Genome-Wide Characterization and Expression Profiling of NBS-LRR-Encoding Gene Family in Radish (Raphanus sativus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Identification of NBS-LRR-Encoding Genes in Radish Genome
2.3. Analysis of Gene Structure and Conserved Domains/Motifs
2.4. Sequence Alignment and Phylogenetic Analysis
2.5. Anchoring NBS-LRR-Encoding Genes to the Radish Chromosomes
2.6. Promoter Analysis and Transcriptome-Based Expression Profiling of RsNBS-LRR Genes
2.7. RNA Isolation and Quantitative Real-Time PCR
3. Results
3.1. Characterization of RsNBS-LRR Genes in Radish
3.2. Sequence Alignment and Gene Structure Analysis
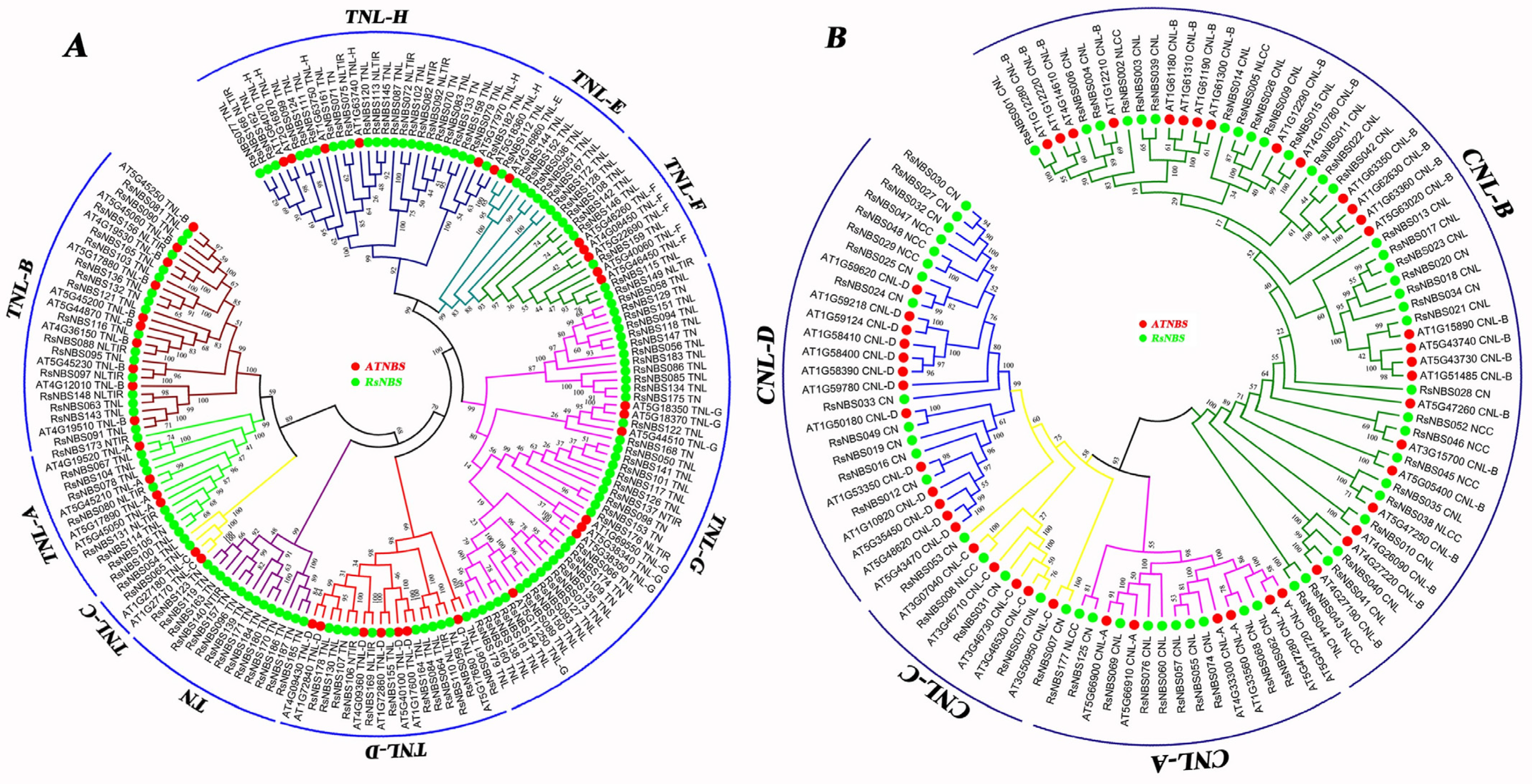
3.3. Phylogenetic Analysis of RsNBS-LRR Proteins
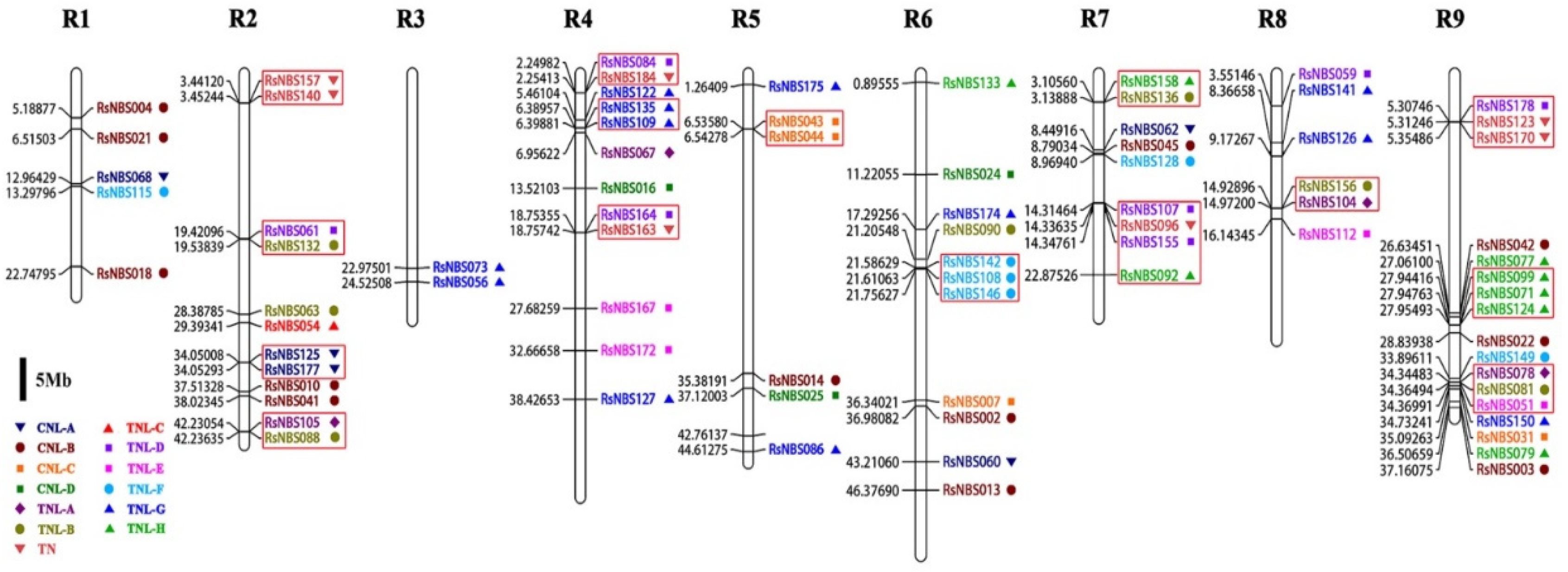
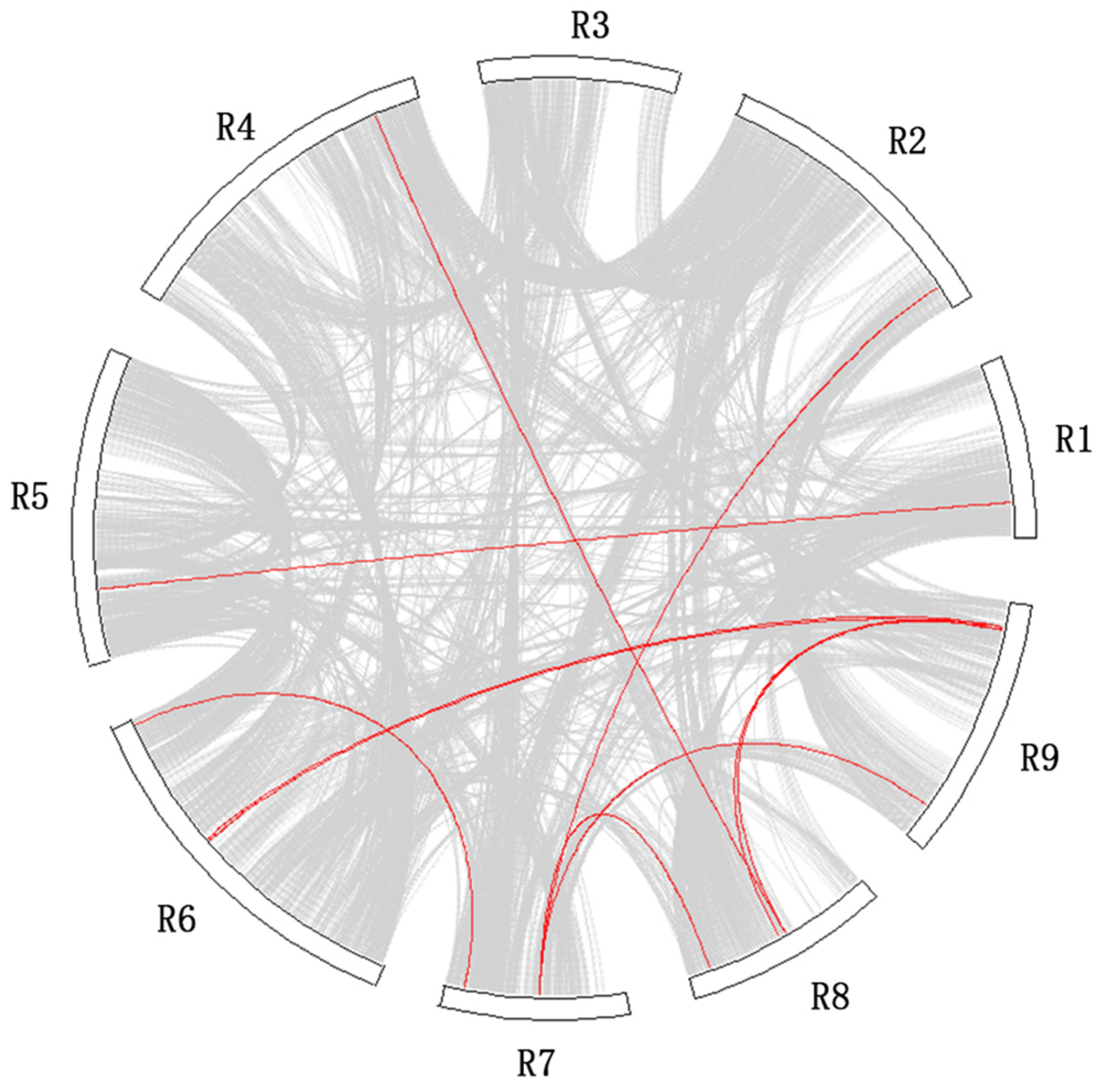
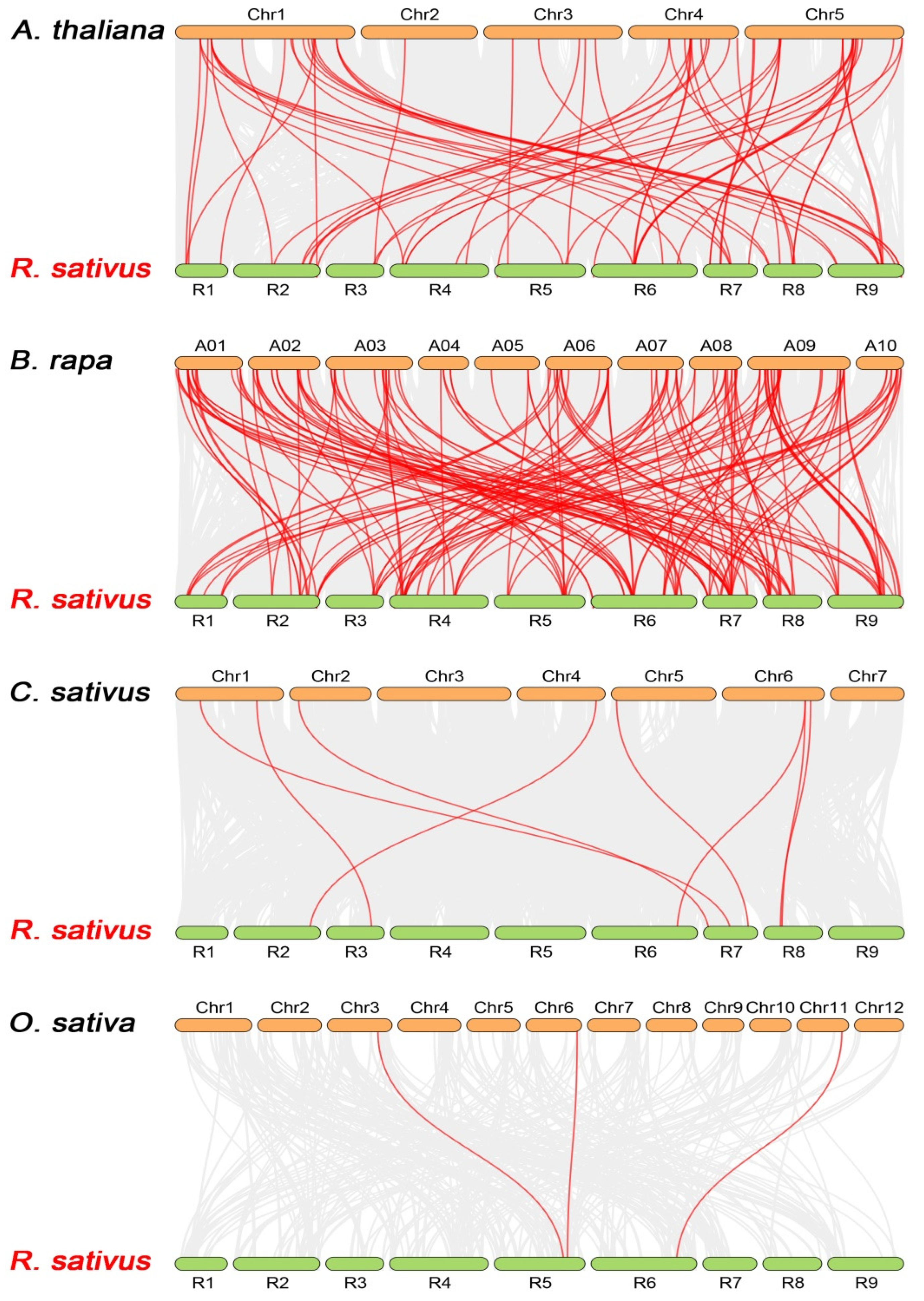
3.4. Chromosomal Location and Evolutionary Analysis of RsNBS-LRR Genes
3.5. Potential CREs Identification from Promoter Region of RsNBS-LRR Genes
3.6. Differential Expression Profiles of RsNBS-LRR Genes in Radish Tissues
3.7. Expression Profiling of RsNBS-LRR Genes under Biotic and Abiotic Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, Y.-M.; Chen, M.; Sun, L.; Wang, Y.; Yin, J.; Liu, J.; Sun, X.-Q.; Huang, Y.-Y. Genome-wide identification and evolutionary analysis of NBS-LRR genes from Dioscorea Rotundata. Front. Genet. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; van der Hoorn, R.A.L. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Hamblin, M.-T.; Prochnik, S.; Jannink, J.-L. Identification and distribution of the NBS-LRR gene family in the Cassava genome. BMC Genom. 2015, 16, 360. [Google Scholar] [CrossRef] [PubMed]
