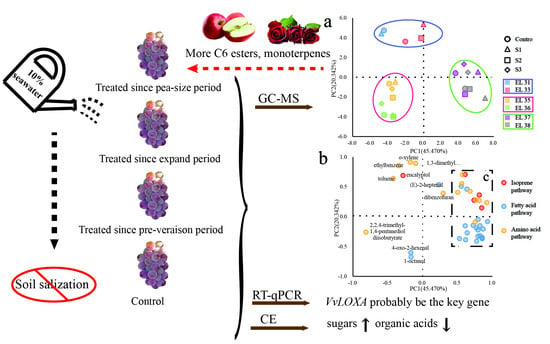
Effect of Seawater Irrigation on the Sugars, Organic Acids, and Volatiles in ‘Reliance’ Grape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Soluble Sugar and Organic Acid Compound Determination
2.3. Extraction of Aromatic Compounds and Analysis
2.4. Determination of Soluble Salt Content, Na+, Cl−, and SO42− Content, Moisture Content, and pH of Soil
2.5. Real-Time Quantitative PCR Assays (RT-qPCR)
2.6. Statistical Analysis
3. Results
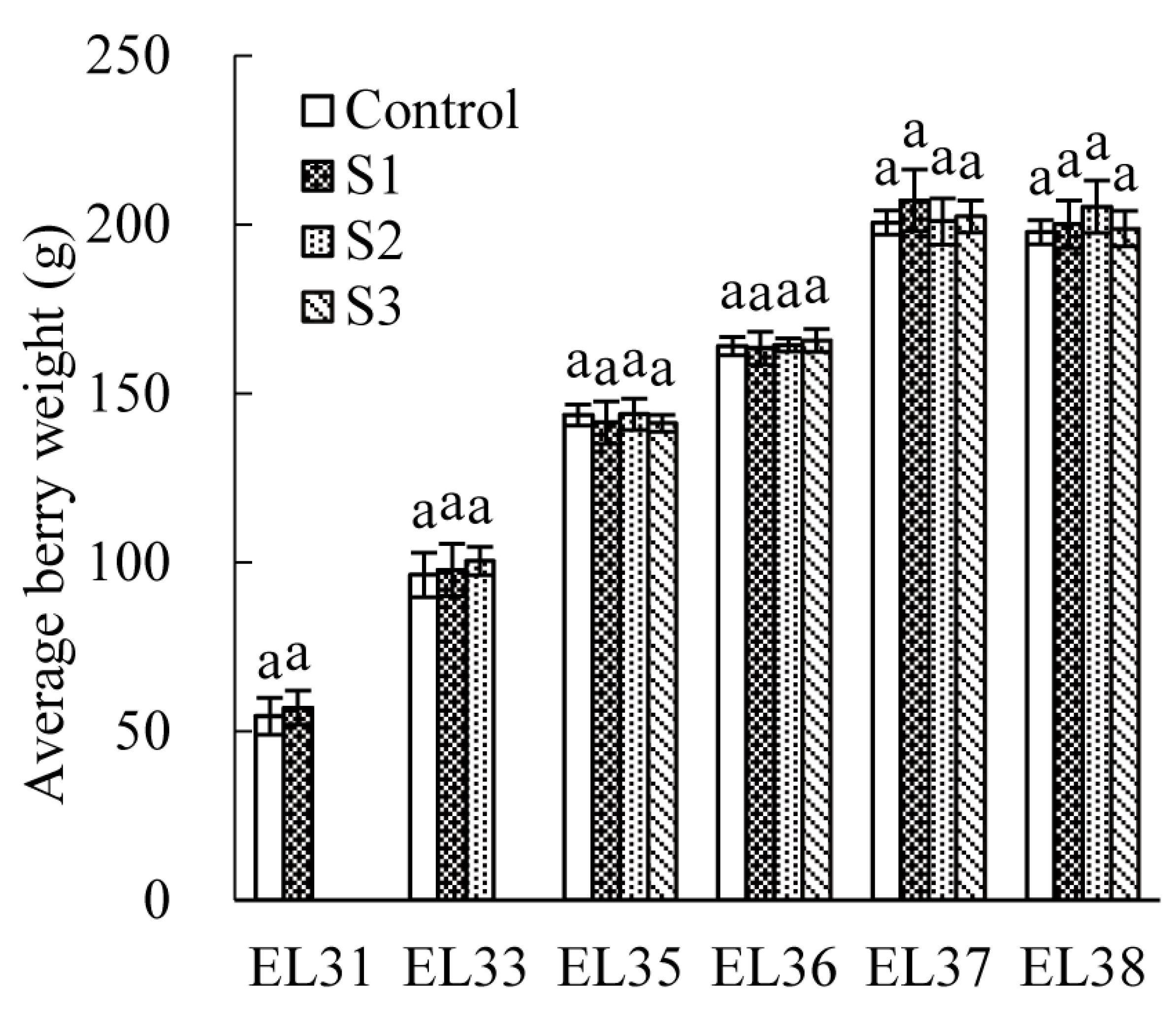
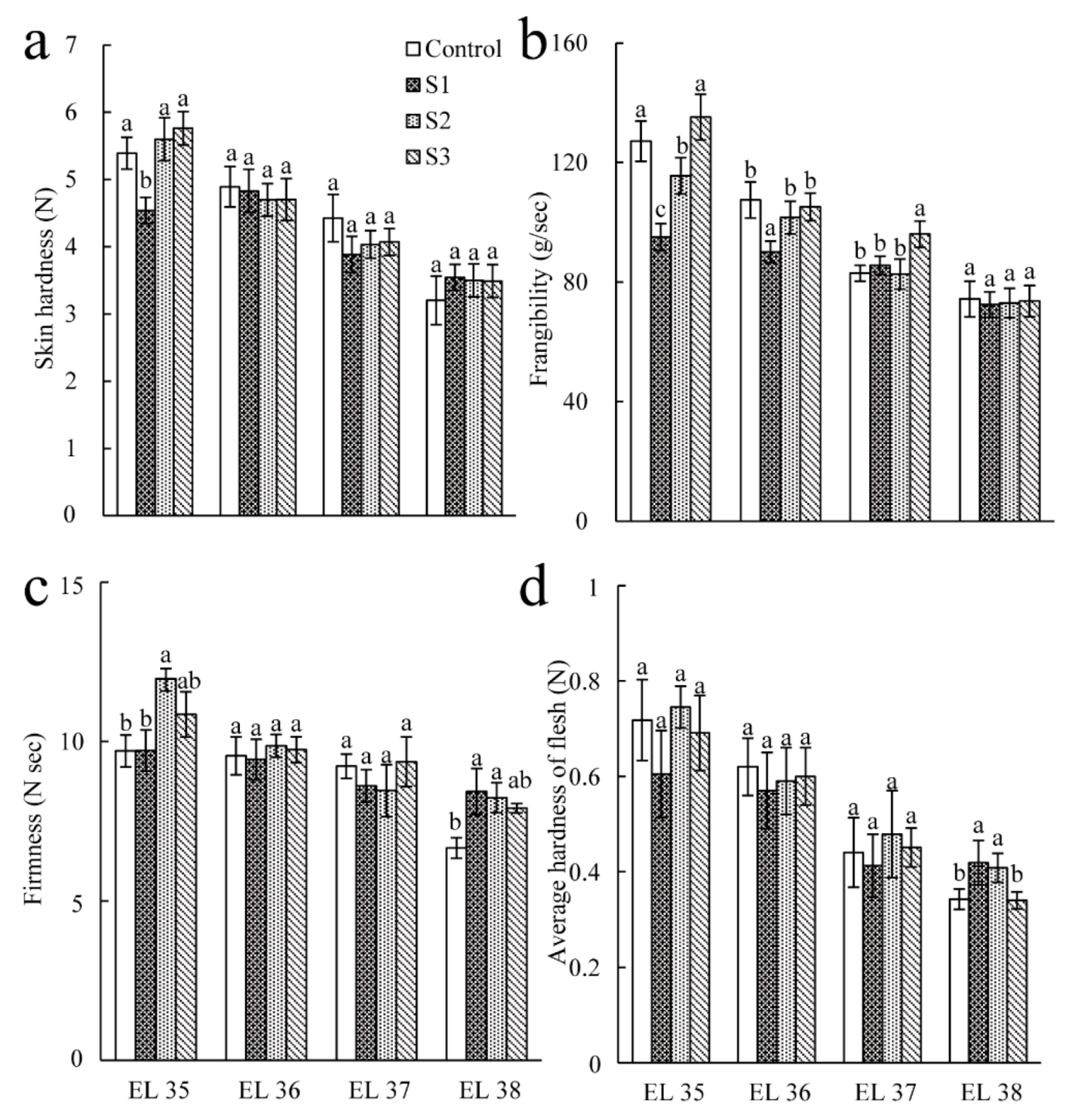
3.1. Effect of Seawater Irrigation on Average Weight and Texture Parameters of the Berry
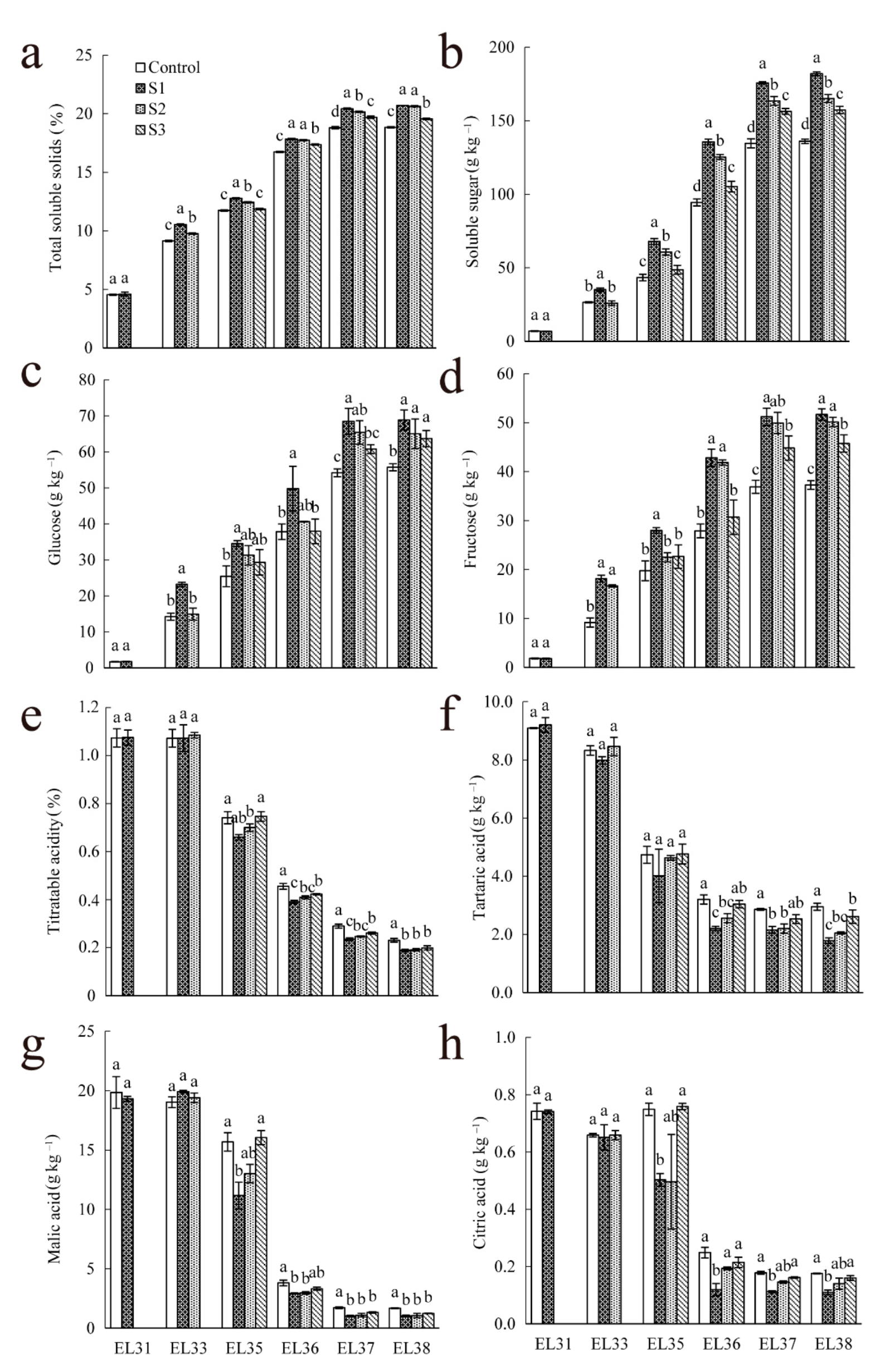
3.2. Effect of 10% Seawater Irrigation on the Concentration of Sugar and Organic Acids
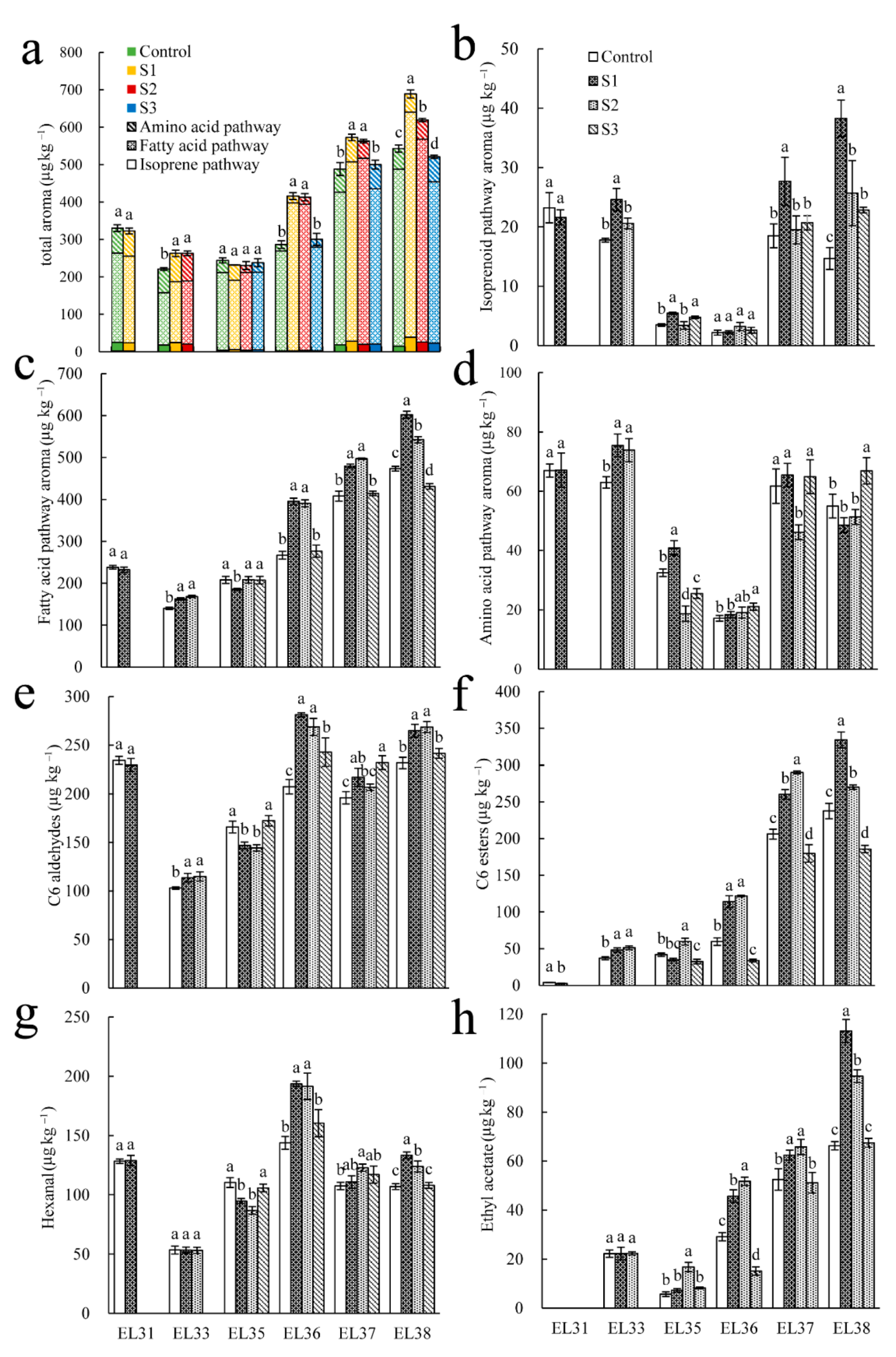
3.3. Effect of Seawater Irrigation on Aroma in ‘Reliance’ Grape at Growing Stages
3.4. Effect of Seawater Irrigation on the Concentrations and Types of Volatiles in ‘Reliance’ Grape at EL 38
3.5. Principal Component Analysis of the Effect of Seawater Irrigation on the Volatiles in ‘Reliance’ Grape during the Berry Development Period
3.6. Relative Expression Levels of Key Genes Responsible for Volatiles in the Fatty Acid Pathway
3.7. Effect of 10% Seawater Treatment on Soil Salinization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Grimplet, J.; Deluc, L.G.; Cramer, G.R.; Cushman, J.C. Integrating Functional Genomics with Salinity and Water Deficit Stress Responses in Wine Grape-Vitis Vinifera; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Liu, C.; Huang, L.; Liu, M.; Hao, S.; Zhai, H.; Shao, X.; Du, Y. Effects of seawater irrigation on fruit quality of grapevine, soil properties and microbial diversity. Sci. Hortic. 2019, 253, 80–86. [Google Scholar] [CrossRef]
- Liu, X.-J.; Dong, Y.-H.; Liu, X.; You, C.-X.; Hao, Y.-J. A C2-domain phospholipid-binding protein MdCAIP1 positively regulates salt and osmotic stress tolerance in apple. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 138, 29–39. [Google Scholar] [CrossRef]
