Aroma Volatiles in Litchi Fruit: A Mini-Review
Abstract
1. Introduction
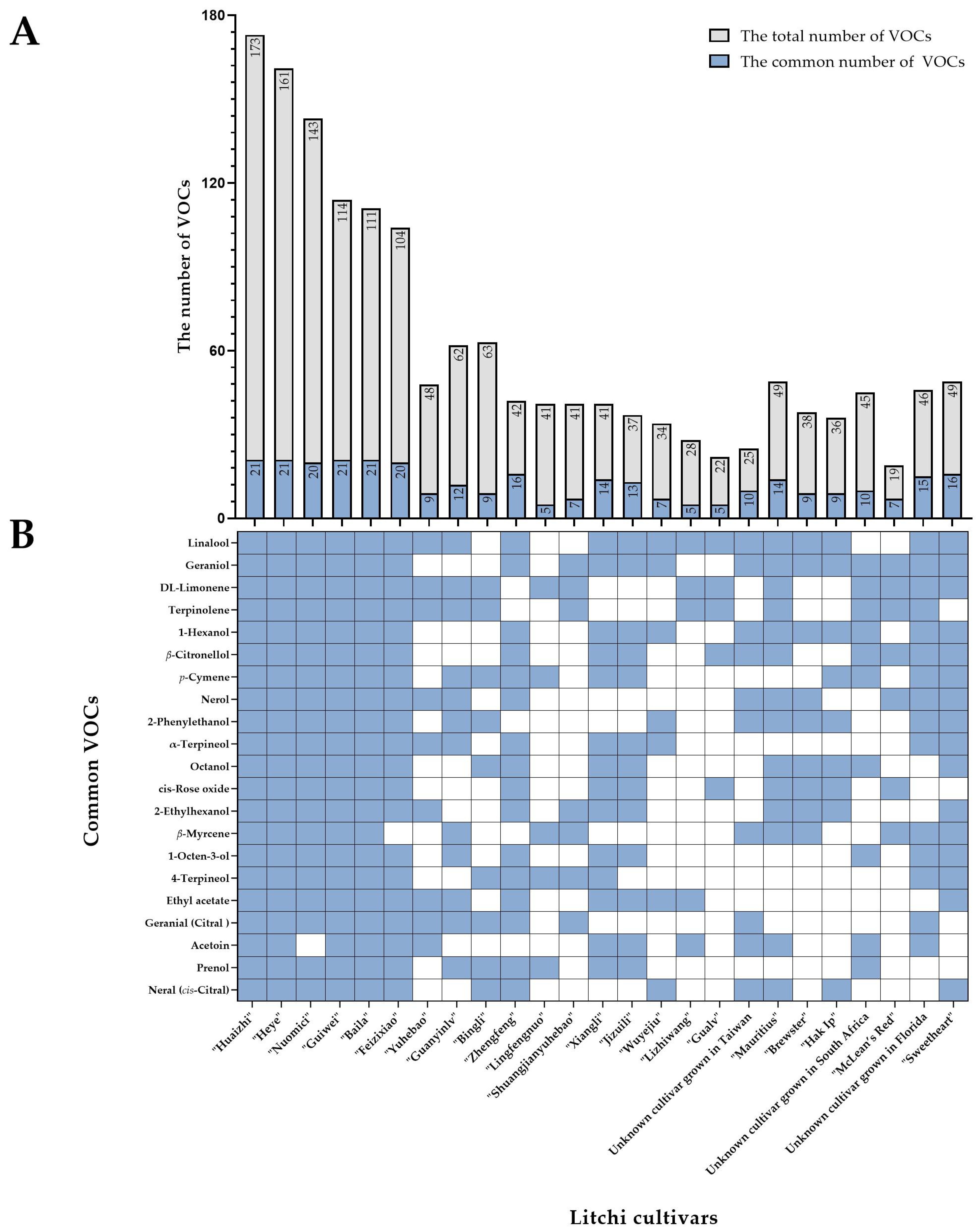
2. The Profiles of VOCs in Litchi Fruit
| Producing Countries | Cultivars | Methods of Detection | Number of VOCs | References |
|---|---|---|---|---|
| China | “Huaizhi” | GC-MS | 173 | Wu et al. (2009) [24], Li et al. (2010) [25], Xu et al. (2010) [28], Yang et al. (2014) [29], Fan et al. (2017) [26], |
| “Heye” | GC-MS | 161 | Hao et al. (2007) [30], Shu et al. (2008) [31], Wu et al. (2009) [24], Xu et al. (2010) [28], Lu (2014) [27], | |
| “Nuomici” | GC-MS | 143 | Ong and Acree (1998) [32], Cai et al. (2007) [33], Chen et al. (2009) [34], Wu et al. (2009) [24], Xu et al. (2010) [28], Fan et al. (2017) [26] | |
| “Guiwei” | GC-MS | 114 | Wu et al. (2009) [24], Xu et al. (2010) [28], Lu (2014) [27] | |
| “Baila” | GC-MS | 111 | Hao et al. (2007) [30], Wu et al. (2009) [24], Yang et al. (2014) [29] | |
| “Feizixiao” | GC-MS | 104 | Hao et al. (2007) [30], Wu et al. (2009) [24], Xu et al. (2010) [28] | |
| “Yuhebao” | GC-MS | 48 | Hao et al. (2007) [30], Xu et al. (2010) [28] | |
| “Guanyinlv” | GC-MS | 62 | Ma et al. (2015) [35] | |
| “Bingli” | GC-MS | 63 | Dong et al. (2022) [36] | |
| “Zhengfeng” | GC-MS | 42 | Wu et al. (2009) [24] | |
| “Lingfengnuo” | GC-MS | 41 | Fan et al. (2017) [26] | |
| “Shuangjianyuhebao” | GC-MS | 41 | Yang et al. (2014) [29] | |
| “Xiangli” | GC-MS | 41 | Wu et al. (2009) [24] | |
| “Jizuili” | GC-MS | 37 | Wu et al. (2009) [24] | |
| “Wuyejiu” | GC-MS | 34 | Xing et al. (1995) [37] | |
| “Lizhiwang” | GC-MS | 28 | Hao et al. (2007) [30] | |
| “Gualv” | GC-MS | 22 | Xu et al. (2010) [28] | |
| An unknown cultivar grown in Taiwan | GC-MS | 25 | Chyau et al. (2003) [38] | |
| South Africa | “Mauritius” | GC-O/MS/PFPD | 49 | Mahattanatawee et al. (2007) [39], Sivakumar et al. (2008) [40] |
| “Brewster” | GC-O/MS/PFPD | 38 | Mahattanatawee et al. (2007) [39] | |
| “Hak Ip” | GC-O/MS/PFPD | 36 | Mahattanatawee et al. (2007) [39] | |
| Unknown cultivar grown in South Africa | GC-MS/FTIR | 34 | Frohlich and Schreier (1986) [41] | |
| “McLean’s red” | GC-MS | 19 | Sivakumar et al. (2008) [40] | |
| America | Unknown cultivar grown in Florida | GC-MS | 42 | Johnston et al. (1980) [22] |
| “Sweetheart” | GC-O/MS | 31 | Feng et al. (2018) [23] |
3. Characteristics of VOCs in Litchi Fruit
4. Sulfur-Containing Volatile Compounds (VSCs) in Litchi Fruit
5. Future Research Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepherd, G.M. Smell images and the flavour system in the human brain. Nature 2006, 444, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun-Waterhouse, D.; Su, G.; Zhao, M. New insight into umami receptor, umami/umami-enhancing peptides and their derivatives: A review. Trends Food Sci. Technol. 2019, 88, 429–438. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Xu, H.; Turlings, T.C.J. Plant volatile as meta-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Campos-Herrera, R.; Kaplan, F.; Duncan, L.; Rodriguez-Saona, C. Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS ONE 2012, 7, e38146. [Google Scholar] [CrossRef]