- Ameline-Torregrosa, C.; Wang, B.-B.; O’Bleness, M.-S.; Deshpande, S.; Zhu, H.; Roe, B.; Young, N.-D.; Cannon, S.-B. Identification and characterization of nucleotide-binding site-leucine-rich repeat genes in the model plant Medicago truncatula. Plant Physiol. 2008, 146, 5–21. [Google Scholar] [CrossRef]
- Meyers, B.-C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.-W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 1683. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Shao, Z.-Q.; Wang, Q.; Hang, Y.-Y.; Xue, J.-Y.; Wang, B.; Chen, J.-Q. Uncovering the dynamic evolution of nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes in Brassicaceae. J. Integr. Plant Biol. 2016, 58, 165–177. [Google Scholar] [CrossRef]
- Meyers, B.-C.; Morgante, M.; Michelmore, R.-W. TIR-X and TIR-NBS proteins: Two new families related to disease resistance TIR-NBS-LRR proteins encoded in Arabidopsis and other plant genomes. Plant J. 2002, 32, 77–92. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Zhang, Y.-M.; Li, Q.; Jiang, X.-M.; Jiang, Z.; Tang, J.-H.; Chen, D.; Wang, Q.; Chen, J.-Q.; et al. An angiosperm NLR atlas reveals that NLR gene reduction is associated with ecological specialization and signal transduction component deletion. Mol. Plant 2021, 14, 2015–2031. [Google Scholar] [CrossRef]
- Goldner, R.; Yaron, A. TIR axons apart: Unpredicted NADase controls axonal degeneration. Neuron 2017, 93, 1239–1241. [Google Scholar] [CrossRef]
- Liu, J.-L.; Liu, X.-L.; Dai, L.-Y.; Wang, G.-L. Recent progress in elucidating the structure, function and evolution of disease resistance genes in plants. J Genet Genom. 2007, 34, 765–776. [Google Scholar] [CrossRef]