- Han, N.; Ji, X.-L.; Du, Y.-P.; He, X.; Zhao, X.-J.; Zhai, H. Identification of a novel alternative splicing variant of VvPMA1 in grape root under salinity. Front. Plant Sci. 2017, 8, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Sgherri, C.; Kadlecová, Z.; Pardossi, A.; Navari-Izzo, F.; Izzo, R. Irrigation with diluted seawater improves the nutritional value of cherry tomatoes. J. Agric. Food Chem. 2008, 56, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Wang, C.R.; Li, X.Y.; Yao, Y.X.; Hao, Y.J. Modifications of Kyoho grape berry quality under long-term NaCl treatment. Food Chem. 2013, 139, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Chalot, G. Chemical composition, bioactive compounds, and volatiles of six table grape varieties (Vitis vinifera L.). Food Chem. 2018, 240, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Liang, Z.; Fan, P.; Wu, B.; Yang, L.; Wang, Y.; Li, S. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC–MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Aldesuquy, H.S.; Ibrahim, A.H. Water Relations, Abscisic Acid and Yield of Wheat Plants in Relation to the Interactive Effect of Seawater and Growth Bioregulators. Agrochimica 2008, 187, 97–104. [Google Scholar] [CrossRef]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8329. [Google Scholar] [CrossRef] [PubMed]
- Roubelakis-Angelakis, K.A. Grapevine Molecular Physiology & Biotechnology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xu, X.Q.; Yu, K.J.; Zhu, B.Q.; Lan, Y.B.; Duan, C.Q.; Pan, Q.H. Varietal Dependence of GLVs Accumulation and LOX-HPL Pathway Gene Expression in Four Vitis vinifera Wine Grapes. Int. J. Mol. Sci. 2016, 17, 1924. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Liu, Y.; Zhang, G.; Yan, A.; Wang, H.; Wang, X.; Pan, Q.; Xu, H.; Sun, L.; Zhu, B. Alcohol acyltransferase gene and ester precursors differentiate composition of volatile esters in three interspecific hybrids of Vitis labruscax V. Vinifera during berry development period. Food Chem. 2019, 295, 234–246. [Google Scholar] [PubMed]