- Hiltpold, I.; Turlings, T.C.J. Manipulation of chemically mediated interactions in agricultural soils to enhance the control of crop pests and to improve crop yield. J. Chem. Ecol. 2012, 38, 641–650. [Google Scholar] [CrossRef]
- Hu, L. Integration of multiple volatile cues into plant defense responses. New Phytol. 2022, 233, 618–623. [Google Scholar] [CrossRef]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-b-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F. Practical applications of research into the regulation of plant volatile emission. Curr. Opin. Plant Biol. 2005, 8, 113–118. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, K.; Wootton, M.; Paton, J.E. Sensory testing of Australian fragrant, imported fragrant, and non-fragrant rice aroma. Int. J. Food Prop. 2002, 7, 27–36. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Luo, Y.; Xiao, L.; Wang, K.; Huang, J.; Liu, Z. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. LWT-Food Sci. Technol. 2020, 127, 109355. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Pereira, L.; Sapkota, M.; Alonge, M.; Zheng, Y.; Zhang, Y.; Razifard, H.; Taitano, N.; Schatz, M.; Ferbue, A.; Wang, Y.; et al. Natural genetic diversity in tomato flavor genes. Front. Plant Sci. 2021, 12, 642828. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Diao, W.; Zhu, H.; Umer, M.; Zhao, S.; He, N.; Lu, X.; Yuan, P.; Anees, M.; Yang, D.; et al. Metabolome and Transcriptome integration reveals insights into flavor formation of ‘Crimson’ watermelon flesh during fruit development. Front. Plant Sci. 2021, 12, 629361. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, N.; Feng, R.; Meng, Z.; Li, Y.; Zhao, Z. Transcriptome and metabolite profling analyses provide insight into volatile compounds of the apple cultivar ‘Ruixue’ and its parents during fruit development. BMC Plant Biol. 2021, 21, 231. [Google Scholar] [CrossRef]
- Zhao, J. Litchi Theme and Image Literature Research—With Han-Song as the Center of Investigation. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2012. [Google Scholar]
- Hu, G.; Feng, J.; Xiang, X.; Wang, J.; Salojarvi, J.; Liu, C.; Wu, Z.; Zhang, J.; Liang, X.; Jiang, Z.; et al. Two divergent haplotypes from a highly heterozygous lychee genome suggest independent domestication events for early and late-maturing cultivars. Nat. Genet. 2022, 54, 73–83. [Google Scholar] [CrossRef]