- Wang, W.; Wen, Y.; Berkey, R.; Xiao, S. Specific targeting of the Arabidopsis resistance protein RPW8.2 to the interfacial membrane encasing the fungal haustorium renders broad-spectrum resistance to powdery mildew. Plant Cell 2009, 21, 2898–2913. [Google Scholar] [CrossRef]
- Xiao, S.; Calis, O.; Patrick, E.; Zhang, G.; Charoenwattana, P.; Muskett, P.; Parker, J.-E.; Turner, J.-G. The atypical resistance gene, RPW8, recruits components of basal defence for powdery mildew resistance in Arabidopsis. Plant J. 2005, 42, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.-B.; Bogdanove, A.-J.; Sessa, G. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 2003, 54, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, A.M.; Argueso, C.T. No hormone to rule them all: Interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, C.; Liu, W.; Wang, Y.J. The WRKY53 transcription factor enhances stilbene synthesis and disease resistance by interacting with MYB14 and MYB15 in Chinese wild grape. J. Exp. Bot. 2020, 71, 3211–3226. [Google Scholar] [CrossRef]
- Van de Weyer, A.-L.; Freddy, M.; Oliver, J.-F.; Marc, T.-N.; Volkan, C.; Kamil, W.; Jonathan, D.-G.-J.; Jeffery, L.-D.; Detlef, W.; Felix, B. A species-wide inventory of NLR genes and alleles in Arabidopsis thaliana. Cell 2019, 178, 1260–1272. [Google Scholar]
- Mun, J.-H.; Yu, H.-J.; Park, S.; Park, B.-S. Genome-wide identification of NBS-encoding resistance genes in Brassica rapa. Mol Genet. Genomics 2009, 282, 617–631. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, H.-Y.; Zhao, Y.; Qian, Y.-X.; Zhu, S.-W.; Cheng, B.-J. A genomic analysis of disease-resistance genes encoding nucleotide binding sites in Sorghum bicolor. Genet. Mol. Biol. 2010, 33, 292–297. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Yue, J.-X.; Tian, D.; Chen, J.-Q. Recent duplications dominate NBS-encoding gene expansion in two woody species. Mol. Genet. Genom. 2008, 280, 187–198. [Google Scholar] [CrossRef]
