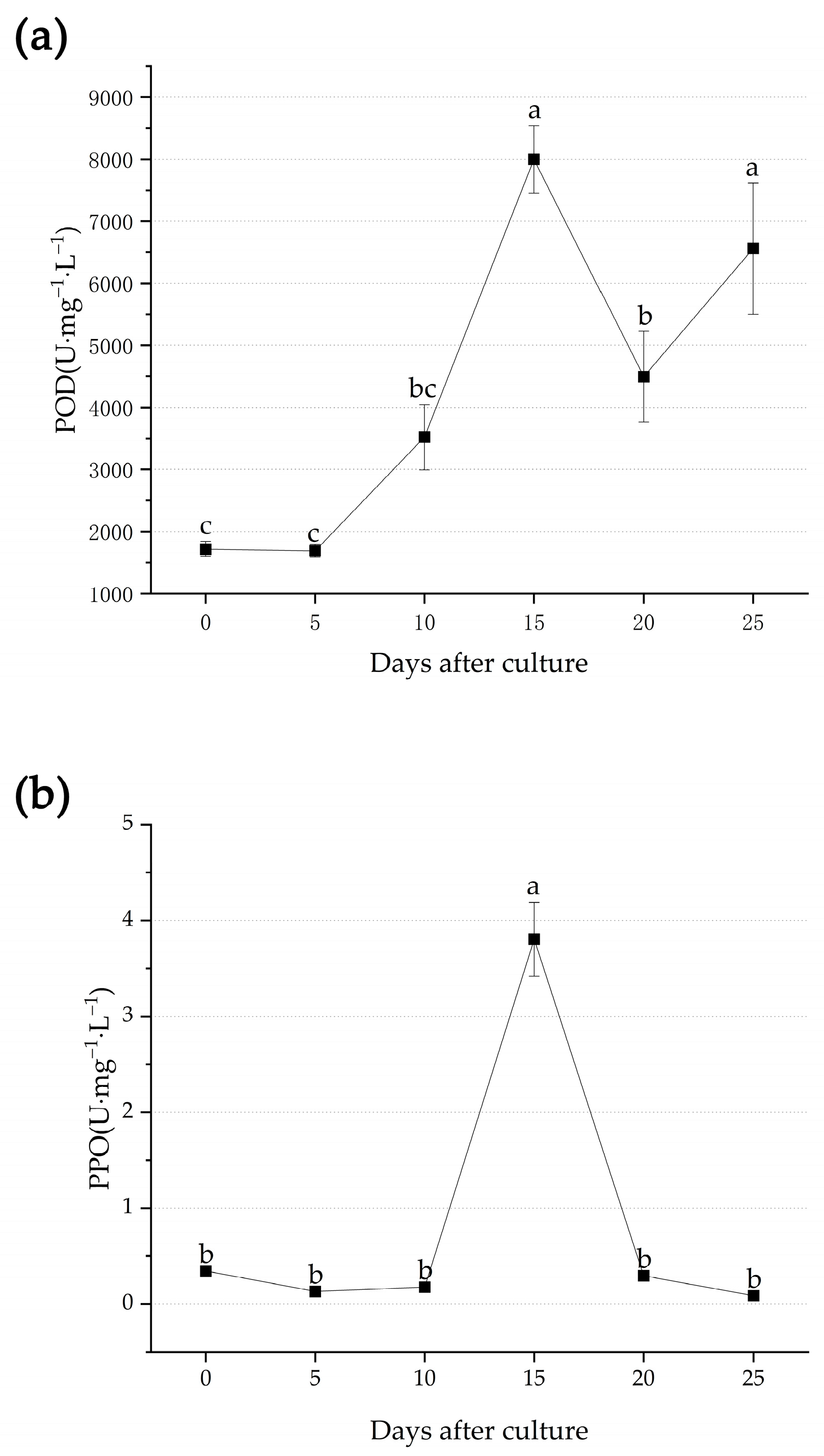
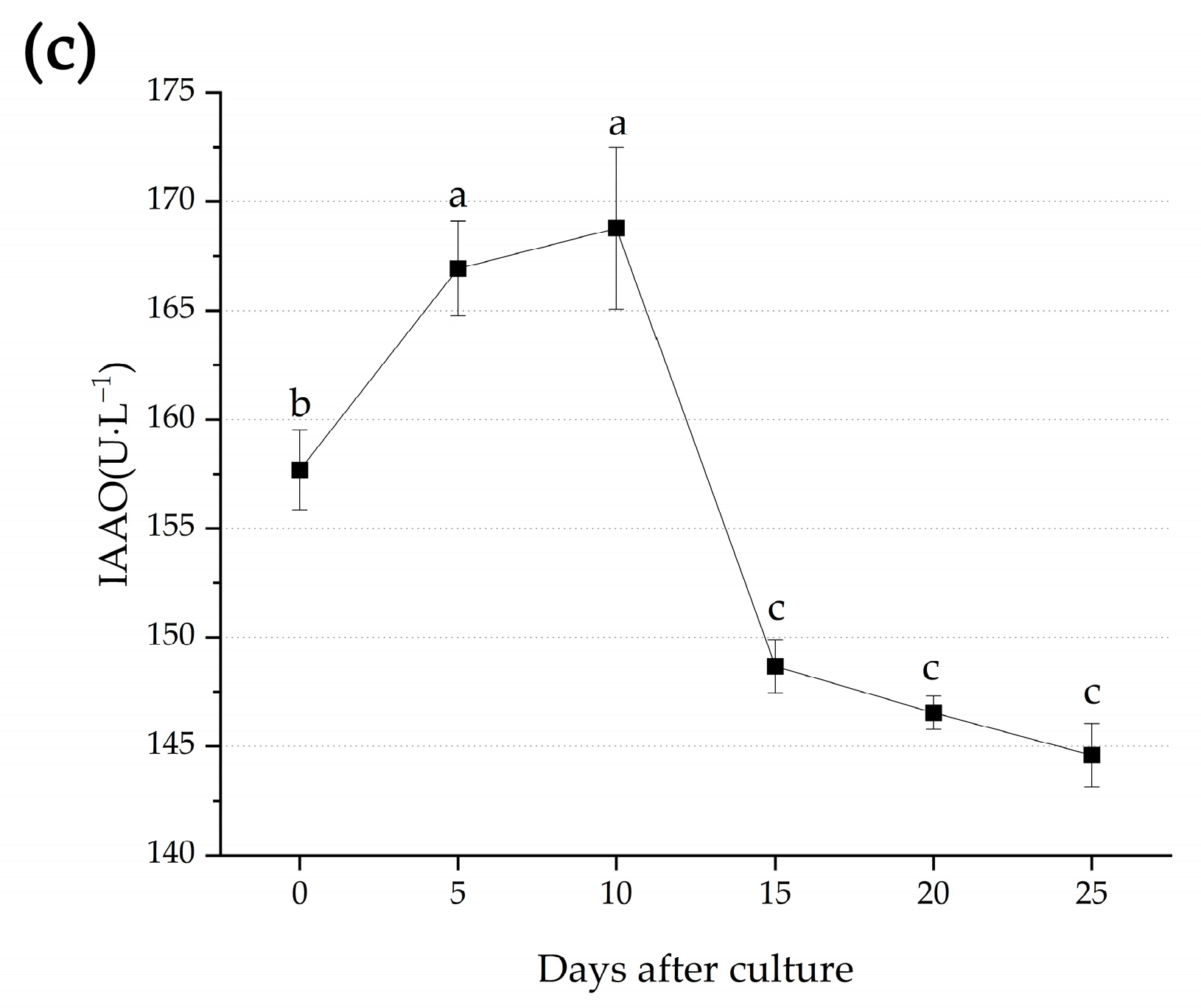
3.2. Metabolic Changes during Adventitious Root Formation
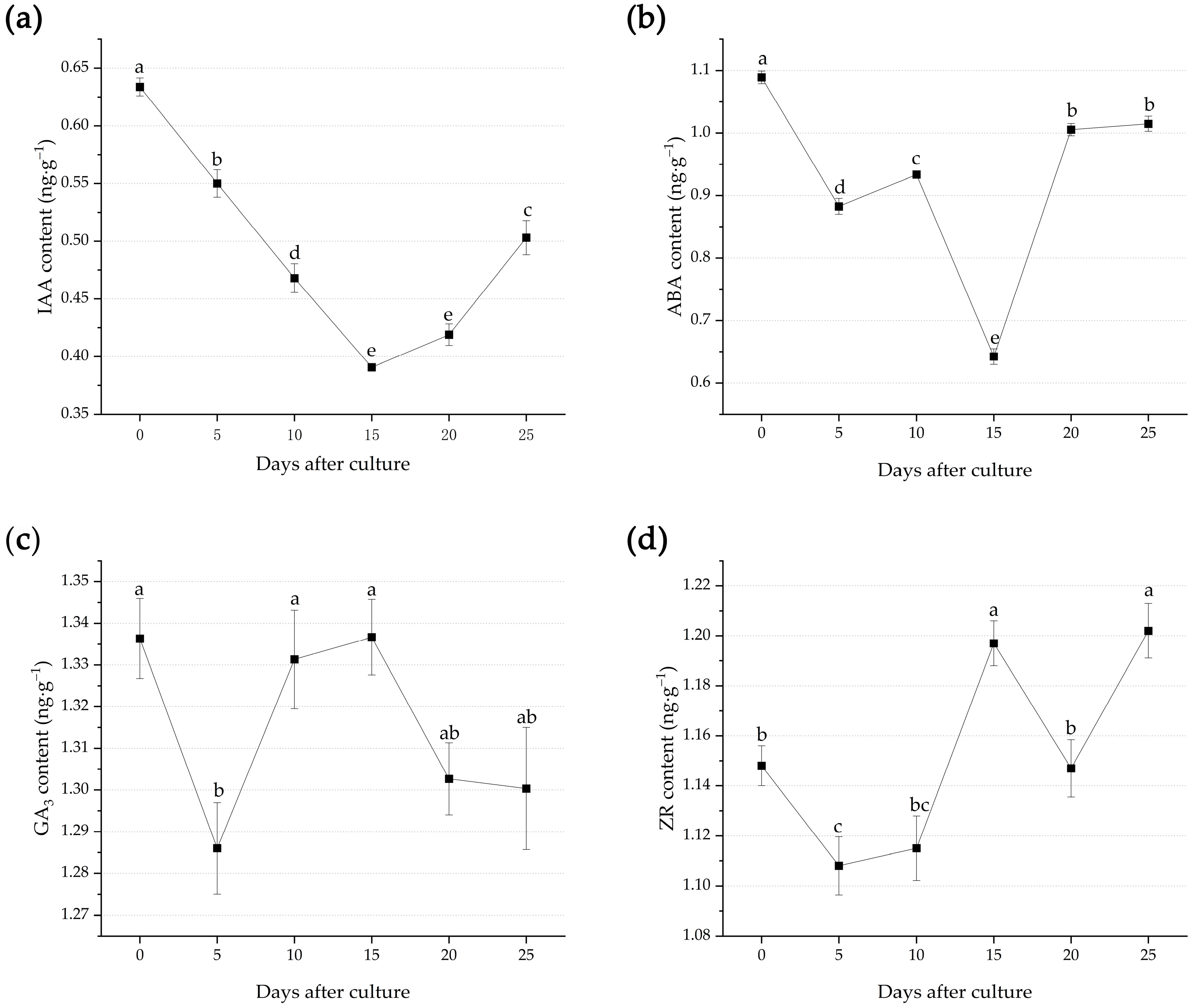
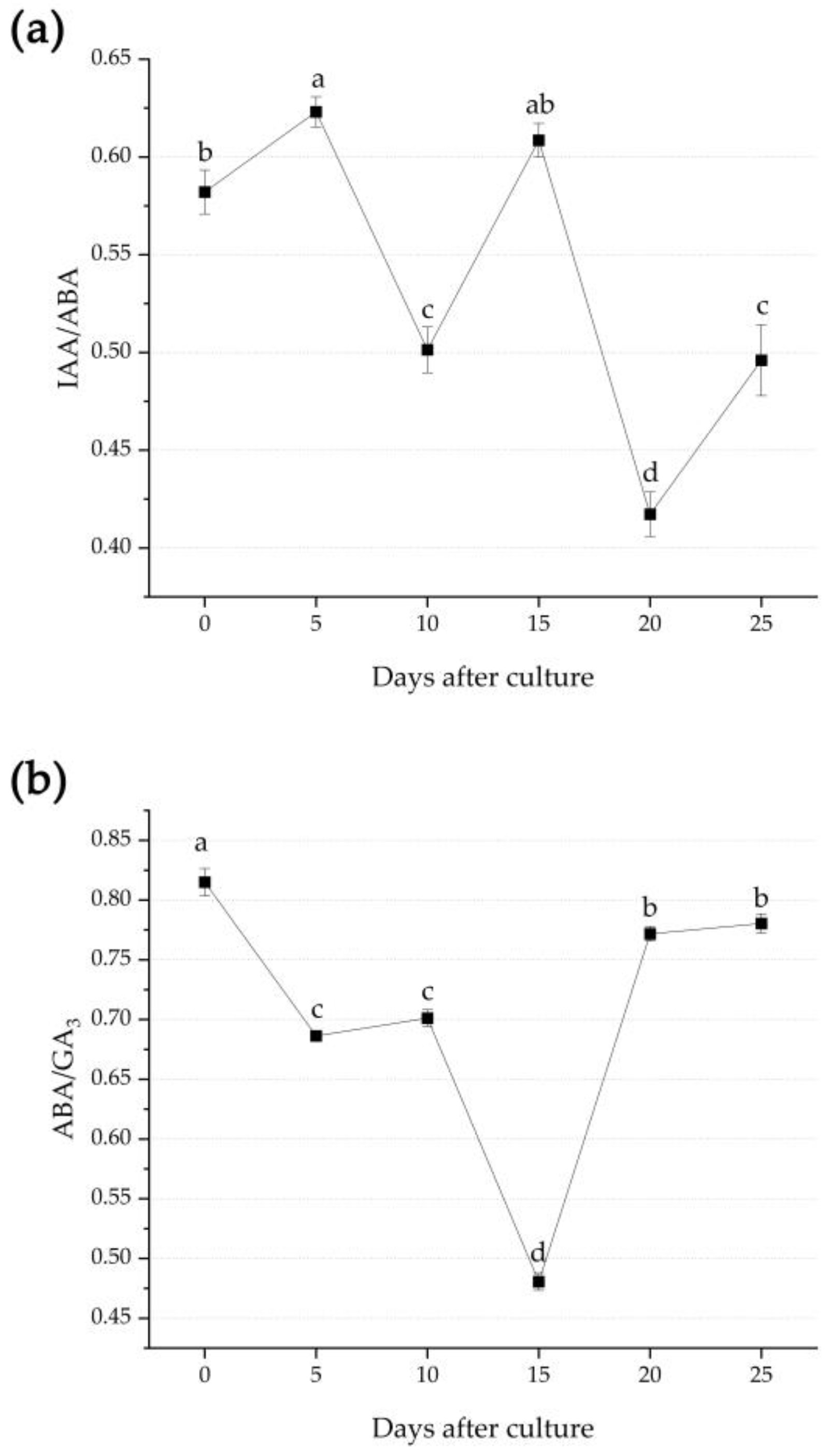
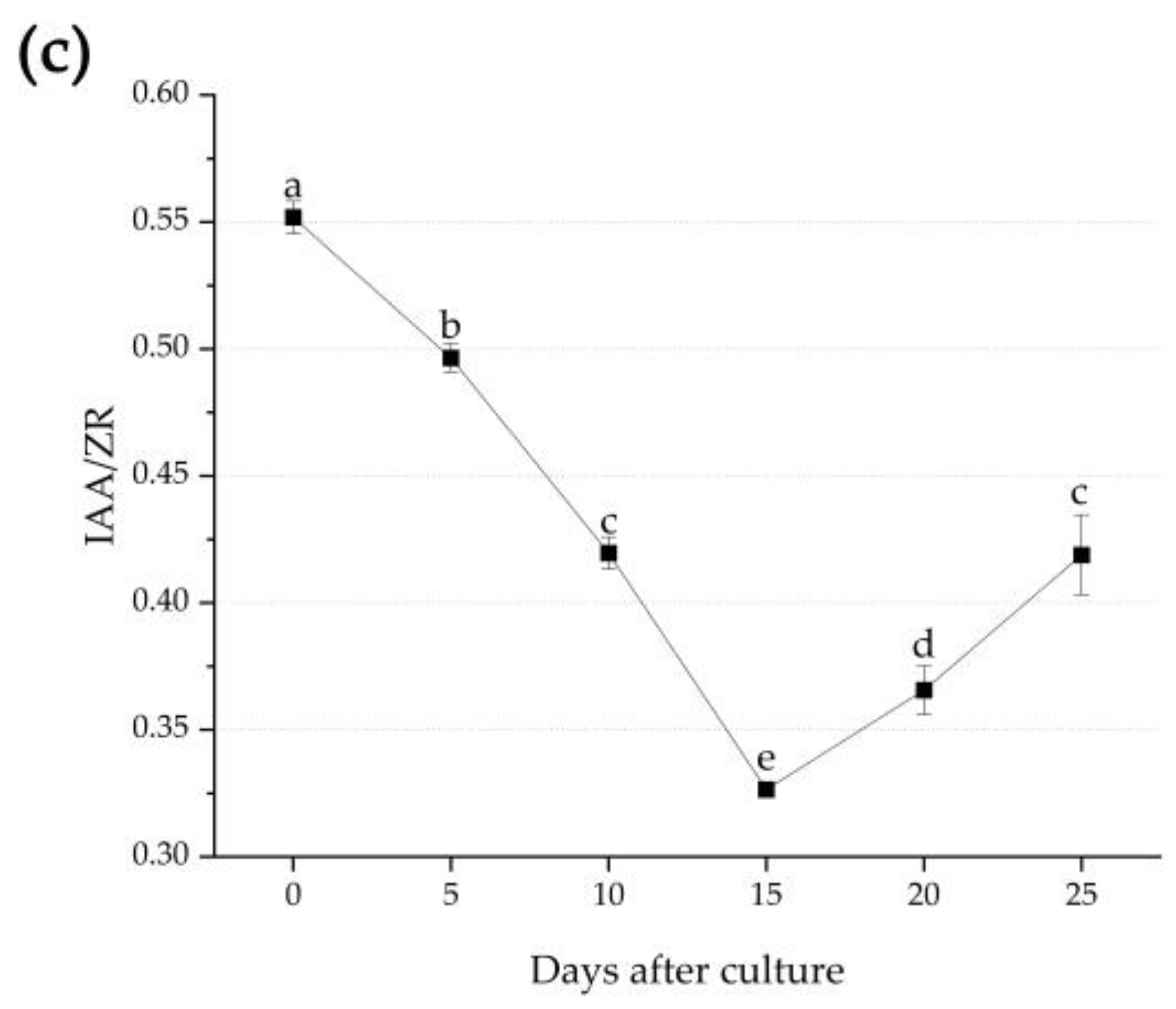
3.3. Endogenous Hormone Changes during Adventitious Root Formation
4. Discussion
4.1. Anatomical Evaluation and Root Primordia Development during Adventitious Root Formation
4.2. Changes of Metabolic Process during Adventitious Root Formation
4.3. Changes of Endogenous Hormones during Adventitious Root Formation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.H. New Flavone from the Aerial Parts of Bougainvillea glabra. Int. J. Comput. Eng. Res. 2014, 4, 1–5. [Google Scholar]
- Jain, R.; Janakiram, T.; Swaroop, K.; Kumar, S.; Kumawat, G.L. Standardization of protocol for in vitro multiplication of Bougainvillea. Indian J. Agric. Sci. 2016, 86, 516–521. [Google Scholar]
- Christopher, B.C.; James, L.G. Influence of Indolebutyric Acid Potassium Salt on Propagation of Semi-hardwood Stem Cuttings of Bougainvillea. HortScience 2006, 41, 983. [Google Scholar]
- Lai, R.Y.; Zhong, Z.H.; Zhang, X.Q.; Lin, L.X.; Su, M.H.; Xie, Z.N. Effects of Remaining Leaf Combining with IBA on Rooting, Physiological and Biochemical Indicators of Leaves from Bougainvillea spectabilis Cuttings. Agric. Sci. Technol. 2010, 11, 120–123. [Google Scholar]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Señorans, J.; Zwiazek, J.J. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012, 12, 99. [Google Scholar] [CrossRef] [Green Version]
- Tran, Y.B.N.; Van-Kieu, N.; Nguyen, P.T.K. Cytotoxic flavonoids from the roots of Bougainvillea spectabilis. Phytochem. Lett. 2021, 42, 117–120. [Google Scholar] [CrossRef]
- Singh, S.; Reddu, S.K.; Sharma, S.K.; Ali, M. New Unsaturated Fatty Acid from Roots of Bougainvillea spectabilis Willd. Asian J. Chem. 2009, 21, 4744–4748. [Google Scholar]
- Balasaraswathi, R.; Sadasivam, S.; Ward, M.; Walker, J.M. An antiviral protein from Bougainvillea spectabilis roots; purification and characterisation. Phytochemistry 1998, 47, 1561–1565. [Google Scholar] [CrossRef]
- Singh, V.; Aggrawal, V. Phytochemical analysis and in vitro antioxidant activities of leaves, stems, flowers, and roots extracts of Bougainvillea spectabilis Willd. Int. J. Green Pharm. 2018, 12, 278–284. [Google Scholar] [CrossRef]
- Jawla, S.; Kumar, Y.; Khan, M.S.Y. Isolation of phytoconstituents and antihyperglycemic activity of Bougainvillea spectabilis root bark extracts. Lat. Am. J. Pharm. 2013, 32, 1389–1395. [Google Scholar]
