In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × josephi-angeli Mansanet & Aguil. (Lamiaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Sterilization and Culture Initiation
2.3. Multiplication and Rooting
2.4. Acclimatization
2.5. Plant Extraction and Chemical Analyses
2.6. Experimental Design and Data Treatment
3. Results
3.1. Plant Sterilization and Culture Initiation
3.2. Multiplication and Rooting
3.3. Acclimatization
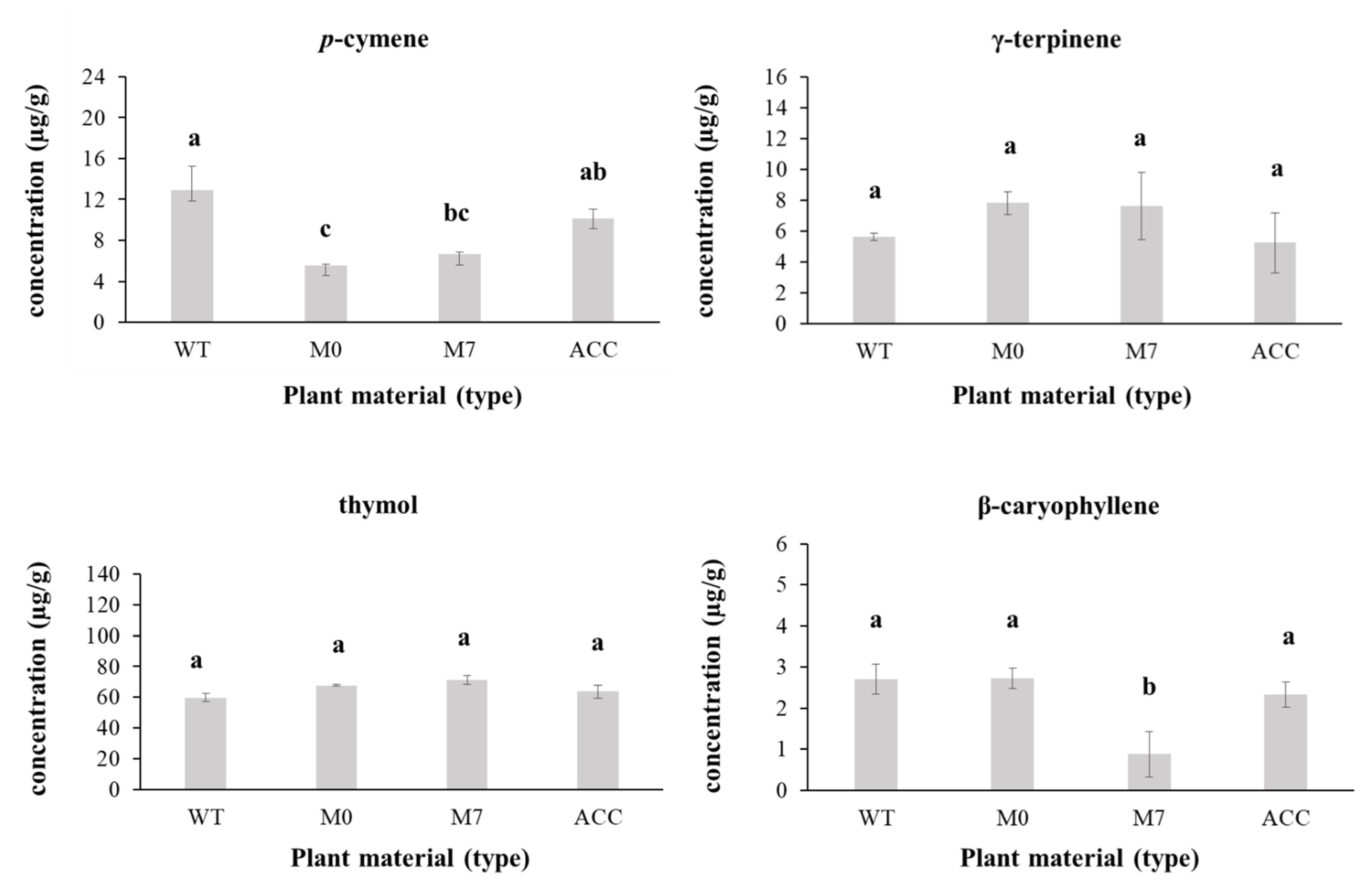
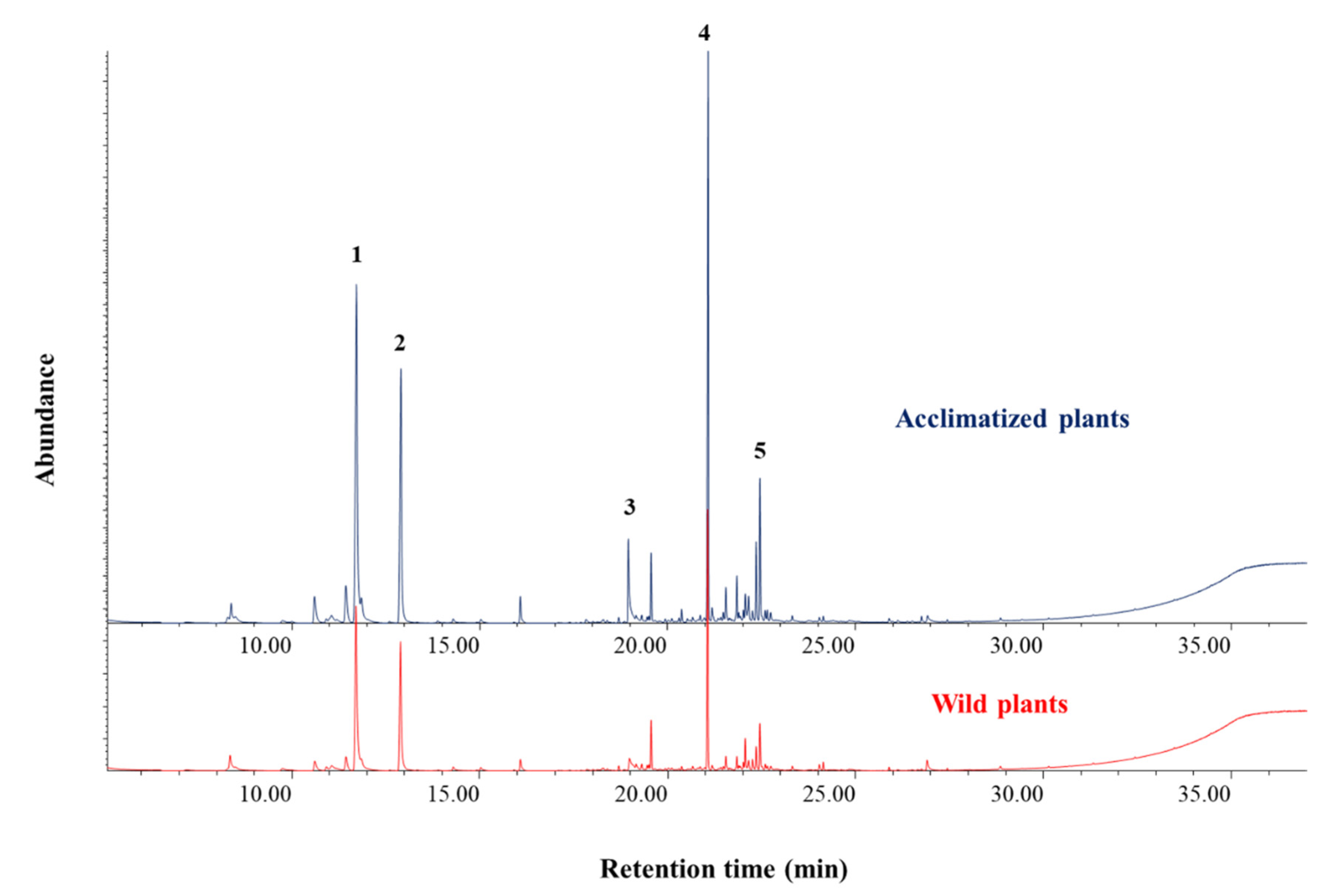
3.4. Plant Extraction and Chemical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales, R. Thymus L. In Flora iberica; Real Jardín Botánico CSIC: Madrid, Spain, 2010; Volume 12, pp. 349–409. [Google Scholar]
- Khajuria, A.K.; Bisht, N.; Bhagat, N. In vitro organogenesis and plant regeneration of Thymus serpyllum L.: An important aromatic medicinal plant. In Vitro Cell. Dev. Biol.—Plant 2020, 56, 652–661. [Google Scholar] [CrossRef]
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pawełczak, A.; Rolewska, K.; Węglarz, Z. Morphological and chemical traits as quality determinants of common thyme (Thymus vulgaris L.), on the example of ‘Standard Winter’ cultivar. Agronomy 2020, 10, 909. [Google Scholar] [CrossRef]
- Font Quer, P. Plantas Medicinales; Editorial Labor: Barcelona, Spain, 1962. [Google Scholar]
- Morales, R.; Tardío, J.; Aceituno, L.; Molina, M.; Pardo-De-Santayana, M. Biodiversidad y Etnobotánica en España; Real Jardín Botánico CSIC: Madrid, Spain, 2011; pp. 157–207. Available online: http://147.96.59.157/rsehn/cont/publis/boletines/130.pdf (accessed on 9 October 2022).