- Prakash, C.; Trognitz, F.C.; Venhuizen, P.; Haeseler, A.; Trognitz, B. A compendium of genome-wide sequence reads from NBS (nucleotide binding site) domains of resistance genes in the common potato. Sci. Rep. 2020, 10, 11392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Weng, Q.-Y.; Song, J.-H.; Ma, H.-L.; Yuan, J.-C.; Dong, Z.-P.; Liu, Y.-H. Bioinformatics analysis of NBS-LRR encoding resistance genes in Setaria italic. Biochem. Genet. 2016, 54, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.-X.; Liu, J.-G.; Wu, C.-F.; Deng, Y.-S.; Cai, C.-W.; Zhang, X.; Cai, Y.-F. Genome-wide comparative analysis of NBS-encoding genes in four Gossypium species. BMC Genom. 2017, 18, 292. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Si, W.; Zhao, L.; Yang, S.; Zhang, X. Dynamic evolution of NBS–LRR genes in bread wheat and its progenitors. Mol. Genet. Genom. 2015, 290, 727–738. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, Q.; Wu, Y.; Zhang, J.; Nie, J. Genome-Wide Identification and Characterization of the CC-NBS-LRR Gene Family in Cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2022, 23, 5048. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.; Tang, M.-J.; Wang, Y.; Dong, J.-H.; Ying, J.-L.; Chen, Y.-L.; Hu, B.; Li, C.; Liu, L.-W. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plant. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.-W.; Wu, J.; Wang, Y.; Gong, Y.-Q.; Wang, X.-L.; Cecilia, L.; Liu, L.-W. Identification and molecular mapping of the RsDmR Locus conferring resistance to downy mildew at seedling stage in radish. J. Integr. Agric. 2014, 13, 2362–2369. [Google Scholar] [CrossRef][Green Version]