- Deshmukh, K.K.; Barad, A.V. Effect of IBA and NAA on Root Initiation of Various Varieties of Bougainvillea Species. Gujarat Agric. Univ. Res. J. 2002, 27, 44–46. [Google Scholar]
- Singh, N.; Singh, B.P.; Singh, H.K. Effect of different concentrations of indole butyric acid (IBA) on rooting potential and root growth of Bougainvillea stem cuttings. J. Ornam. Hortic. 2010, 13, 41–44. [Google Scholar]
- Kuruwanshi, V.B.; Deo, A.K.; Pal, D.P.; Sarnaik, D.A. Effect of treatment of stem cutting with IBA and NAA on sprouting, rooting and root biomass in Bougainvillea var. Refulgence. Adv. Plant Sci. 2009, 21, 557–558. [Google Scholar]
- Singh, P.; Singh, K. Effects of NAA and IBA on Rooting of Stem Cuttings in Bougainvillea CV. Cherry Blossom. Agric. Res. J. 2003, 40, 205–206. [Google Scholar]
- Abd, I.A.; Meklef, A.A.; Neamah, S.I. Microbial technique for the production of growth regulator compounds Indole Butyric Acid (IBA) and its role on the rooting and growth of Bougainvillea spectabilis L. stem cuttings. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 761, p. 012003. [Google Scholar]
- Atzmon, N.; Wiesman, Z.; Fine, P. Biosolids improve rooting of bougainvillea (Bougainvillea glabra) cuttings. J. Environ. Hortic. 1997, 15, 1–5. [Google Scholar] [CrossRef]
- Costa, E.M.; Loss, A.; Pereira, H.P.N.; Almeida, J.F. Rooting of Bougainvillea spectabilis Willd. stem cuttings using indol butyric acid. Acta Agron. 2015, 64, 205–210. [Google Scholar]
- Iddrisu, I.; Adzraku, H.-V.; Tandoh, P.K. Effects of Different Soil-biochar on Physico-Chemical Soil Properties, Rooting and Growth of Bougainvillea glabra and Ficus benjemena Using Stem Cuttings. Asian J. Agric. Hortic. Res. 2018, 1, 1–16. [Google Scholar] [CrossRef]
- Kale, P.N.; Bhujbal, B.G. Use of Growth Regulators in Rooting of Cuttings of Bougainvillea Var. Mary Palmer. Indian J. Hortic. 1972, 29, 307–309. [Google Scholar]
- Li, C.N.; Deng, J.L.; Zhou, J.Y.; Li, X.L.; Zen, S.J.; Bu, C.Y. lmpact Factors of Root Growth Under Hardwood Cuttage of Bougainvillea glabra. J. Agric. 2016, 6, 47–52. [Google Scholar]
- Kumari, P.; Swaroop, K.; Janakiram, T.; Singh, S.K.; Prasad, K.V.; Jain, R. In vitro Sterilization, Rooting and Acclimatization of Difficult-to-root Bougainvillea Cultivars. Int. J. Bio-Resour. Stress Manag. 2016, 7, 412–419. [Google Scholar] [CrossRef]
- Datta, S.K.; Mandal, A.K.A. Standardization of in vitro Multiplication of Two Difficult-to-root Bougainvillea Cultivars for Commercial Exploitation. Sci. Cult. 2012, 78, 251–254. [Google Scholar]
