Decreased Solution pH and Increased K+ Uptake Are Related to Ammonium Tolerance in Hydroponically Cultured Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Environmental Conditions
2.2. Hydroponic Cultivation Experiment
2.2.1. Preparation of the Plants
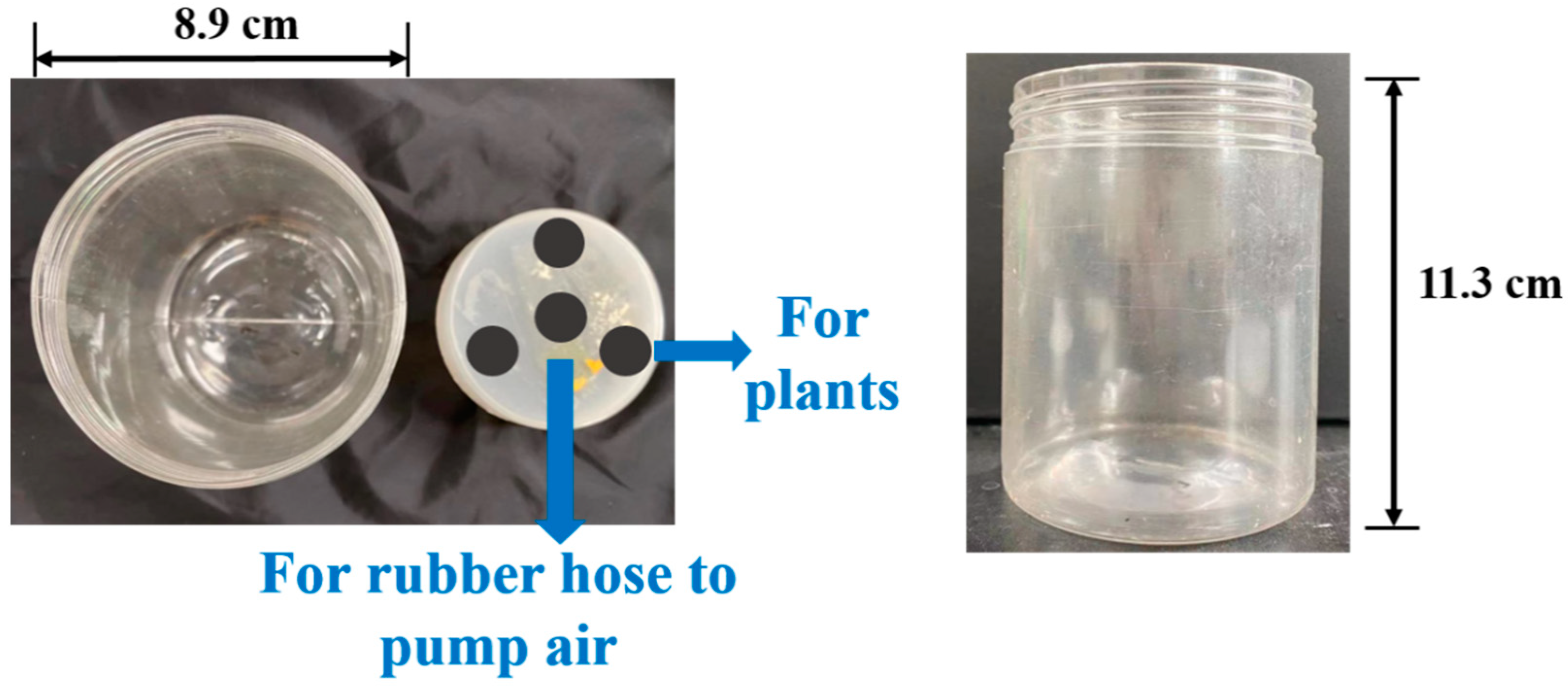
2.2.2. Preparation of the Hydroponic System
2.3. Treatment Solutions and Layout of the Plants
2.4. Monitoring of the pH and EC Variations in the Hydroponic Solutions
2.5. Determinations of Potassium (K), Calcium (Ca), and Magnesium (Mg) Concentrations in Hydroponic Solutions
2.6. Ammonium Tolerances of Salvia, Petunia, Ageratum, Cabbage and Lettuce
2.7. Statistical Analysis and Graphing
3. Results
3.1. Major pH Changes of the Hydroponic Solutions during the 84 h Cultivation
3.2. Ion Concentrations in the Hydroponic Solutions as Affected by the NH4+:NO3− Ratios and Plant Species
3.3. Minor EC Changes of the Hydroponic Solutions during the 84 h Cultivation
4. Discussion
4.1. Declined pH of Hydroponic Solutions May Be Attributed to H+ Extrusion from Roots
4.2. Net Influx of K+ by Ageratum May Help Prevent NH4+ Toxicity
4.3. Minor EC Changes of Hydroponic Solutions Is a Negligible Indicator of the NH4+ Tolerance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Miller, A.; Cramer, M. Root nitrogen acquisition and assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Coruzzi, G.; Bush, D.R. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol. 2001, 125, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Middleton, K.; Smith, G. A comparison of ammoniacal and nitrate nutrition of perennial ryegrass through a thermodynamic model. Plant Soil 1979, 53, 487–504. [Google Scholar] [CrossRef]
- Guerrero, M.G.; Vega, J.M.; Losada, M. The assimilatory nitrate-reducing system and its regulation. Annu. Rev. Plant Physiol. 1981, 32, 169–204. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Root GS and NADH-GDH Play Important Roles in Enhancing the Ammonium Tolerance in Three Bedding Plants. Int. J. Mol. Sci. 2022, 23, 1061. [Google Scholar] [CrossRef]
- Roosta, H.R.; Schjoerring, J.K. Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. J. Plant Nutr. 2007, 30, 1933–1951. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef] [Green Version]
- Ariz, I.; Cruz, C.; Moran, J.F.; González-Moro, M.B.; García-Olaverri, C.; González-Murua, C.; Martins-Loução, M.A.; Aparicio-Tejo, P.M. Depletion of the heaviest stable N isotope is associated with NH4+/NH3− toxicity in NH4+-fed plants. BMC Plant Biol. 2011, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Britto, D.T.; Davenport, R.J.; Tester, M. Ammonium toxicity and the real cost of transport. Trends Plant Sci. 2001, 6, 335–337. [Google Scholar] [CrossRef]