- Wan, H.-J.; Yuan, W.; Bo, K.-L.; Shen, J.; Pang, X.; Chen, J.-F. Genome-wide analysis of NBS-encoding disease resistance genes in Cucumis sativus and phylogenetic study of NBS-encoding genes in Cucurbitaceae crops. BMC Genom. 2013, 14, 109. [Google Scholar] [CrossRef]
- Kitashiba, H.; Li, F.; Hirakawa, H.; Kawanabe, T.; Zou, Z.-W.; Hasegawa, Y.; Tonosaki, K.; Shirasawa, S.; Fukushima, A.; Yokoi, S.; et al. Draft sequences of the radish (Raphanus sativus L.) genome. DNA Res. 2014, 21, 481–490. [Google Scholar] [CrossRef]
- Mitsui, Y.; Shimomura, M.; Komatsu, K.; Namiki, N.; Shibata-Hatta, M.; Imai, M.; Katayose, Y.; Mukai, Y.; Kanamori, H.; Kurita, K.; et al. The radish genome and comprehensive gene expression profile of tuberous root formation and development. Sci Rep. 2015, 5, 10835. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-M.; Kim, N.; Ahn, B.-O.; Oh, M.; Chung, W.-H.; Chung, H.; Jeong, S.; Lim, K.-B.; Hwang, Y.-J.; Kim, G.-B.; et al. Elucidating the triplicated ancestral genome structure of radish based on chromosome-level comparison with the Brassica genomes. Theor. Appl. Genet. 2016, 129, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, T.; Wang, J.; Wang, P.; Qiu, Y.; Zhao, W.; Pang, S.; Li, X.; Wang, H.; Song, J.; et al. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol. Plant 2021, 14, 2032–2055. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Jang, H.; Baek, S.; Kim, M.-J.; Yim, B.; ·Huh, S.; Kwon, S.-H.; Yu, H.-J.; Mun, J.-H. An improved Raphanus sativus cv. WK10039 genome localizes centromeres, uncovers variation of DNA methylation and resolves arrangement of the ancestral Brassica genome blocks in radish chromosomes. Theor. Appl. Genet. 