- Huang, T.; Zhang, H.H.; Zhao, R.N.; Zhu, Z.L. Establishing an Efficient Regeneration System for Tissue Culture in Bougainvillea buttiana ‘Miss Manila’. Plants 2022, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Swaroop, K.; Janakiram, T.; Jain, R.; Singh, S.K.; Singh, N. In vivo propagation of difficult-to-root Bougainvillea cultivars by air layering. J. Ornam. Hortic. 2014, 17, 82–87. [Google Scholar] [CrossRef]
- Correa, L.R.; Troleis, J.; Mastroberti, A.A.; Mariath, J.E.; Fett-Neto, A.G. Distinct modes of adventitious rooting in Arabidopsis thaliana. Plant Biol. 2012, 14, 100–109. [Google Scholar] [PubMed]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef]
- Xiao, Z.A.; Xiong, H. Research Progress in Mechanism of Adventitious root Organgenesis for Microporpagated Shoots. Biotechnol. Bull. 2002, 3, 31–34. [Google Scholar]
- Zhang, H.X.; Dong, C.J.; Li, F.K.; Wang, H.F.; Shang, Q.M. Progress on the Regulatory Mechanism of Adventitious Rooting. Acta Bot. Boreali-Occident. Sin. 2017, 37, 1457–1464. [Google Scholar]
- Xu, A.H.; Wei, C.X. Comprehensive comparison and applications of different sections in investigating the microstructure and histochemistry of cereal kernels. Plant Methods 2020, 16, 8. [Google Scholar] [CrossRef]
- Li, S.J.; Yang, N.; Chen, L.Q. Paraffin section observation of flower bud differentiation of Chimonanthus praecox in Kunming and comparison of the differentiation processes in different regions, China. Hortic. Plant J. 2022, 8, 221–229. [Google Scholar] [CrossRef]
- Liu, G.B.; Zhao, J.Z.; Zhang, Y.P.; Liao, T.; Guo, L.Q.; Cao, J. Study on the occurrence pattern of Platycladus orientalis. Acta Bot. Boreali-Occident. Sin. 2020, 40, 987–996. [Google Scholar]
- Zhang, Z.L.; Qu, W.J. Experimental Course of Plant Physiology; China Forestry Press: Beijing, China, 2003; pp. 88–104. [Google Scholar]
- Lu, W.J.; Li, Y.S. Experimental Course of Plant Physiology; China Forestry Press: Beijing, China, 2012; pp. 43–45. [Google Scholar]
- Zhang, W.X.; Fan, J.J.; Tan, Q.Q.; Zhao, M.M.; Cao, F.L. Mechanisms Underlying the Regulation of Root Formation in Malus hupehensis Stem Cuttings by Using Exogenous Hormones. J. Plant Growth Regul. 2017, 36, 174–185. [Google Scholar] [CrossRef]
- Yang, Y.M.; Xu, C.N.; Wang, B.M.; Jia, J.Z. Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regul. 2001, 35, 233–237. [Google Scholar]
- Liu, Y.; Xiao, D.X.; Huang, C.F.; Lei, X.G. The Anatomical Feasture Study on the Rooting of the Tender Branch Cuttings of Castanea mollissima Blume. Acta Hortic. Sin. 1997, 24, 8–12. [Google Scholar]
- Zhou, R.T.; Liu, Y.; Leng, S.S.; Wang, N.; Yan, S.P. Morphological and Anatomical Features during the Formation of Adventitious Roots of Populus tremula × P. tremuloides by Micro-cuttage Multiplication. Acta Bot. Boreali-Occident. Sin. 2013, 33, 2184–2188. [Google Scholar]