- Yang, C.; Wang, Y.; Wu, B.; Fang, J.; Li, S. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, Z.; Gao, Y.; Bian, L.; Sun, X.; Chen, Z. Volatiles from non-host aromatic plants repel tea green leafhopper E mpoasca vitis. Entomol. Exp. Appl. 2014, 153, 156–169. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, S.; Lu, H.; Zhang, D.; Liu, F.; Lin, J.; Zhou, C.; Mu, W. Effects of the plant volatile trans-2-hexenal on the dispersal ability, nutrient metabolism and enzymatic activities of Bursaphelenchus xylophilus. Pestic. Biochem. Physiol. 2017, 143, 147–153. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jimenez, M. Monoterpenes in grape juice and wines. J. Chromatogr. 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Camps, C.; Guillermin, P.; Mauget, J.C.; Bertrand, D. Data analysis of penetrometric force/displacement curves for the characterization of whole apple fruits. J. Texture Stud. 2005, 36, 387–401. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, Q.; Du, Y.; Yang, X.; Zhai, H. Induction of cyclic electron flow around photosystem I during heat stress in grape leaves. Plant Sci. 2017, 256, 65–71. [Google Scholar] [CrossRef]
- Antolinos, V.; Sánchez-Martínez, M.J.; Maestre-Valero, J.F.; López-Gómez, A.; Martínez-Hernández, G.B. Effects of Irrigation with Desalinated Seawater and Hydroponic System on Tomato Quality. Water 2020, 12, 518. [Google Scholar] [CrossRef] [Green Version]
- Ghaste, M.; Narduzzi, L.; Carlin, S.; Vrhovsek, U.; Shulaev, V.; Mattivi, F. Chemical composition of volatile aroma metabolites and their glycosylated precursors that can uniquely differentiate individual grape cultivars. Food Chem. 2015, 188, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Evolution of volatile compounds during the development of Muscat grape ‘Shine Muscat’ (Vitis labruscax V. vinifera). Food Chem. 2020, 309, 125778. [Google Scholar] [CrossRef] [PubMed]
- Ul Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Jamali, B.; Rowshan, V. Headspace Analysis of Aroma Composition and Quality Changes of Selva Strawberry (Fragariaxananassa Duch.), Fruits as Influenced by Salinity Stress and Application Timing of Nitric Oxide. Anal. Chem. Lett. 2014, 4, 178–189. [Google Scholar] [CrossRef]
- Scacco, A.; Verzera, A.; Lanza, C.M.; Sparacio, A.; Genna, G.; Raimondi, S.; Tripodi, G.; Dima, G. Influence of Soil Salinity on Sensory Characteristics and Volatile Aroma Compounds of Nero d’Avola Wine. Am. J. Enol. Vitic. 2010, 61, 498–505. [Google Scholar] [CrossRef]