- Pardo de Santayana, M.; Morales, R.; Tardío, J.; Molina, M. Inventario Español de los Conocimientos Tradicionales Relativos a la Biodiversidad. Fase II; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2018.
- Ballester, C. Composición Química y Propiedades Antibacterianas y Antioxidantes de Aceites Esenciales de Especies de Thymus Procedentes de Cultivo Ecológico y su Aplicación a Películas de Quitosano. Ph.D. Thesis, Universidad Miguel Hernández, Elche, Spain, 2016. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=64432 (accessed on 9 October 2022).
- Carlen, C.; Schaller, M.; Carron, C.A.; Vouillamoz, J.F.; Baroffio, C.A. The new Thymus vulgaris L. hybrid cultivar ‘Varico 3’ compared to five established cultivars from Germany, France and Switzerland. Acta Hortic. 2010, 860, 161–166. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Sefidkon, F.; Hejazi, M. Essential oil composition of Thymus x citriodorus L. cultivated in Iran. Flavour Fragr. J. 2005, 20, 237–238. [Google Scholar] [CrossRef]
- Jurevičiūtė, R.; Ložienė, K.; Bruno, M.; Maggio, A.; Rosselli, S. Composition of essential oil of lemon thyme (Thymus × citriodorus) at different hydrodistillation times. Nat. Prod. Res. 2018, 33, 80–88. [Google Scholar] [CrossRef]
- Vaičiulyte, V.; Ložiene, K.; Sivicka, I. Effect of organic matter fertilizers on the composition of volatiles, morphometrical and anatomical parameters of essential oil-bearing Thymus × citriodorus cultivated in an open field conditions. Horticulturae 2022, 8, 917. [Google Scholar] [CrossRef]
- Loziene, K.; Vaiciuniene, J.; Venskutonis, P.R. Chemical composition of the essential oil of an interspecific hybrid of thyme (Thymus x oblongifolius Opiz) growing wild in Lithuania. J. Essent. Oil Res. 2002, 14, 308–311. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomàs, X.; Cañigueral, S.; Paiva, J.; Proença da Cunha, A.; Adzet, T. Essential oil composition and variability of Thymus lotocephalus and Thymus x mourae. Biochem. Syst. Ecol. 2000, 28, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.; Baher, Z.F. Chemical composition of essential oil from Thymus vulgaris Hybrid. J. Essent. Oil Res. 2003, 15, 404–405. [Google Scholar] [CrossRef]
- Mansanet, J.; Aguilella-Palasís, A.; Mateu-Andrés, I. Dos especies híbridas nuevas: Thymus x Josephi Angeli J. Mansanet & A. Aguilella y Helianthemum x Carmen Joanae J. Mansanet & I. Mateu. Mediterr. Ser. Estud. Biol. 1985, 8, 83–88. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitro Cell. Dev. Biol.—Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Pharm. Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Marco-Medina, A.; Casas, J.L. In vitro multiplication and essential oil composition of Thymus moroderi Pau ex Martinez, an endemic Spanish plant. Plant Cell Tiss. Organ Cult. 2015, 120, 99–108. [Google Scholar] [CrossRef]
- Leal, F.; Taghouti, M.; Nunes, F.; Silva, A.; Coelho, A.C.; Matos, M. Thymus plants: A review—Micropropagation, molecular and antifungal activity. In Active Ingredients from Aromatic and Medicinal Plants; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Sáez, F.; Sánchez, P.; Piqueras, A. Micropropagation of Thymus piperella. Plant Cell Tissue Organ Cult. 1994, 39, 269–272. [Google Scholar] [CrossRef]