- Zhou, X.M.; Liu, Y.T.; Zhao, X.Z.; Song, Z.W.; Wang, S. Rooting Anatomy of Hardwood Cutting for Albizia julibrissin Duraxx. and Activity Change of Related Enzymes During Rooting Process. Bull. Bot. Res. 2016, 36, 58–61. [Google Scholar]
- Zhang, J.H.; Shi, J.J.; Xia, G.H.; Huang, Y.J.; Huang, J.X.; Wang, Z.J. Research of lignified cuttings and rooting mechanism of Carya illinoensis. J. Anhui Agric. Univ. 2014, 41, 203–208. [Google Scholar]
- Bai, X.Y.; Wang, L.R.; Wang, X.W.; Zhu, G.R.; Fang, W.C.; Cao, K.; Chen, C.W.; Li, Y. Microscopic observation on adventitious root development of micropropagation and cutting propagation on peach rootstocks. J. Fruit Sci. 2015, 32, 74–78+175. [Google Scholar]
- Zheng, Q.Q.; Lan, S.R.; Liu, X.D.; Feng, Y.C.; Peng, D.H. Effects of growth regulators on rooting and correlative enzyme activities of Michelia figo ‘Xiangfei’ cuttings. J. Cent. South Univ. For. Technol. 2020, 40, 67–76. [Google Scholar]
- Jin, H.D.; Zheng, C.Y.; Hua, B.; Yu, C.L.; Li, K.Y.; Yu, W.W. Rooting anatomy and physiological enzyme activity of Torreya grandis cuttings. Acta Agric. Zhejiangensis 2022, 34, 1955–1966. [Google Scholar]
- Vielba, J.M.; Vidal, N.; José, M.C.S.; Rico, S.; Sánchez, C. Recent Advances in Adventitious Root Formation in Chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant. 2014, 151, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kevers, C.; Hausman, J.F.; Faivre-Rampant, O.; Evers, D.; Gaspar, T. Hormonal control of adventitious rooting: Progress and questions. J. Appl. Bot. 1997, 71, 71–79. [Google Scholar]
- Ballester, A.; San-José, M.C.; Vidal, N.; Fernández-Lorenzo, J.L.; Vieitez, A.M. Anatomical and Biochemical Events during in vitro Rooting of Microcuttings from Juvenile and Mature Phases of Chestnut. Ann. Bot. 1999, 83, 619–629. [Google Scholar] [CrossRef]
- Sharma, A.K.; Prasad, R.N.; Chaturvedi, H.C. Clonal propagation of Bougainvillea glabra ‘Magnifica’ through shoot apex culture. Plant Cell Tissue Organ Cult. 1981, 1, 33–38. [Google Scholar] [CrossRef]
- Banda, J.; Bellande, K.; Wangenheim, D.V.; Goh, T.; Guyomarc, S.; Laplaze, L.; Bennett, M.J. Lateral Root Formation in Arabidopsis: A Well-Ordered LRexit. Trends Plant Sci. 2019, 24, 826–839. [Google Scholar] [CrossRef]
- Xie, Z.N.; Lai, R.Y.; Lin, L.X.; Zhong, Z.H. Anatomical observation on the rooting of the cutting of Bougainvillea spectabilis. J. Minxi Vocat. Tech. Coll. 2008, 10, 97–99. [Google Scholar]