2022, 135, 1731–1750. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.-H.; Zhang, W.; Xu, L.; Su, X.-J.; Muleke, E.-M.; Liu, L.-W. Identification and characterization of expressed TIR- and non-TIR-NBS-LRR resistance gene analogous sequences from radish (Raphanus sativus L.) de novo transcriptome. Sci. Hortic. 2017, 216, 284–292. [Google Scholar] [CrossRef]
- Finn, R.-D.; Clements, J.; Eddy, S.-R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.-P.; Guo, A.-Y.; Zhang, H.; Luo, J.-C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Finn, R.-D.; Coggill, P.; Eberhardt, R.-Y.; Eddy, S.-R.; Mistry, J.; Mitchell, A.-L.; Potter, S.-C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2015, 44, D279–D285. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef]
- Delorenzi, M.; Speed, T. An HMM model for coiled-coil domains and a comparison with PSSM-based predictions. Bioinformatics 2002, 18, 617–625. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.-D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Chen, H.; Zhang, Y.; Thomas, H.-R.; Frank, M.-H.; He, Y.-H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.-V.; Choe, G.; Zheng, Y.; Aliaga Fandino, A.-C.; Sung, I.; Hur, J.; Kamran, M.; Park, C.; Kim, H.; Ahn, H.; et al. Identification of conserved gene-regulatory networks that integrate environmental sensing and growth in the root cambium. Curr. Biol. 2020, 30, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.-R.; Baker, B.-J. The evolution of resistance genes in multi-protein plant resistance systems. Curr. Opin. Genet. Dev. 2007, 17, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Castel, B.; Ngou, P.-M.; Cevik, V.; Redkar, A.; Kim, D.-S.; Yang, Y.; Ding, P.; Jones, J.-D.-G. Diverse NLR immune receptors activate defence via the RPW8-NLR NRG1. New Phytol. 2019, 222, 966–980. [Google Scholar] [CrossRef]