- Cheng, D.; Vrieling, K.; Klinkhamer, P.G. The effect of hybridization on secondary metabolites and herbivore resistance: Implications for the evolution of chemical diversity in plants. Phytochem. Rev. 2011, 10, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Gary, F.P. Breeding and engineering trees to accumulate high levels of terpene metabolites for plant defense and renewable chemicals. Front. Plant Sci. 2018, 9, 1672. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wrona, M.; Blasco, S.; Becerril, R.; Nerín, C.; Sales, E.; Asensio, E. Antioxidant and antimicrobial markers by UPLC®–ESI-Q-TOF-MSE of a new multilayer active packaging based on Arctostaphylos uva-ursi. Talanta 2019, 196, 498–509. [Google Scholar] [CrossRef]
- Asensio, E.; Vitales, D.; Pérez, I.; Peralba, L.; Viruel, J.; Montaner, C.; Vallès, J.; Garnatje, T.; Sales, E. Phenolic compounds content and genetic diversity at population level across the natural distribution range of bearberry (Arctostaphylos uva-ursi, Ericaceae) in the Iberian Peninsula. Plants 2020, 9, 1250. [Google Scholar] [CrossRef]
- Song, X.-C.; Canellas, E.; Asensio, E.; Nerín, C. Predicting the antioxidant capacity and total phenolic content of bearberry leaves by data fusion of UV-Vis spectroscopy and UHPLC/Q-TOF-MS. Talanta 2020, 213, 120831. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Ramírez-Luna, J.E.; Piqueras, A.; Casas, J.L. Micropropagation and cryopreservation by vitrification of the Spanish endemic medicinal plant Sideritis leucantha Cav. subsp. leucantha (Lamiaceae). In Vitro Cell. Dev. Biol.—Plant 2021, 57, 1057–1065. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Serrano-Martínez, F.; Cano-Castillo, M.; Casas, J.L. In vitro propagation, genetic assessment, and medium-term conservation of the coastal endangered epecies Tetraclinis articulata (Vahl) Masters (Cupressaceae) from adult trees. Plants 2022, 11, 187. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer: New Delhi, India, 2013; ISBN 9788132210269. [Google Scholar]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book 2014, 12, e0168. [Google Scholar] [CrossRef] [Green Version]
- Hart, D.S.; Keightley, A.; Sappington, D.; Nguyen, T.M.P.; Chritton, C.; Seckinger, G.R.; Torres, K.C. Stability of adenine-based cytokinins in aqueous solution. In Vitro Cell. Dev. Biol.—Plant 2016, 52, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol.—Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Nataraj, M.; Mafatlal, M.K.; Teixeira da Silva, J.A. Micropropagation of Clerodendrum L. species: A review. Rend. Fis. Acc. Lincei 2016, 27, 169–179. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Seed germination and in vitro propagation of Sideritis athoa. Acta Hortic. 2009, 813, 471–476. [Google Scholar] [CrossRef]
- Shtereva, L.A.; Vassilevska-Ivanova, R.D.; Kraptchev, B.V. In vitro cultures for micropropagation, mass multiplication and preservation of an endangered medicinal plant Sideritis scardica Griseb. Bot. Serb. 2015, 39, 111–120. Available online: https://botanicaserbica.bio.bg.ac.rs/arhiva/pdf/2015_39_2_633_full.pdf (accessed on 9 October 2022).