- Hu, R. Anatomical Structure of the Stem of Bougainvillea glabra. J. Neijiang Norm. Univ. 2015, 30, 15–19. [Google Scholar]
- Ibáñez, S.; Ruiz-Cano, H.; Fernández, M.Á.; Sánchez-García, A.B.; Villanova, J.; Micol, J.L.; Pérez-Pérez, J.M. A Network-Guided Genetic Approach to Identify Novel Regulators of Adventitious Root Formation in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 461. [Google Scholar] [CrossRef]
- Zhou, N.F.; Zhang, J.P.; Liu, H.; Cha, W.W.; Pei, D. New Protocols for Paraffin Sections of Heterogeneous Tissues of Woody Plants. Chin. Bull. Bot. 2018, 53, 653–660. [Google Scholar]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; Forchetti, S.M.; Tigier, H.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000, 122, 1119–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheniany, M.; Ebrahimzadeh, H.; Masoudi-nejad, A.; Vahdati, K.; Leslie, C. Effect of endogenous phenols and some antioxidant enzyme activities on rooting of Persian walnut (Juglans regia L.). Afr. J. Plant Sci. 2010, 4, 479–487. [Google Scholar]
- Elmongy, M.S.; Zhou, H.; Cao, Y.; Liu, B.; Xia, Y.P. The effect of humic acid on endogenous hormone levels and antioxidant enzyme activity during in vitro rooting of evergreen azalea. Sci. Hortic. 2018, 227, 234–243. [Google Scholar] [CrossRef]
- Li, S.W.; Xue, L.G.; Xu, S.J.; Feng, H.Y.; An, L.Z. IBA-induced changes in antioxidant enzymes during adventitious rooting in mung bean seedlings: The role of H2O2. Environ. Exp. Bot. 2009, 66, 442–450. [Google Scholar] [CrossRef]
- Porfirio, S.; Calado, M.L.; Noceda, C.; Cabrita, M.J.; da Silva, M.G.; Azadi, P.; Peixe, A. Tracking biochemical changes during adventitious root formation in olive (Olea europaea L.). Sci. Hortic. 2016, 204, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Tehranifar, A.; Tabar, S.M.; Selahvarzi, Y. Biochemical changes in barberries during adventitious root formation: The role of indole-3-butyric acid and hydrogen peroxide. Span. J. Agric. Res. 2014, 12, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.X.; Fan, J.J.; Tan, Q.Q.; Zhao, M.M.; Zhou, T.; Cao, F.L. The effects of exogenous hormones on rooting process and the activities of key enzymes of Malus hupehensis stem cuttings. PLoS ONE 2017, 12, e0172320. [Google Scholar] [CrossRef] [Green Version]
- He, S.L. Dynamic changes in enzyme activities and phenolic content during in vitro rooting of tree peony (Paeonia suffruticosa Andr.) plantlets. Maejo Int. J. Sci. Technol. 2011, 5, 252–265. [Google Scholar]
- Meng, X.Y.; Wang, Z.; He, S.L.; Shi, L.Y.; Song, Y.L.; Lou, X.Y.; He, D. Endogenous hormone levels and activities of IAA-modifying enzymes during adventitious rooting of tree peony cuttings and grafted scions. Hortic. Environ. Biotechnol. 2019, 60, 187–197. [Google Scholar] [CrossRef]