- Hachiya, T.; Inaba, J.; Wakazaki, M.; Sato, M.; Toyooka, K.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Nakagawa, T.; Kiba, T. Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 2021, 12, 4944. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Yan, F. Nitrate and ammonium nutrition of plants: Effects on acid/base balance and adaptation of root cell plasmalemma H+-ATPase. Z. Pflanz. Bodenkd. 1997, 160, 275–281. [Google Scholar] [CrossRef]
- Kirk, G.; Bajita, J. Root-induced iron oxidation, pH changes and zinc solubilization in the rhizosphere of lowland rice. N. Phytol. 1995, 131, 129–137. [Google Scholar] [CrossRef]
- Brix, H.; Dyhr-Jensen, K.; Lorenzen, B. Root-zone acidity and nitrogen source affects Typha latifolia L. growth and uptake kinetics of ammonium and nitrate. J. Exp. Bot. 2002, 53, 2441–2450. [Google Scholar] [CrossRef] [Green Version]
- Ariz, I.; Artola, E.; Asensio, A.C.; Cruchaga, S.; Aparicio-Tejo, P.M.; Moran, J.F. High irradiance increases NH4+ tolerance in Pisum sativum: Higher carbon and energy availability improve ion balance but not N assimilation. J. Plant Physiol. 2011, 168, 1009–1015. [Google Scholar] [CrossRef]
- Ohmori, M.; Kanda, J. H+ and K+ efflux associated with NH4+ uptake in Anabaena cylindrica cells in the dark. J. Gen. Appl. Microbiol. 1987, 33, 311–319. [Google Scholar] [CrossRef]
- Szczerba, M.W.; Britto, D.T.; Kronzucker, H.J. Rapid, futile K+ cycling and pool-size dynamics define low-affinity potassium transport in barley. Plant Physiol. 2006, 141, 1494–1507. [Google Scholar] [CrossRef] [Green Version]
- Zou, N.; Li, B.; Dong, G.; Kronzucker, H.J.; Shi, W. Ammonium-induced loss of root gravitropism is related to auxin distribution and TRH1 function, and is uncoupled from the inhibition of root elongation in Arabidopsis. J. Exp. Bot. 2012, 63, 3777–3788. [Google Scholar] [CrossRef] [Green Version]
- Santa-Marıa, G.E.; Danna, C.H.; Czibener, C. High-affinity potassium transport in barley roots. Ammonium-sensitive and-insensitive pathways. Plant Physiol. 2000, 123, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Balkos, K.D.; Britto, D.T.; Kronzucker, H.J. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ. 2010, 33, 23–34. [Google Scholar]
- Xian, L.; Zhang, Y.; Cao, Y.; Wan, T.; Gong, Y.; Dai, C.; Ochieng, W.A.; Nasimiyu, A.T.; Li, W.; Liu, F. Glutamate dehydrogenase plays an important role in ammonium detoxification by submerged macrophytes. Sci. Total Environ. 2020, 722, 137859. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Growth, Quality, and Nitrogen Assimilation in Response to High Ammonium or Nitrate Supply in Cabbage (Brassica campestris L.) and Lettuce (Lactuca sativa L.). Agronomy 2021, 11, 2556. [Google Scholar] [CrossRef]