- He, M.; Zhou, Y.; Zhu, H.; Jiang, Y.; Qu, H. Metabolome, transcriptome and physiological analyses provide insight into the color transition of litchi pericarp. Postharvest Biol. Technol. 2022, 192, 112031. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef]
- Johnston, J.C.; Welch, R.C.; Hunter, G.L.K. Volatile constitutes of litchi (Litchi chinensis Sonn.). J. Agric. Food Chem. 1980, 28, 859–861. [Google Scholar] [CrossRef]
- Feng, S.; Huang, M.; Crane, J.; Wang, Y. Characterization of key aroma-active compounds in lychee (Litchi chinensis Sonn.). J. Food Drug Anal. 2018, 26, 497–503. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Q.; Qu, W.; Duan, C. Comparison of volatile profiles of nine litchi (Litchi chinensis Sonn.) cultivars from southern China. J. Agric. Food Chem. 2009, 57, 9676–9681. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hao, J.; Zhong, H.; Xu, Y. Free and glycosidically bound volatile flavor compounds in fruit of Litchi chinensis ‘Huaizhi’. Food Sci. 2010, 31, 268–271. [Google Scholar]
- Fan, Y.; Huang, X.; Mo, W.; Miao, B.; Wu, W.; Li, W. Analysis of aromatic compounds of three litchi cultivars by SPME/GC-MS. Chin. J. Trop. Agric. 2017, 37, 72–78. [Google Scholar]
- Lu, K. The Characteristic Aroma Composition Analysis of Lychee and Lychee Wine. Master’s Thesis, Northwest Agriculture and Forestry University, Yanglin, China, 2014. [Google Scholar]
- Xu, H.; Yu, X.; Hu, Z.; Chen, H. Study on extraction and analyzing of aroma compounds in seven cultivars of litchi fruits. Food Mach. 2010, 26, 26–26+39. [Google Scholar] [CrossRef]
- Yang, B.; Yao, L.; Li, G.; Zhou, C.; He, Z.; Tu, S. Analysis of amino acids and aromatic components of pulps for different litchi variety. Chin. J. Trop. Crops 2014, 35, 1228–1234. [Google Scholar]
- Hao, J.; Xu, Y.; Li, C.; Dou, H.; Gu, H.; Hu, W. Analysis of aromatic compositions in different breeds of litchi by SPME/GC-MS methods. Food Sci. 2007, 28, 404–408. [Google Scholar]
- Shu, Z.; Qin, Y.; Xiao, W.; Han, D.; Wu, J. Aromatic compounds of fresh juice and fermented wine made of Litchi (cv. Heiye). Guangdong Agric. Sci. 2008, 1, 67–69. [Google Scholar]
- Ong, P.K.C.; Acree, T.E. Gas chromatography olfactory analysis of lychee (Litchi chinensis Sonn.). J. Agric. Food Chem. 1998, 46, 2282–2286. [Google Scholar] [CrossRef]
- Cai, C.; Guo, J.; Zeng, Q. Study on aromatic components of fresh litchi and dried litchi. Food Sci. 2007, 28, 455–461. [Google Scholar]
- Chen, Y.; Cai, C.; Zeng, X. Analysis of aroma components in fresh litchi by HS-SPME-GC-MS. Mod. Food Sci. Technol. 2009, 25, 91–95. [Google Scholar] [CrossRef]
- Ma, K.; Gu, C.; Yin, J.; Hu, R.; Luo, S.; Wang, Z.; Li, J. An analysis of volatile components in ‘Guanyinlü’ litchi by headspace solid-phase microextraction with GC-MS. J. S. China Agric. Univ. 2015, 36, 113–116. [Google Scholar]