- Ilczuk, A.; Jacygrad, E. The effect of IBA on anatomical changes and antioxidant enzyme activity during the in vitro rooting of smoke tree (Cotinus coggygria Scop.). Sci. Hortic. 2016, 210, 268–276. [Google Scholar] [CrossRef]
- Moncousin, C.H.; Gaspar, T.H. Peroxidase as a Marker for Rooting Improvement of Cynara scolymus L. Cultured in vitro. Biochem. Und Physiol. Pflanz. 1983, 178, 263–271. [Google Scholar] [CrossRef]
- Song, J.Y.; He, W.L.; Li, S.B.; Liu, Y.J.; Sun, Y.J. Analysis of physiological and biochemical characteristics related to cutting and rooting of chimera in Populous tomentosa carr. Sci. Silvae Sin. 2001, 37, 64–67. [Google Scholar]
- GÜNEŞ, T. Peroxidase and IAA-Oxidase Activities During Rooting in Cuttings of Three Poplar Species. Turk. J. Bot. 2000, 24, 97–102. [Google Scholar]
- Tong, Z.Q. Studies on the Softwood Cutting Propagation Technique and Rooting Mechanism of Euonymus alatus (Thunb.) Sieb. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 12 June 2017. [Google Scholar]
- Husen, A.; Pal, M. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New For. 2007, 33, 309–323. [Google Scholar] [CrossRef]
- Fett-Neto, A.; Teixeira, S.L.; Silva, E.A.A.D.; Anna, R.S. Biochemical and Morphological Changes during in vitro Rhizogenesis in Cuttings of Sequoia sempervirens (D. Don) Endl. J. Plant Physiol. 1992, 140, 720–728. [Google Scholar] [CrossRef]
- Mato, M.C.; Rúa, M.L.; Enrique, F. Changes in levels of peroxidases and phenolics during root formation in Vitis cultured in vitro. Physiol. Plant. 1988, 72, 84–88. [Google Scholar] [CrossRef]
- Tian, M.; Rao, L.B.; Li, J.Y. Reactive Oxygen Species (ROS)and lts Physiological Functions in Plant Cells. Plant Physiol. J. 2005, 37, 235–241. [Google Scholar]
- Druege, U.; Hilo, A.; Pérez-Pérez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef] [Green Version]
- Osterc, G.; Štampar, F. Differences in endo/exogenous auxin profile in cuttings of different physiological ages. J. Plant Physiol. 2011, 168, 2088–2092. [Google Scholar] [CrossRef]
- Kumar, P.; Patel, P.K.; Sonkar, M.K. Propagation through juvenile shoot cuttings in difficult-to-root Dalbergia latifolia—examining role of endogenous IAA in adventitious rooting. Plant Physiol. Rep. 2022, 27, 242–249. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Javid, M.G.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffens, B.; Wang, J.X.