- Phatak, S.V.; Heble, M.R. Organogenesis and terpenoid synthesis in Mentha arvensis. Fitoterapia 2002, 73, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, A.; Parshuram, S.; Manoranjan, K. Micro-propagation and biochemical analysis of Spear Mint (Mentha spicata). Indian J. Innov. Dev. 2015, 1, 489–493. [Google Scholar]
- Mehta, J.; Naruka, R.; Sain, M.; Dwivedi, A.; Sharma, D.; Mirza, J. An efficient protocol for clonal micropropagation of Mentha piperita L. (Pipperment). Asian J. Plant Sci. Res. 2012, 2, 518–523. [Google Scholar]
- Ozudogru, E.A.; Kaya, E.; Kirdok, E.; Issever-Ozturk, S. In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell. Dev. Biol.—Plant 2011, 47, 309–320. [Google Scholar] [CrossRef]
- Arrebola, M.L.; Socorro, O.; Barcelò-Muńoz, A.; Simón-Pérez, E.; Pliego-Alfaro, F. Micropropagation of Satureja obovata Lag. Hortic Sci. 1997, 32, 1278–1280. [Google Scholar] [CrossRef]
- Vaidya, B.N.; Asanakunov, B.; Shahin, L.; Jernigan, H.L.; Joshee, N.; Dhekney, S.A. Improving micropropagation of Mentha × piperita L. using a liquid culture system. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 71–80. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N. In vitro seed and clonal propagation of the Mediterranean aromatic and medicinal plant Teucrium capitatum. HortScience 2016, 51, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Coelho, N.; Gonçalves, S.; González-Benito, M.E.; Romano, A. Establishment of an in vitro propagation protocol for Thymus lotocephalus, a rare aromatic species of the Algarve (Portugal). Plant Growth Regul. 2012, 66, 69–74. [Google Scholar] [CrossRef]
- Stals, H.; Inzé, D. When plant cells decide to divide. Trends Plant Sci. 2001, 6, 359–364. [Google Scholar] [CrossRef]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef]
- Romanov, G.A.; Lomin, S.N.; Schmülling, T. Biochemical characteristics and ligand-binding properties of Arabidopsis cytokinin receptor AHK3 compared to CRE1/AHK4 as revealed by a direct binding assay. J. Exp. Bot. 2006, 57, 4051–4058. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Isidron, L.; Valencia-Lozano, E.; Rosiles-Loeza, P.Y.; Robles-Hernández, M.G.; Napsuciale-Heredia, A.; Cabrera-Ponce, J.L. Gene expression analysis of microtubers of potato Solanum tuberosum L. induced in cytokinin containing medium and osmotic stress. Plants 2021, 10, 876. [Google Scholar] [CrossRef]
- Romanov, G.A.; Lomin, S.N.; Schmülling, T. Cytokinin signaling: From the ER or from the PM? That is the question! New Phytol. 2018, 218, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantilla, G.; Lorenzo, G.A.; Mascarini, L. Hormonal endogenous changes in response to the exogenous 6-benzylaminopurine application in pre- and post-harvesting lilium flower stalks. Ornam. Hortic. 2021, 27, 357–364. [Google Scholar] [CrossRef]
- Hussey, G. In vitro propagation of Narcissus. Ann. Bot. 1982, 49, 707–719. [Google Scholar] [CrossRef]