- Capone, D.L.; Van Leeuwen, K.; Taylor, D.K.; Jeffery, D.W.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A. Evolution and occurrence of 1, 8-cineole (Eucalyptol) in Australian wine. J. Agric. Food Chem. 2011, 59, 953–959. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Handa, A.K.; Mattoo, A.K. Transcript Abundance Patterns of 9- and 13-Lipoxygenase Subfamily Gene Members in Response to Abiotic Stresses (Heat, Cold, Drought or Salt) in Tomato (Solanum lycopersicum L.) Highlights Member-Specific Dynamics Relevant to Each Stress. Genes 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Merillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, Y.L.; Liu, M.; Tu, T.Y.; Zhao, X.F.; Yue, X.F.; Zhang, J.X.; Fang, Y.L.; Meng, J.F. Effect of regulated deficit irrigation on fatty acids and their derived volatiles in ‘Cabernet Sauvignon’ grapes and wines of Ningxia, China. Food Chem. 2018, 245, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Boyko, H.; Boyko, E. Experiments of plant growing under irrigation with saline waters from 2000 mg/litre TDS (Total diluted solids) up to Sea-water of oceanic concentration, without desalination. In Salinity and Aridity; Springer: Berlin/Heidelberg, Germany, 1966; pp. 214–282. [Google Scholar]


| Na+ (mg L−1) | Ca2+ (mg L−1) | Mg2+ (mg L−1) | Cl− (mg L−1) | SO42− (mg L−1) | Degree of Mineralization (g L−1) | pH |
|---|---|---|---|---|---|---|
| 11,041 | 300 | 1627 | 19,241 | 2498 | 1200 | 7.9 |
| Date | May 29th | May 31st | Jun. 12th | Jun. 14th | Jun. 26th | Jun. 28th | Jul. 5th | Jul. 12th | Jul. 19th |
|---|---|---|---|---|---|---|---|---|---|
| initial irrigating time | Control, S1 | S2 | S3 | ||||||
| sampling time | EL 31 | EL 33 | EL 35 | EL 36 | EL 37 | EL 38 | |||
| Compounds | RT | RI | m/z | Treatment | |||
|---|---|---|---|---|---|---|---|
| (µg kg−1 FW) | Control | S1 | S2 | S3 | |||
| Isoprene pathway | 14.68 ± 1.84 c | 38.3 ± 3.06 a | 25.69 ± 5.48 b | 22.84 ± 0.48 bc | |||
| Terpenoids | 11.44 ± 1.63 c | 33.14 | 21.6 | 18.83 ± 0.25 b | |||
| Limonene | 12.84 | 1018 | 68, 93 | 5.42 ± 0.97 a | 4.94 ± 0.47 a | 4.79 ± 1.11 a | 5.06 ± 0.09 a |