- Dong, C.; Li, J.; Zheng, X.; Wang, G.; Li, W.; Hu, G. Analysis on the flesh fragrance ingredient of ‘Bingli’, the new litchi specie by adopting HS SPME/GC-MS. S. China Agric. 2022, 16, 12–16. [Google Scholar]
- Xing, Q.; Jin, S.; Lin, Z.; Wang, X. A study on the composition of top note of litchi in Fujian. Acta Sci. Nat. Univ. Pekin. 1995, 31, 159–165. [Google Scholar]
- Chyau, C.C.; Ko, P.T.; Chang, C.H.; Mau, J.L. Free and glycosidically bound aroma compounds in lychee (Litchi chinensis Sonn.). Food Chem. 2003, 80, 387–392. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Perez-Cacho, P.R.; Davenport, T.; Rouseff, R. Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. J. Agric. Food Chem. 2007, 55, 1939–1944. [Google Scholar] [CrossRef]
- Sivakumar, D.; Naude, Y.; Rohwer, E.; Korsten, L. Volatile compounds, quality attributes, mineral composition and pericarp structure of South African litchi export cultivars Mauritius and McLean’s Red. J. Sci. Food Agric. 2008, 88, 1074–1081. [Google Scholar] [CrossRef]
- Frohlich, O.; Schreler, P. Additional Neutral Volatiles from Litchi (Litchi chinensis Sonn.) Fruit. Flavour Fragr. J. 1986, 1, 149–153. [Google Scholar] [CrossRef]
- Katsukawa, M.; Nakata, R.; Koeji, S.; Hori, K.; Takahashi, S.; Inoue, H. Citronellol and geraniol, components of rose oil, activate peroxisome proliferator-activated receptor α and γ and suppress cyclooxygenase-2 expression. Biosci. Biotechnol. Biochem. 2011, 75, 1010–1012. [Google Scholar] [CrossRef]
- Tang, Z.; Zeng, X.; Brennan, M.; Han, Z.; Niu, D.; Huo, Y. Characterization of aroma profile and characteristic aroma during lychee wine fermentation. J. Food Process Preserv. 2019, 43, e14003. [Google Scholar] [CrossRef]
- Magnard, J.; Roccia, A.; Caissard, J.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Oyant, L.; Jullien, F.; Nicole, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Suh, J.; Gmitter, F.; Wang, Y. Differentiation between flavors of sweet orange (Citrus sinensis) and mandarin (Citrus reticulata). J. Agric. Food Chem. 2018, 66, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Shen, S.; Yin, X.; Klee, H.; Zhang, B.; Chen, K. Trancription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-Geraniol in sweet orange fruit. J. Exp. Bot. 2017, 68, 4929–4938. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Aubourg, S.; Schouwey, M.B.; Daviet, L.; Schalk, M.; Toub, O.; Lund, S.T.; Bohlmann, J. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FlcDNA cloning, and enzyme assays. BMC Plant Biol. 2010, 10, 226. [Google Scholar] [CrossRef]