- Monteiro, F.; Nishimura, M.T. Structural, functional, and genomic diversity of plant NLR proteins: An evolved resource for rational engineering of plant immunity. Annu. Rev. Phytopathol. 2018, 56, 243–267. [Google Scholar] [CrossRef]
- Collier, S.-M.; Hamel, L.-P.; Moffett, P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol. Plant-Microbe Interact. 2011, 24, 918–931. [Google Scholar] [CrossRef]
- Ma, Y.; Chhapekar, S.-S.; Lu, L.; Oh, S.; Singh, S.; Kim, C.-S.; Kim, S.; Choi, G.-J.; Lim, Y.-P.; Choi, S.-R. Genome-wide identification and characterization of NBS-encoding genes in Raphanus sativus L. and their roles related to Fusarium oxysporum resistance. BMC Plant Biol. 2021, 21, 47. [Google Scholar] [CrossRef]
- Ma, Y.; Chhapekar, S.-S.; Lu, L.; Yu, X.; Kim, S.; Lee, S.-M.; Gan, T.-H.; Choi, G.-J.; Lim, Y.-P.; Choi, S.-R. QTL mapping for Fusarium wilt resistance based on the whole-genome resequencing and their association with functional genes in Raphanus sativus. Theor. Appl. Genet. 2021, 134, 3925–3940. [Google Scholar] [CrossRef]


| Predicted Domains | Letter Code | Number | Proportion |
|---|---|---|---|
| CNL Type | |||
| CC-NBS-LRR | CNL | 24 | 12.83% |
| CC-NBS | CN | 24 | 12.83% |
| NBS-LRR | NL | 6 | 3.21% |
| NBS | N | 6 | 3.21% |
| TNL Type | |||
| TIR-NBS-LRR | TNL | 79 | 42.25% |
| TIR-NBS | TN | 28 | 14.97% |
| NBS-LRR | NL | 15 | 8.02% |
| NBS | N | 5 | 2.67% |
| Total | 187 |
| Class | Motif | Consensus Sequence |
|---|---|---|
| TNL-NBS-LRR | P-loop/Kin1 | IGIWGPAGIGKTTIARALYNQ |
| TNL RNBS-A | DDYGLKLRLQEQLLSKILN | |