| 2-Carene | 12.28 | 948 | 41, 121, 93 | 1.55 ± 0.40 a | 1.55 ± 0.53 a | 1.55 ± 0.35 a | - |
| γ-Terpinene | 14.33 | 998 | 93, 136 | 1.33 ± 0.32 a | 1 ± 0.36 a | 1.19 ± 0.30 a | 1.42 ± 0.20 a |
| β-Pinene | 10.36 | 943 | 93, 41, 69 | 1.32 ± 0.36 a | 1.93 ± 0.59 a | 2.79 ± 0.99 a | 3.06 ± 0.63 a |
| Sabinene | 10.28 | 897 | 93, 41, 77 | 1.06 ± 0.24 a | 0.82 ± 0.18 a | 1.82 ± 0.96 a | 1.46 ± 0.44 a |
| α-Pinene | 8.51 | 948 | 93, 77 | 0.76 ± 0.16 a | 1.13 ± 0.05 a | 1.04 ± 0.19 a | 1.3 ± 0.49 a |
| Citronellol | 22.53 | 1179 | 69, 41, 81 | - | 9.57 ± 0.72 a | 3.51 ± 0.98 b | 1.85 ± 0.26 b |
| β-Myrcene | 11.28 | 958 | 93, 41, 69 | - | 2.69 ± 0.62 | - | - |
| α-Terpineol | 20.60 | 1143 | 59, 93, 121 | - | 3.73 ± 0.60 a | 2.59 ± 0.38 a | 2.11 ± 0.87 a |
| 4-Thujanol | 19.93 | 897 | 93 | - | 4.12 ± 1.07 a | 2.31 ± 0.24 b | 2.01 ± 0.28 b |
| Trans-rose oxide | 20.24 | 1114 | 139, 69 | - | 0.97 ± 0.14 | - | - |
| Eucalyptol | 12.97 | 1059 | 43, 81, 71 | - | 0.7 ± 0.09 a | - | 0.56 ± 0.09 a |
| Norisoprenopids | 3.25 ± 0.22 a | 5.15 ± 0.44 a | 4.09 ± 1.35 a | 4.01 ± 0.49 a | |||
| Methyl heptenone | 11.13 | 938 | 43, 69, 108 | 3.25 ± 0.22 a | 4.29 ± 0.39 a | 4.09 ± 1.35 a | 4.01 ± 0.49 a |
| Geranylacetone | 31.63 | 1420 | 43, 69, 151 | - | 0.86 ± 0.05 | - | - |
| Fatty acid pathway | 473.13 ± 5.60 c | 601.99 ± 8.36 a | 542.33 ± 7.42 b | 430.99 ± 6.59 d | |||
| C6 alcohols | 3.74 ± 0.85 a | 3.01 ± 0.58 a | 3.57 ± 0.32 a | 3.67 ± 0.40 a | |||
| 1-Octanol | 15.14 | 1059 | 56, 70, 84 | 0.87 ± 0.39 a | 1.54 ± 0.57 a | 1.37 ± 0.31 a | 1.56 ± 0.20 a |
| 1-Hexanol | 6.19 | 860 | 56, 69 | 2.86 ± 0.46 a | 0.83 ± 0.22 b | 1.31 ± 0.19 b | 0.93 ± 0.22 b |
| 1-Octen-3-ol | 10.79 | 969 | 57, 72 | - | 0.64 ± 0.09 b | 0.89 ± 0.18 ab | 1.18 ± 0.29 a |
| C6 Aldehydes | 231.71 ± 5.79 b | 264.83 ± 6.58 a | 268.53 ± 5.73 a | 241.52 ± 5.07 b | |||
| Hexanal | 3.92 | 806 | 44, 56 | 106.97 ± 2.46 c | 133.44 ± 2.73 a | 123.88 ± 4.72 b | 108.01 ± 2.46 c |
| (E)-2-Hexenal | 7.47 | 814 | 55, 83, 69 | 95.05 ± 4.49 b | 98.56 ± 4.80 b | 113.04 ± 0.82 a | 95.68 ± 2.33 b |
| Nonanal | 16.63 | 1104 | 57, 70 | 19.38 ± 0.79 b | 21.53 ± 2.37 ab | 22.43 ± 1.96 ab | 23.83 ± 0.80 a |