- Zhu, B.; Cai, J.; Wang, Z.; Xu, X.; Duan, C.; Pan, Q. Identification of a plastid-localized bifunctional nerolidol/linalool synthase in relation to linalool biosynthesis in young grape berries. Int. J. Mol. Sci. 2014, 15, 21992–22010. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.J.; Green, S.A.; Chen, X.; Bailleul, E.J.D.; Matich, A.J.; Wang, M.Y.; Atkinson, R.G. Functional genomics reveals that a compact terpene synthase gene family can account for terpene volatile production in apple. Plant Physiol. 2013, 161, 787–804. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Y.; Nieuwenhuizen, N.J.; Atkinson, R.G. TPS-b family genes involved in signature aroma terpenes emission in ripe kiwifruit. Plant Signal. Behav. 2021, 16, 1962657. [Google Scholar] [CrossRef]
- Fang, C.; Sun, C.; Qiao, F.; Gu, Y.; Zhang, X.; Zhang, S. Determination of aromatic compositions in three kinds of litchi flowers by Headspace Gas Chromatography Coupled to Mass Spectrometry Method. Acad. Period. Farm Prod. Process. 2011, 241, 16–19. [Google Scholar]
- Guo, S. Analysis of Volatile Components and Content of Litchi Flowers in Different Cultivars at Different Blooming Stages. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- Li, J. Analysis of Compositional Differences of Volatile Scent in Different Cultivars of Litchi Flowers and Cloning and Expression of TPS Genes. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Defilippi, B.; Manriquez, D.; Luengwilai, K.; Gonzalez-Aguero, M. Aroma volatiles: Biosynthesis and mechanisms of modulation during fruit ripening. Adv. Bot. Res. 2009, 50, 1–37. [Google Scholar] [CrossRef]
- Tieman, D.; Taylor, M.; Schauer, N.; Klee, H. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, M.; Peng, Y.; Yang, W.; Shi, J. Antifungal activity of 1-octen-3-ol against Monilinia fructicola and its ability in enhancing disease resistance of peach fruit. Food Control 2022, 135, 108804. [Google Scholar] [CrossRef]
- Berendsen, R.; Kalkhove, S.; Lugones, L.; Baars, J.J.P.; Wosten, H.A.B.; Bakker, P.A.H.M. Effects of the mushroom-volatile 1-octen-3-ol on dry bubble disease. Appl. Microbiol. Biotechnol. 2013, 97, 5535–5543. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Song, M.; Rouseff, R. Identification of new strawberry sulfur volatiles and changes during maturation. J. Agric. Food Chem. 2011, 59, 1293–1300. [Google Scholar] [CrossRef]
- Teh, B.T.; Lim, K.; Yong, C.H.; Ng, C.C.Y.; Rao, S.R.; Rajasegaran, V.; Lim, W.K.; Ong, C.K.; Chan, K.; Cheng, V.K.Y.C.; et al. The draft genome of tropical fruit durian (Durio zibethinus). Nat. Genet. 2017, 49, 1633–1641. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Teranishi, R.; Flath, R.A.; Guentert, M. Identification of additional pineapple volatiles. J. Agric. Food Chem. 1991, 39, 1848–1851. [Google Scholar] [CrossRef]
- Engel, K.H.; Tressl, R. Identification of new sulfur-containing volatiles in yellow passionfruit (Passiflora edulis f. flavicarpa). J. Agric. Food Chem. 1991, 39, 2249–2252. [Google Scholar] [CrossRef]