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.L.; Liang, J.H.; Qiao, Y.C.; Yan, Y.C.; Li, L.; Dai, Y.H. Involvement of G1-to-S transition and AhAUX-dependent auxin transport in abscisic acid-induced inhibition of lateral root primodia initiation in Arachis hypogaea L. J. Plant Physiol. 2012, 169, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Yang, Y.W.; Lur, H.S.; Tsai, Y.G.; Chang, M.C. A novel function of abscisic acid in the regulation of rice (Oryza sativa L.) root growth and development. Plant Cell Physiol. 2006, 47, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.J.; Liu, M.G.; Dai, F.; Wu, Y.L.; Shan, S.T.; Ding, R.J. Variation of endogenous hormone contents in softwood cuttings of Armeniaca sibirica during adventitious root formation. Non-Wood For. Res. 2013, 31, 108–114. [Google Scholar]
- Pacifici, E.; Polverari, L.; Sabatini, S. Plant hormone cross-talk: The pivot of root growth. J. Exp. Bot. 2015, 66, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, C.T.; De Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.L.; Qian, Y.P.; Jin, J.B.; Zhou, Q. Physiology and biochemistry dynamics in Carpinus betulus during rooting culture. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2017, 46, 43–49. [Google Scholar]
- Palmer, M.V.; Wong, O.C. Identification of Cytokinins from Xylem Exudate of Phaseolus vulgaris L. Plant Physiol. 1985, 79, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Davey, J.E.; Staden, J.V. Cytokinin translocation: Changes in zeatin and zeatin-riboside levels in the root exudate of tomato plants during their development. Planta 1976, 130, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Antón, M.Á.; Ferrández-Ayela, A.; Fernández-García, N.; Nicolás, C.; Albacete, A.; Pérez-Alfocea, F.; Sánchez-Bravo, J.; Pérez-Pérez, J.M.; Acosta, M. Early steps of adventitious rooting: Morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol. Plant. 2014, 150, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Wang, K.; Feng, Y.L. Endogenous hormones levels in cuttings of Larix olgensis and their relations to rooting. Bull. Bot. Res. 2002, 22, 190–195. [Google Scholar]
- He, H.W. Studies on The Changes of Structure, Physiology and GeneExpression in Chromolaena odorata Adventitious Rooting. Master’s Thesis, Guangxi University, Nanning, China, 25 May 2015. [Google Scholar]
- Ross, A.R.S.; Ambrose, S.J.; Cutler, A.J.; Feurtado, J.A.; Kermode, A.R.; Nelson, K.; Zhou, R.; Abrams, S.R. Determination of endogenous and supplied deuterated abscisic acid in plant tissues by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry with multiple reaction monitoring. Anal. Biochem. 2004, 329, 324–333. [Google Scholar] [CrossRef]