| Decanal | 21.42 | 1204 | 57, 82, 70 | 7.56 ± 0.53 b | 9.84 ± 0.52 a | 7.38 ± 0.71 b | 8.63 ± 0.76 ab |
| 4-Oxo-2-hexenal | 9.95 | 950 | 83, 55 | 2.26 ± 0.87 a | - | - | 2.41 ± 0.44 a |
| (E)-2-Heptenal | 9.68 | 913 | 41, 83 | 0.49 ± 0.12 b | - | 0.67 ± 0.14 b | 1.31 ± 0.16 a |
| (E)-2-Octenal | 14.41 | 1013 | 70, 55 | - | 1.47 ± 0.18 ab | 1.13 ± 0.19 b | 1.64 ± 0.20 a |
| C6 Esters | 237.69 ± 10.54 c | 334.15 ± 10.87 a | 270.23 ± 2.91 b | 185.8 ± 5.02 d | |||
| Ethyl acetate | 1.38 | 586 | 43, 70 | 66.3 ± 1.67 c | 113.12 ± 4.71 a | 94.67 ± 2.64 b | 67.48 ± 1.83 c |
| Ethyl butanoate | 4.09 | 785 | 71, 43, 88 | 47.85 ± 2.76 a | 39.07 ± 1.33 b | 41.39 ± 1.16 b | 36.55 ± 3.21 b |
| Ethyl caprylate | 21.12 | 1183 | 88, 57 | 40.65 ± 1.48 b | 46.93 ± 2.33 a | 30.69 ± 0.23 c | 18.75 ± 0.77 d |
| Ethyl 2-butenoate | 5.28 | 793 | 69, 41, 99 | 13.08 ± 0.36 d | 20.49 ± 1.46 c | 34.98 ± 2.01 a | 24.4 ± 1.44 b |
| Ethyl heptanoate | 16.44 | 1083 | 88, 43, 70 | 12.85 ± 1.33 b | 15.94 ± 1.04 a | 10.92 ± 0.07 b | 7.87 ± 0.46 c |
| Ethyl valerate | 7.38 | 884 | 88, 57 | 9.34 ± 0.41 a | 7.49 ± 0.15 b | 6.93 ± 0.14 b | 8.25 ± 1.09 ab |
| Ethyl (E,Z)-2,4-decadienoate | 31.76 | 1397 | 125, 97 | 7.34 ± 1.01 c | 28.02 ± 2.00 a | 15.55 ± 4.75 b | 4.83 ± 0.77 c |
| Ethyl 2-hexenoate | 13.83 | 992 | 97, 55, 73 | 6.5 ± 0.62 b | 12.07 ± 0.13 a | 6.83 ± 0.40 b | 4.86 ± 0.83 c |
| Ethyl caprate | 29.89 | 1381 | 88, 70 | 7.16 ± 1.07 b | 12.3 ± 1.37 a | 4.29 ± 0.16 c | 1.53 ± 0.21 d |
| Ethyl sorbate | 16.22 | 1000 | 67, 95, 41 | 5.91 ± 0.37 a | 3.96 ± 0.72 b | 2.37 ± 0.38 c | 1.61 ± 0.34 c |
| Ethyl (E)-2-octenoate | 23.36 | 1191 | 55, 125, 73 | 4.53 ± 0.93 a | 5.79 ± 0.45 a | 2.55 ± 0.20 b | 1.63 ± 0.56 b |
| Ethyl 4-octenoate | 20.72 | 1191 | 55, 82 | 3.59 ± 0.38 c | 7.6 ± 0.74 a | 5.93 ± 0.65 c | 4.5 ± 0.52 bc |
| Ethyl (E)-4-hexenoate | 12.20 | 992 | 68, 55, 81 | 2.93 ± 0.58 a | 2.65 ± 0.55 a | 2.53 ± 0.29 a | - |
| Ethyl 3-hexenoate | 12.07 | 992 | 69, 41 | 3.15 ± 0.09 ab | 2.75 ± 0.53 ab | 3.4 ± 0.62 a | 2.07 ± 0.38 b |
| Ethyl (E)-4-decenoate | 29.20 | 1389 | 88, 110, 69 | 2.71 ± 0.14 c | 11.21 ± 1.33 a | 5.9 ± 1.28 b | - |
| Ethyl propanoate | 2.23 | 686 | 57, 75 | 1.23 ± 0.54 a | 0.77 ± 0.35 a | 1.29 ± 0.33 a | - |