- El Omari, R.; Rueda-López, M.; Avila, C.; Crespillo, R.; Nhiri, M.; Cánovas, F.M. Ammonium tolerance and the regulation of two cytosolic glutamine synthetases in the roots of sorghum. Funct. Plant Biol. 2010, 37, 55–63. [Google Scholar] [CrossRef]
- Rupp, L.; Dudley, L. Iron availability in rockwool may affect rose nutrition. HortScience 1989, 24, 258–260. [Google Scholar]
- Thi, L.T.; Park, Y.G.; Jeong, B.R. Growth and development of carnation ‘Dreambyul’ plantlets in a temporary immersion system and comparisons with conventional solid culture methods. Vitr. Cell Dev. Biol.-Plant 2019, 55, 539–548. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Son, J.E.; Kim, H.J.; Ahn, T.I. Hydroponic systems. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–283. [Google Scholar]
- Xu, G.; Magen, H.; Tarchitzky, J.; Kafkafi, U. Advances in chloride nutrition of plants. Adv. Agron. 1999, 68, 97–150. [Google Scholar]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a beneficial macronutrient in higher plants: New roles and regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.R.; Lee, C.W. Growth suppression and raised tissue Cl− contents in NH4+-fed marigold, petunia, and salvia. J. Am. Soc. Hortic. Sci. 1992, 117, 762–768. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.R.; Lee, C.W. Influence of ammonium, nitrate, and chloride on solution pH and ion uptake by ageratum and salvia in hydroponic culture. J. Plant Nutr. 1996, 19, 1343–1360. [Google Scholar] [CrossRef]
- Pearson, J.; Stewart, G.R. The deposition of atmospheric ammonia and its effects on plants. New Phytol. 1993, 125, 283–305. [Google Scholar] [CrossRef]
- Roos, W.; Luckner, M. Relationships between proton extrusion and fluxes of ammonium ions and organic acids in Penicillium cyclopium. Microbiology 1984, 130, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Hackette, S.L.; Skye, G.; Burton, C.; Segel, I.H. Characterization of an ammonium transport system in filamentous fungi with methylammonium-14C as the substrate. J. Biol. Chem. 1970, 245, 4241–4250. [Google Scholar] [CrossRef]
- Bar-Yosef, B.; Mattson, N.; Lieth, H. Effects of NH4+: NO3−: Urea ratio on cut roses yield, leaf nutrients content and proton efflux by roots in closed hydroponic system. Sci. Hortic. 2009, 122, 610–619. [Google Scholar] [CrossRef]
- Michelet, B.; Boutry, M. The plasma membrane H+-ATPase (A highly regulated enzyme with multiple physiological functions). Plant Physiol. 1995, 108, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Feuerle, R.; Schäffer, S.; Fortmeier, H.; Schubert, S. Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiol. 1998, 117, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Di, T.; Xu, G.; Chen, X.; Zeng, H.; Yan, F.; Shen, Q. Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ. 2009, 32, 1428–1440. [Google Scholar] [CrossRef]