- Langen-Gerrits, M.M.; de Klerk, G.J. Micropropagation of flower bulbs: Lily and Narcissus. Methods Mol. Biol. 1999, 111, 141–147. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Pavlov, A.; Ríos, S.; Casas, J.L. In vitro culture and micropropagation of the Baetic-Moroccan endemic plant Lapiedra martinezii Lag. (Amaryllidaceae). In Vitro Cell. Dev. Biol.-Plant 2019, 55, 725–732. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Pavlov, A.; Ríos, S.; Casas, J.L. Micropropagation of five endemic, rare and/or endangered Narcissus species from the Iberian Peninsula (Spain and Portugal). Acta Biol. Crac. Ser. Bot. 2021, 63, 55–61. [Google Scholar] [CrossRef]
- Escobar, E.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Blanquer, A.; Boira, H.; Soler, V.; Perez, I. Variability of the essential oil of Thymus piperella. Phytochemistry 1998, 47, 1271–1276. [Google Scholar] [CrossRef]
- Bekircan, T.; Yaşar, A.; Yıldırım, S.; Sökmen, M.; Sökmen, A. Effect of cytokinins on in vitro multiplication, volatiles composition and rosmarinic acid content of Thymus leucotrichus Hal. shoots. 3 Biotech 2018, 8, 180. [Google Scholar] [CrossRef]
- Juan-Méndez, R.; Asensio-Casas, E.; Juan-Vicedo, J. Thymol elicitation during in vitro regeneration of axillary bud explants from a Thymus piperella L. commercial hybrid. In MOL2NET’22, Conference on Molecular, Biomedical & Computational Sciences and Engineering, Proceedings of the Congress NIECXSM-08: North-Ibero-America-Europe Congress on Exp. & Simul. Methods, Valencia, Spain; Miami, FL, USA, 2022, 8th ed.; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]

| Treatment | Shoot Elongation (cm) | Leaves Produced (n°) | Growth Restoration (%) | Contamination (%) |
|---|---|---|---|---|
| S1 (30 s EtOH 70% + 20 min NaOCl 7%) | 1.88 ± 0.39 a | 9.31 ± 0.30 a | 91.50 ± 5.34 a | 12.78 ± 3.09 a |
| S2 (30 s EtOH 70% + 15 min NaOCl 15%) | 1.92 ± 0.45 a | 8.73 ± 0.65 a | 91.29 ± 6.87 a | 14.66 ± 4.16 a |
| Treatment | Shoots per Explant (n°) | Shoot Elongation (cm) | Nodes (n°) | Leaves (n°) | Micropropagation Rate | Roots per Explant (n°) | Root Elongation (cm) |
|---|---|---|---|---|---|---|---|
| M0 (PGR free) | 1.15 ± 0.31 c | 2.07 ± 0.98 a | 4.50 ± 1.46 b | 19.50 ± 3.25 a | 5:18 | 5.68 ± 2.01 a | 1.04 ± 0.21 b |
| M1 (BAP = 6.57 µM) | 2.31 ± 0.45 ab | 1.21 ± 0.11 bcde | 4.03 ± 1.52 bc | 10.93 ± 2.19 bc | 9.31 | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| M2 (BAP = 6.57 µM + IAA = 3.25 µM) | 1.45 ± 0.62 bc | 0.87 ± 0.33 de | 2.14 ± 1.11 d | 7.65 ± 2.25 d | 3.10 | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| M3 (BAP = 6.57 µM + 2iP = 0.064 µM) | 1.41 ± 0.60 bc | 0.82 ± 0.24 e | 2.88 ± 1.00 cd | 8.04 ± 1.11 d | 4.06 | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| M4 (BAP = 6.57 µM + 2iP = 0.34 µM) | 1.35 ± 0.82 bc | 0.80 ± 0.29 e | 2.93 ± 0.97 cd | 8.73 ± 3.06 d | 3.96 | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| M5 (BAP = 6.57 µM + 2iP = 0.69 µM) | 1.50 ± 0.57 bc | 1.13 ± 0.40 cde | 3.15 ± 0.76 cd | 9.15 ± 1.67 d | 4.73 | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| M6 (BAP = 6.57 µM + 2iP = 3.44 µM) | 1.42 ± 0.72 bc | 1.17 ± 0.53 bcd | 3.91 ± 1.25 bcd | 10.15 ± 1.98 bc | 5.55 | 0.29 ± 1.11 c | 0.35 ± 0.50 cd |