| TNL Kin2 | DKKVLVVLDDVDDLEQLEALA | |
| TNL RNBS-B | WFGPGSRIIITTQDKKLLKAH | |
| TNL RNBS-C | EVKFPSDDEALQIFCRYAF | |
| TNL GLPL | EVVELAGGLPLGLRVLGSYLRGKSK | |
| TNL RNBS-D | GEIESYLRVSYDALSDEDKDLFLHIACFF | |
| TNL MHDL | IEMHDLLQQMGREIV | |
| CNL-NBS-LRR | P-loop/Kin1 | VGIYGMGGYGKTTLARQINNK |
| CNL RNBS-A | HFDVVIWVVVSKEFQIKKIQQ | |
| CNL Kin2 | KKFLLVLDDIW | |
| CNL RNBS-B | NGSKVVFTTRSEEVC | |
| CNL RNBS-C | MEVECLTEEEAWELFQKKAGP | |
| CNL GLPL | DIEELAKKIAKKCGGLPLALKVIGGLM | |
| CNL RNBS-D | PEHLKSCFLYCALFPEDYEIEKEKLIEYW | |
| CNL MHDL | VKMHDVVREMALWIA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhang, W.; Tang, M.; Zhang, X.; Wang, J.; Wang, Y.; Liu, L. Genome-Wide Characterization and Expression Profiling of NBS-LRR-Encoding Gene Family in Radish (Raphanus sativus L.). Horticulturae 2022, 8, 1164. https://doi.org/10.3390/horticulturae8121164
Xu L, Zhang W, Tang M, Zhang X, Wang J, Wang Y, Liu L. Genome-Wide Characterization and Expression Profiling of NBS-LRR-Encoding Gene Family in Radish (Raphanus sativus L.). Horticulturae. 2022; 8(12):1164. https://doi.org/10.3390/horticulturae8121164
Chicago/Turabian StyleXu, Liang, Wei Zhang, Mingjia Tang, Xiaoli Zhang, Juanjuan Wang, Yan Wang, and Liwang Liu. 2022. "Genome-Wide Characterization and Expression Profiling of NBS-LRR-Encoding Gene Family in Radish (Raphanus sativus L.)" Horticulturae 8, no. 12: 1164. https://doi.org/10.3390/horticulturae8121164
APA StyleXu, L., Zhang, W., Tang, M., Zhang, X., Wang, J., Wang, Y., & Liu, L. (2022). Genome-Wide Characterization and Expression Profiling of NBS-LRR-Encoding Gene Family in Radish (Raphanus sativus L.). Horticulturae, 8(12), 1164. https://doi.org/10.3390/horticulturae8121164