| Ethyl (E)-2-decenoate | 31.50 | 1389 | 55, 73, 101 | 1.41 ± 0.15 a | 1.17 ± 0.08 a | - | - |
| Ethyl 2-pentenoate | 9.43 | 892 | 83, 55 | 0.6 ± 0.07 | - | - | - |
| Propyl butyrate | 7.35 | 884 | 71, 43, 89 | 0.55 ± 0.11 | - | - | - |
| Butyl butanoate | 12.10 | 992 | 55, 73 | - | 1.29 ± 0.55 a | - | 1.47 ± 0.37 a |
| Ethyl 3-hydroxybutyrate | 8.81 | 947 | 43, 60, 88 | - | 1.53 ± 0.22 | - | - |
| Amino acid pathway | 54.97 ± 4.01 b | 48.59 ± 2.48 b | 51.31 ± 2.51 b | 66.86 ± 4.45 a | |||
| Benzene derivatives | 32.41 ± 3.66 b | 30.56 ± 2.34 b | 31.07 ± 1.12 b | 41.78 ± 2.43 a | |||
| Fluorene | 34.18 | 1494 | 166 | 5.9 ± 0.88 a | 6.38 ± 0.86 a | 5.36 ± 0.88 a | 6.74 ± 0.75 a |
| o-Xylene | 6.03 | 907 | 91, 106 | 5.15 ± 0.86 a | 3.61 ± 0.18 b | 4.96 ± 0.35 a | 4.47 ± 0.29 ab |
| Naphthalene | 20.00 | 1231 | 128 | 4.51 ± 0.53 a | 2.72 ± 0.78 b | 3.88 ± 0.18 ab | 4.98 ± 0.82 a |
| 1,3-Dimethyl benzene | 6.85 | 907 | 91, 106 | 3.12 ± 0.08 a | 2.03 ± 0.57 b | 2.85 ± 0.13 ab | 2.8 ± 0.58 ab |
| Dibenzofuran | 32.90 | 1483 | 168, 139 | 3.06 ± 0.37 b | 4.71 ± 0.80 a | 3.25 ± 0.26 b | 2.8 ± 0.33 b |
| 2-Ethyl toluene | 9.72 | 1006 | 105, 120 | 2.4 ± 0.62 a | - | 2.35 ± 0.21 a | - |
| o-Cymene | 12.67 | 1042 | 119, 134 | 2.29 ± 0.18 a | 2.3 ± 0.18 a | 2.51 ± 0.58 a | 2.43 ± 0.12 a |
| Phenanthrene | 36.95 | 1782 | 178, 76, 152 | 1.74 ± 0.10 a | 1.78 ± 0.39 a | 0.9 ± 0.11 b | 1.86 ± 0.27 a |
| Benzeneacetaldehyde | 13.58 | 1081 | 91, 120 | 1.88 ± 0.48 ab | 3.1 ± 0.17 a | 1.09 ± 0.13 b | 3.48 ± 1.10 a |
| Toluene | 3.13 | 794 | 91, 65 | 0.88 ± 0.14 a | 0.85 ± 0.04 a | 0.91 ± 0.11 a | 0.86 ± 0.08 a |
| Ethylbenzene | 5.74 | 893 | 91, 107 | 0.58 ± 0.18 a | 0.37 ± 0.12 a | 0.69 ± 0.11 a | 0.54 ± 0.08 a |
| Styrene | 6.83 | 883 | 104, 78 | - | 1.73 ± 0.40 | - | - |
| 1-Methylethyl benzene | 13.06 | 804 | 91 | - | - | 1.43 ± 0.49 | - |
| Ethyl benzoate | 19.71 | 1160 | 105, 77 | - | - | - | 9.25 ± 0.68 |
| Branched volatiles | 22.56 ± 0.38 ab | 18.02 ± 1.42 c | 20.24 ± 2.37 bc | 25.08 ± 2.02 a | |||
| 2-Ethyl-1-hexanol | 13.10 | 995 | 57, 70, 83 | 14.21 ± 0.44 b | 17.03 ± 1.28 ab | 16.99 ± 1.32 ab | 19.73 ± 1.67 a |
| 3-Methyl butanal | 1.65 | 643 | 44, 71 | 0.89 ± 0.44 a | 0.99 ± 0.20 a | 0.89 ± 0.56 a | 1.58 ± 0.40 a |