- Chen, L.Y.; Zheng, Y.; Chen, L.G.; Rong, J.D.; Zheng, Y.S. Study on Rhododendron hybridum’s rooting culture and hormones contents changes. J. For. Environ. 2011, 31, 131–135. [Google Scholar]
- Guo, S.J.; Ling, H.Q.; Li, F.L. Physiological and biochemical basis of rooting of Pinus bungeana cuttings. J. Beijing For. Univ. 2004, 26, 43–47. [Google Scholar]
- Cline, M.; Wesse, T.; Iwamura, H. Cytokinin/Auxin Control of Apical Dominance in Ipomoea nil. Plant Cell Physiol. 1997, 38, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Nag, S.; Saha, K.; Choudhuri, M. Role of Auxin and Polyamines in Adventitious Root Formation in Relation to Changes in Compounds Involved in Rooting. J. Plant Growth Regul. 2001, 20, 182–194. [Google Scholar] [CrossRef]
- Dagla, H. Plant tissue culture. Resonance 2012, 17, 759–767. [Google Scholar] [CrossRef]
- Feng, X. The Study of Basis of Adventitious Roots on (P. simonii × P. nigra) × P.15 ACL Tissue Culuture Leaves. Master’s Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]


| Proportioning and Dewatering Order | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
|---|---|---|---|---|---|
| Distilled water:ethanol | 1:1 | 3:7 | 3:17 | 1:19 | Only ethanol |
| Dehydration time/h | 0.5 | 0.5 | 0.5 | 0.5 | 1.0 |
| Proportioning and Dewatering Order | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
|---|---|---|---|---|---|
| xylene:Distilled water:ethanol | Only xylene | 1:1:0 | Only ethanol | 0:1:19 | 0:3:17 |
| Dehydration time/min | 60–90 | 5–10 | 5–10 | 5–10 | 5–10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Zhang, H.; Sheng, Q.; Zhu, Z. Morphological, Anatomical, Physiological and Biochemical Changes during Adventitious Roots Formation of Bougainvillea buttiana ‘Miss Manila’. Horticulturae 2022, 8, 1156. https://doi.org/10.3390/horticulturae8121156
Huang T, Zhang H, Sheng Q, Zhu Z. Morphological, Anatomical, Physiological and Biochemical Changes during Adventitious Roots Formation of Bougainvillea buttiana ‘Miss Manila’. Horticulturae. 2022; 8(12):1156. https://doi.org/10.3390/horticulturae8121156
Chicago/Turabian StyleHuang, Tao, Huihui Zhang, Qianqian Sheng, and Zunling Zhu. 2022. "Morphological, Anatomical, Physiological and Biochemical Changes during Adventitious Roots Formation of Bougainvillea buttiana ‘Miss Manila’" Horticulturae 8, no. 12: 1156. https://doi.org/10.3390/horticulturae8121156
APA StyleHuang, T., Zhang, H., Sheng, Q., & Zhu, Z. (2022). Morphological, Anatomical, Physiological and Biochemical Changes during Adventitious Roots Formation of Bougainvillea buttiana ‘Miss Manila’. Horticulturae, 8(12), 1156. https://doi.org/10.3390/horticulturae8121156