| M7 (2iP = 0.064 µM) | 3.25 ± 0.65 a | 2.01 ± 0.25 a | 6.40 ± 1.93 a | 19.77 ± 4.32 a | 20.80 | 5.39 ± 1.95 a | 1.64 ± 0.44 a |
| M8 (2iP = 0.34 µM) | 1.34 ± 0.61 bc | 1.67 ± 0.51 ab | 3.70 ± 1.45 bc | 11.23 ± 3.19 cd | 4.96 | 2.09 ± 1.35 b | 0.75 ± 0.80 bc |
| M9 (2iP = 0.69 µM) | 1.80 ± 0.43 abc | 1.54 ± 0.39 abc | 4.49 ± 1.63 b | 12.55 ± 1.45 bcd | 8.08 | 1.86 ± 1.44 b | 1.01 ± 0.80 ab |
| M10 (2iP = 3.44 µM) | 1.08 ± 0.15 c | 1.48 ± 0.19 abc | 3.13 ± 0.96 bcd | 10.23 ± 1.99 cd | 3.38 | 0.73 ± 0.491 bc | 0.20 ± 0.19 cd |
| Acclimatization Scheme | Primary Acclimatization (Days) | Survival (%) | Hardening (Days) | Survival (Accumulated %) |
|---|---|---|---|---|
| AC1 | 30 | 86.49 ± 3.74 a | 30 | 82.78 ± 3.74 a |
| AC2 | 30 | 79.41 ± 4.01 a | 15 | 77.78 ± 4.28 a |
| AC3 | 15 | 77.78 ± 4.22 a | 15 | 75.22 ± 4.54 a |
| Components | RT (min) | Wild-Type (% PA) | In Vitro Cultured | Acclimatized (%PA) | |
|---|---|---|---|---|---|
| M0 (% PA) | M7 (% PA) | ||||
| β-thujene | 8334 | 0.45 ±0.06 | 0.49 ± 0.04 | 0.64 ± 0.14 | 0.44 ± 0.09 |
| α-pinene 1 | 8530 | 0.19 ± 0.02 | 0.20 ± 0.01 | 0.32 ± 0.16 | 0.19 ± 0.04 |
| β-pinene | 9966 | 0.21 ± 0.02 | 0.17 ± 0.00 | 0.10 ± 0.01 | 0.20 ± 0.02 |
| 1-octene-3-ol | 10,424 | 0.89 ± 0.11 | 0.76 ± 0.01 | 0.54 ± 0.04 | 0.86 ± 0.16 |
| β-myrcene | 10,626 | 1.00 ± 0.09 | 1.40 ± 0.01 | 1.11 ± 0.31 | 1.06 ± 0.30 |
| α-phellandrene | 11,079 | 0.56 ± 0.08 | 0.53 ± 0.11 | 0.65 ± 0.22 | 0.59 ± 0.04 |
| α-terpinene 1 | 11,463 | 1.03 ± 0.03 | 1.19 ± 0.01 | 1.05 ± 0.30 | 0.98 ± 0.30 |
| p-cymene 1 | 11,731 | 12.88 ± 2.40 | 5.52 ± 0.12 | 6.62 ± 0.26 | 10.11 ± 0.91 |
| D-limonene 1 | 11,875 | 1.01 ± 0.08 | 0.82 ± 0.05 | 0.78 ± 0.03 | 0.97 ± 0.05 |
| eucalyptol | 12,126 | 0.29 ± 0.07 | 0.08 ± 0.01 | 0.35 ± 0.15 | 0.25 ± 0.06 |
| β-ocimene | 12,607 | 0.09 ± 0.00 | 0.10 ± 0.01 | 0.09 ± 0.04 | 0.09 ± 0.03 |
| γ-terpinene 1 | 12,911 | 5.63± 0.23 | 7.82 ± 0.76 | 7.61 ± 2.19 | 5.23 ± 1.95 |
| cis-sabinene hydrate | 13,382 | 0.52 ± 0.01 | 0.36 ± 0.02 | 0.17 ± 0.01 | 0.47 ± 0.06 |
| terpinolene 1 | 13,897 | 0.20 ± 0.07 | 0.13 ± 0.01 | ND | 0.15 ± 0.03 |
| linalool 1 | 14,412 | 3.30 ± 0.28 | 1.73 ± 0.04 | 1.18 ± 0.59 | 3.03 ± 0.38 |
| endo-borneol | 16,414 | 0.18 ± 0.08 | 0.06 ± 0.00 | ND | 0.22 ± 0.26 |
| terpinen-4-ol 1 | 16,634 | 0.24 ± 0.02 | 0.12 ± 0.01 | 0.09 ± 0.02 | 0.22 ± 0.03 |
| umbellulone | 16,992 | 0.22 ± 0.00 | 0.08 ± 0.02 | ND | 0.17 ± 0.01 |
| isothymol methyl ether | 17,837 | 0.13 ± 0.02 | 0.08 ± 0.01 | 0.09 ± 0.00 | 0.12 ± 0.05 |
| thymoquinone | 18,466 | 2.68 ± 0.51 | 0.74 ± 0.04 | 0.80 ± 0.36 | 1.77 ± 0.30 |
| thymol | 19,005 | 59.77 ± 2.59 | 67.72 ± 0.66 | 71.45 ± 2.96 | 63.63 ± 4.07 |