- Hachiya, T.; Watanabe, C.K.; Fujimoto, M.; Ishikawa, T.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar] [CrossRef]
- Kirkby, E.A.; Knight, A.H. Influence of the level of nitrate nutrition on ion uptake and assimilation, organic acid accumulation, and cation-anion balance in whole tomato plants. Plant Physiol. 1977, 60, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K.; Liu, J.; Xiong, L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell 1998, 10, 1181–1191. [Google Scholar] [CrossRef] [Green Version]
- Hoopen, F.t.; Cuin, T.A.; Pedas, P.; Hegelund, J.N.; Shabala, S.; Schjoerring, J.K.; Jahn, T.P. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J. Exp. Bot. 2010, 61, 2303–2315. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Song, H.; Li, B.; Wang, M.; Di, D.; Lin, X.; Kronzucker, H.J.; Shi, W.; Li, G. Induction of S-nitrosoglutathione reductase protects root growth from ammonium toxicity by regulating potassium homeostasis in Arabidopsis and rice. J. Exp. Bot. 2021, 72, 4548–4564. [Google Scholar] [CrossRef]
- Samarakoon, U.; Weerasinghe, P.; Weerakkody, W. Effect of electrical conductivity (EC) of the nutrient solution on nutrient uptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006, 18, 13–21. [Google Scholar]
- James, E.C.; van Iersel, M.W. Fertilizer concentration affects growth and flowering of subirrigated petunias and begonias. HortScience 2001, 36, 40–44. [Google Scholar] [CrossRef] [Green Version]




| Nutrient Source | Ammonium to Nitrate Ratios in the Nutrient Solutions | ||
|---|---|---|---|
| 0:100 | 50:50 | 100:0 | |
| Ca(NO3)2·4H2O | 6.9 | 5.9 | - |
| KNO3 | 4.8 | - | - |
| Mg(NO3)2·6H2O | 1.3 | 0.6 | - |
| MgSO4·7H2O | 1.0 | 1.4 | 1.7 |
| KH2PO4 | 1.0 | - | 2.0 |
| NH4H2PO4 | - | 2.0 | - |
| (NH4)2SO4 | - | 4.5 | 13.0 |
| K2SO4 | - | 4.5 | 1.2 |
| CaCl2·6H2O | - | - | 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Yang, J.; Jeong, B.R. Decreased Solution pH and Increased K+ Uptake Are Related to Ammonium Tolerance in Hydroponically Cultured Plants. Horticulturae 2022, 8, 228. https://doi.org/10.3390/horticulturae8030228
Song J, Yang J, Jeong BR. Decreased Solution pH and Increased K+ Uptake Are Related to Ammonium Tolerance in Hydroponically Cultured Plants. Horticulturae. 2022; 8(3):228. https://doi.org/10.3390/horticulturae8030228
Chicago/Turabian StyleSong, Jinnan, Jingli Yang, and Byoung Ryong Jeong. 2022. "Decreased Solution pH and Increased K+ Uptake Are Related to Ammonium Tolerance in Hydroponically Cultured Plants" Horticulturae 8, no. 3: 228. https://doi.org/10.3390/horticulturae8030228
APA StyleSong, J., Yang, J., & Jeong, B. R. (2022). Decreased Solution pH and Increased K+ Uptake Are Related to Ammonium Tolerance in Hydroponically Cultured Plants. Horticulturae, 8(3), 228. https://doi.org/10.3390/horticulturae8030228