- Collin, S.; Nizet, S.; Muls, S.; Iraqi, R.; Bouseta, A. Characterization of odor-active compounds in extracts obtained by simultaneous extraction/distillation from Moroccan black olives. J. Agric. Food Chem. 2008, 56, 3273–3278. [Google Scholar] [CrossRef]
- Inga, M.; Garcia, J.M.; Aguilar-Galvez, A.; Campos, D.; Osorio, C. Chemical characterization of odour-active volatile compounds during lucuma (Pouteria lucuma) fruit ripening. CyTA J. Food 2019, 17, 494–500. [Google Scholar] [CrossRef]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Munoz, P.D. A Review of the fruit volatiles found in blueberry and other vaccinium species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef]
- Ferrao, L.F.V.; Sater, H.; Lyrene, P.; Amadeu, R.R.; Sims, C.A.; Tieman, D.M.; Munoz, P.R. Terpene volatiles mediates the chemical basis of blueberry aroma and consumer acceptability. Food Res. Int. 2021, 158, 111468. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Nawaz, M.; Liu, R.; Sun, R.; Jiang, J.; Fan, X.; Liu, C.; Zhang, Y. Evaluation of volatile aroma compounds from Chinese wild grape berries by headspace-SPME with GC-MS. Food Sci. Technol. 2021, 42, e54320. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.T.; Resende, M.F.R., Jr.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tian, J.; Chen, Y.; Chen, M.; Liu, Y.; Wei, A. Volatile oil profile of prickly Ash (Zanthoxylum) pericarps from different locations in China. Food 2021, 10, 2386. [Google Scholar] [CrossRef] [PubMed]
- Molina-corral, F.J.; Espino-diaz, M.; Jacobo, J.L.; Mattinson, S.D.; Fellman, J.K.; Sepulbeda, D.R.; Gonzalez-Aguilar, G.A.; Salas-Salazar, N.A.; Olivas, G.I. Quality attributes during maturation of ‘Golden Delicious’ and ‘Red Delicious’ apples grown in two geographical regions with different environmental conditions. Not. Bot. Horti Agrobo. 2021, 49, 12241. [Google Scholar] [CrossRef]
- Meng, X.; Hu, J.; Li, Y.; Dai, J.; Guo, M.; Ouyang, G. The preference choices of Conopomorpha sinensis Bradley (Lepidoptera: Gracilariidae) for litchi based on its host surface characteristics and volatiles. Sci. Rep. 2018, 8, 2013. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, S.; Dong, Y.; Lu, K.; Chen, B. Identification and characterization of two general odourant-binding proteins from the litchi fruit borer, Conopomorpha sinensis Bradley. Pest Manag. Sci. 2016, 72, 877–887. [Google Scholar] [CrossRef]
| CAS | Volatile Organic Compounds | Number of Hits | Scent Descriptions | Substance Classifications |
|---|---|---|---|---|
| 78-70-6 | Linalool | 21 | Floral scent of lilac, lily of the valley and rose | Monoterpenes |
| 106-24-1 | Geraniol | 20 | Rose-like aroma | Monoterpenes |
| 138-86-3 | DL-Limonene | 18 | Orange and lemon-like aroma | Monoterpenes |