| carvacrol 1 | 19,176 | 4.32 ± 0.16 | 4.39 ± 0.07 | 4.45 ± 0.00 | 4.88 ± 0.41 |
| thymol acetate | 20,024 | 0.32 ± 0.05 | 0.46 ± 0.01 | ND | 0.36 ± 0.15 |
| α-cubebene | 20,389 | 0.06 ± 0.00 | 0.05 ± 0.00 | ND | 0.05 ± 0.01 |
| β-caryophyllene 1 | 21,075 | 2.71 ± 0.36 | 2.73 ± 0.25 | 0.88 ± 0.56 | 2.33 ± 0.30 |
| humulene 1 | 21,559 | 0.23 ± 0.02 | 0.15 ± 0.02 | ND | 0.27 ± 0.04 |
| γ-muurolene | 21,860 | ND | 0.09 ± 0.02 | ND | ND |
| 2,6,10,15-tetramethylheptadecane | 22,027 | 0.56 ± 0.14 | 0.70 ± 0.80 | ND | 0.74 ± 0.17 |
| 2,4-di-tert-butylphenol | 22,274 | ND | 0.42 ± 0.01 | 0.84 ± 0.24 | ND |
| γ-muurolene | 22,374 | ND | 0.21 ± 0.04 | ND | ND |
| (+)-δ-Cadinene | 22,472 | 0.33 ± 0.00 | 0.34 ± 0.08 | ND | 0.30 ± 0.01 |
| cubenene | 22,607 | ND | 0.11 ± 0.02 | 0.20 ± 0.10 | ND |
| (−)-α-cadinene | 22,676 | ND | 0.03 ± 0.00 | ND | ND |
| α-calacorene | 22,761 | ND | 0.02 ± 0.03 | ND | ND |
| hexadecane | 23,318 | ND | ND | ND | 0.35 ± 0.44 |
| guaiene | 23,980 | ND | 0.08 ± 0.01 | ND | ND |
| Components | RT (min) | Wild-Type (µg/g) | Acclimatized (µg/g) |
|---|---|---|---|
| α-pinene | 8.567 | 1.21 ± 1.07 | |
| camphene | 9.07 | ||
| β-pinene | 10.061 | <LQ | <LQ |
| α-terpinene * | 11.507 | 3.33 ± 2.31 | 4.37 ± 2.62 |
| p-cymene | 11.8 | 32.41 ± 15.59 | 37.64 ± 15.96 |
| D-limonene | 11.924 | <LQ | 0.60 ± 0.85 |
| γ-terpinene | 13.101 | 30.84 ± 12.78 | 34.24 ± 14.22 |
| terpinolene | 13.922 | <LQ | 0.16 ± 0.13 |
| linalool | 14.385 | ||
| camphor | 15.601 | ||
| endo-borneol ** | 16.23 | ||
| terpinen-4-ol | 16.544 | ||
| methyl-carvacrol *** | 18.024 | ||
| carvacrol | 19.125 | ||
| Thymol *** | 19.125 | 459.36 ± 174.68 | 576.07 ± 196.60 |
| carvacrol acetate *** | 20.285 | ||
| β-caryophyllene | 21.183 | 17.46 ± 10.61 | 23.01 ± 11.37 |
| humulene | 21.609 | 1.03 ± 0.95 | 1.50 ± 1.04 |
| 2,4-di-tert-butylphenol | 22.263 | 128.81 ± 97.91 | 77.75 ± 13.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asensio, E.; de Medinacelli Juan-Méndez, R.; Juan-Vicedo, J. In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × josephi-angeli Mansanet & Aguil. (Lamiaceae). Horticulturae 2022, 8, 1188. https://doi.org/10.3390/horticulturae8121188
Asensio E, de Medinacelli Juan-Méndez R, Juan-Vicedo J. In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × josephi-angeli Mansanet & Aguil. (Lamiaceae). Horticulturae. 2022; 8(12):1188. https://doi.org/10.3390/horticulturae8121188
Chicago/Turabian StyleAsensio, Esther, Roberto de Medinacelli Juan-Méndez, and Jorge Juan-Vicedo. 2022. "In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × josephi-angeli Mansanet & Aguil. (Lamiaceae)" Horticulturae 8, no. 12: 1188. https://doi.org/10.3390/horticulturae8121188
APA StyleAsensio, E., de Medinacelli Juan-Méndez, R., & Juan-Vicedo, J. (2022). In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × josephi-angeli Mansanet & Aguil. (Lamiaceae). Horticulturae, 8(12), 1188. https://doi.org/10.3390/horticulturae8121188