| Isopropyl hexanoate | 13.54 | 1019 | 43, 99, 60 | 6.45 ± 0.32 a | - | 2.36 ± 0.84 b | 2.69 ± 0.36 b |
| 2-Ethyl furan | 2.08 | 742 | 81, 45 | 1 ± 0.20 a | - | - | 1.07 ± 0.21 a |
| SUM | 541.89 ± 9.72 c | 687.88 ± 10.79 a | 618.44 ± 4.45 b | 519.11 ± 4.37 d | |||
| Varieties | Control | S1 | S2 | S3 | |||
| Isoprene pathway | 7 | 14 | 10 | 10 | |||
| Terpenoids | 6 | 12 | 9 | 9 | |||
| Norisoprenopids | 1 | 2 | 1 | 1 | |||
| Fatty acid pathway | 27 | 27 | 26 | 24 | |||
| C6 Alcohols | 2 | 3 | 3 | 3 | |||
| C6 Aldehydes | 6 | 5 | 6 | 7 | |||
| C6 Esters | 19 | 19 | 17 | 14 | |||
| Amino acid pathway | 15 | 13 | 14 | 15 | |||
| Benzene derivatives | 11 | 11 | 11 | 11 | |||
| Branched volatiles | 4 | 2 | 3 | 4 | |||
| SUM | 49 | 54 | 50 | 49 | |||
 | |||||||
| Control | S1 | S2 | S3 | |
|---|---|---|---|---|
| pH | 6.80 ± 0.06 c | 7.01 ± 0.05 a | 6.89 ± 0.02 b | 6.90 ± 0.04 b |
| Soluble salt content (g kg−1) | 0.189 ± 0.12 d | 0.704 ± 0.04 a | 0.542 ± 0.08 b | 0.403 ± 0.14 c |
| Na+ content (g kg−1) | 0.0158 ± 0.0007 b | 0.0176 ± 0.0008 a | 0.0169 ± 0.0004 ab | 0.0159 ± 0.002 b |
| Cl− content (g kg−1) | 0.0235 ± 0.0005 b | 0.0268 ± 0.0008 a | 0.0261 ± 0.0002 a | 0.0244 ± 0.0001 a |
| SO42− content (g kg−1) | 0.0230 ± 0.0002 b | 0.0270 ± 0.0010 a | 0.0259 ± 0.0005 a | 0.0246 ± 0.0006 a |
| Soil moisture content (%) | 16.13 ± 0.6 a | 17.10 ± 1.3 a | 16.15 ± 0.9 a | 16.55 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Yu, M.; Yao, Y.; Zhai, H.; Tang, M.; Gao, Z.; Du, Y. Effect of Seawater Irrigation on the Sugars, Organic Acids, and Volatiles in ‘Reliance’ Grape. Horticulturae 2022, 8, 248. https://doi.org/10.3390/horticulturae8030248
Liu M, Yu M, Yao Y, Zhai H, Tang M, Gao Z, Du Y. Effect of Seawater Irrigation on the Sugars, Organic Acids, and Volatiles in ‘Reliance’ Grape. Horticulturae. 2022; 8(3):248. https://doi.org/10.3390/horticulturae8030248
Chicago/Turabian StyleLiu, Menglong, Meng Yu, Yuxin Yao, Heng Zhai, Meiling Tang, Zhen Gao, and Yuanpeng Du. 2022. "Effect of Seawater Irrigation on the Sugars, Organic Acids, and Volatiles in ‘Reliance’ Grape" Horticulturae 8, no. 3: 248. https://doi.org/10.3390/horticulturae8030248