| 586-62-9 | Terpinolene | 17 | Lemon, woody and slightly sweet citrus-like aroma | Monoterpenes |
| 111-27-3 | 1-Hexanol | 17 | fruity-like aroma | Alcohols |
| 106-22-9 | β-Citronellol | 17 | Sweet rose-like aroma | Monoterpenes |
| 99-87-6 | p-Cymene | 16 | Aromatic smell | Monoterpenes |
| 106-25-2 | Nerol | 16 | Rose and orange-like aroma | Monoterpenes |
| 60-12-8 | 2-Phenylethanol | 16 | Rose-like aroma | Primary alcohol benzenes |
| 98-55-5 | α-Terpineol | 15 | Clove aroma | Monoterpenes |
| 111-87-5 | Octanol | 15 | Strong oily and citrus aroma | Saturated fatty alcohol |
| 876-17-5 | cis-Rose oxide | 15 | Rose-like aroma | Monoterpenes |
| 104-76-7 | 2-Ethylhexanol | 15 | Special aroma | Primary alcohol |
| 123-35-3 | β-Myrcene | 14 | Light balsamic aroma | Monoterpenes |
| 3391-86-4 | 1-Octen-3-ol | 14 | Mushroom, lavender, rose, and hay aroma | Aliphatic unsaturated alcohol |
| 562-74-3 | 4-Terpineol | 14 | Warm peppery, lighter earthy, and aged wood aroma | Monoterpenes |
| 5392-40-5 | Citral | 14 | Intense lemon scent | Monoterpenes |
| 141-78-6 | Ethyl acetate | 14 | Fruity aroma | Esters |
| 513-86-0 | Acetoin | 13 | Pleasant creamy aroma | Methyl ketone |
| 556-82-1 | Prenol | 13 | Fruity aroma | Alkenyl alcohol |
| 106-26-3 | Neral (cis-Citral) | 13 | Lemon-like aroma | Monoterpenes |
| Method of Detection | Volatile Sulfur Compounds | Scent Descriptions | References |
|---|---|---|---|
| Electrolytic conductivity detector and mass spectrometer | Benzothiazole | Rubber-like odor | Johnston et al. (1980) [22] |
| GC-PFPD/O | Hydrogen sulfide | Sulfur, fetid | Mahattanatawee et al. (2007) [39] |
| Dimethyl sulfide | Cabbage | ||
| Diethyl disulfide | Moldy, sulfur | ||
| 2-Acetyl-2-thiazoline | Dry fruit, nutty | ||
| 2-Methyl thiazole | Roasted garlic | ||
| 2,4-Dithiopentane | Burning tire, cabbage | ||
| Dimethyl trisulfide (DMTS) | Cabbage, sulfur | ||
| Methional | Cooked potato | ||
| GC-MS | 2,4-Dithiopentane | Cabbage | Wu et al. (2009) [24] |
| 2,3,5-Trithiahexane | N/A | ||
| GC-MS/O and AEDA | DMTS Methional | Pickled vegetable Cooked potato | Feng et al. (2018) [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhao, M.; Li, J. Aroma Volatiles in Litchi Fruit: A Mini-Review. Horticulturae 2022, 8, 1166. https://doi.org/10.3390/horticulturae8121166
Liu Z, Zhao M, Li J. Aroma Volatiles in Litchi Fruit: A Mini-Review. Horticulturae. 2022; 8(12):1166. https://doi.org/10.3390/horticulturae8121166
Chicago/Turabian StyleLiu, Zhuoyi, Minglei Zhao, and Jianguo Li. 2022. "Aroma Volatiles in Litchi Fruit: A Mini-Review" Horticulturae 8, no. 12: 1166. https://doi.org/10.3390/horticulturae8121166
APA StyleLiu, Z., Zhao, M., & Li, J. (2022). Aroma Volatiles in Litchi Fruit: A Mini-Review. Horticulturae, 8(12), 1166. https://doi.org/10.3390/horticulturae8121166







