The Agro-Economic Feasibility of Growing the Medicinal Plant Euphorbia peplus in a Modified Vertical Hydroponic Shipping Container
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Vertical Hydroponic Container
- -
- Dimensions: 12,192 mm × 2438 mm × 2896 mm
- -
- Germination area: Eight PE food-grade plastic trays (1220 mm × 560 mm) at 4 levels equipped with separate manual irrigation valves and LED lights adjustable in intensity allowing a maximum of 2400 seeds to germinate.
- -
- Cultivation area: Thirty-six food-grade plastic trays (1220 mm × 560 mm) with 2 irrigation systems and at 3 levels, each 600 mm high, equipped with automatic irrigation valves and LED lights adjustable in intensity. Each cultivation tray had a capacity of 24 plants (Figure 2).
- -
- Irrigation system: A deep-water irrigation system supplied irrigation for 1 min every 5 min, with water recirculation to the water reserve. The irrigation system included a water reserve (800 L), a connection to the reserve of concentrated nutrient solutions, peristaltic pumps, the piping assembly sized according to the required flow rates, the flow control valve, filters (UV and physical filters) for water recirculation, and a control station connected to the LED control circuit and to various measuring instruments—including a pH-meter, an EC-meter, a kWh meter, and a CO2 concentration sensor.
- -
- LED lighting: For the substrate experiment (see Section 2.4), LEDs with an irradiance of 150 μmol m−2 s−1 at a distance of 30 cm were used. The LED spectrum was composed of 35% blue (450 nm) and 65% red (660 nm). For the light intensity experiment (see Section 2.5), LEDs adjustable up to 500 μmol m−2 s−1 were used. The spectrum was composed of 20.8% blue, 22.7% green, 52.5% red, and 4% far red.
2.3. Euphorbia peplus Growing Conditions
2.4. Substrate Experiment
2.5. Light and Localization Experiment
2.6. Biomass Accumulation Measurements
2.7. Apical Growth Measurements
2.8. Total Ingenol Measurements
2.9. Extraction Procedures
2.10. Economical Evaluation of E. peplus Production
2.11. Data Analysis
3. Results and Discussion
3.1. Substrate Experiment
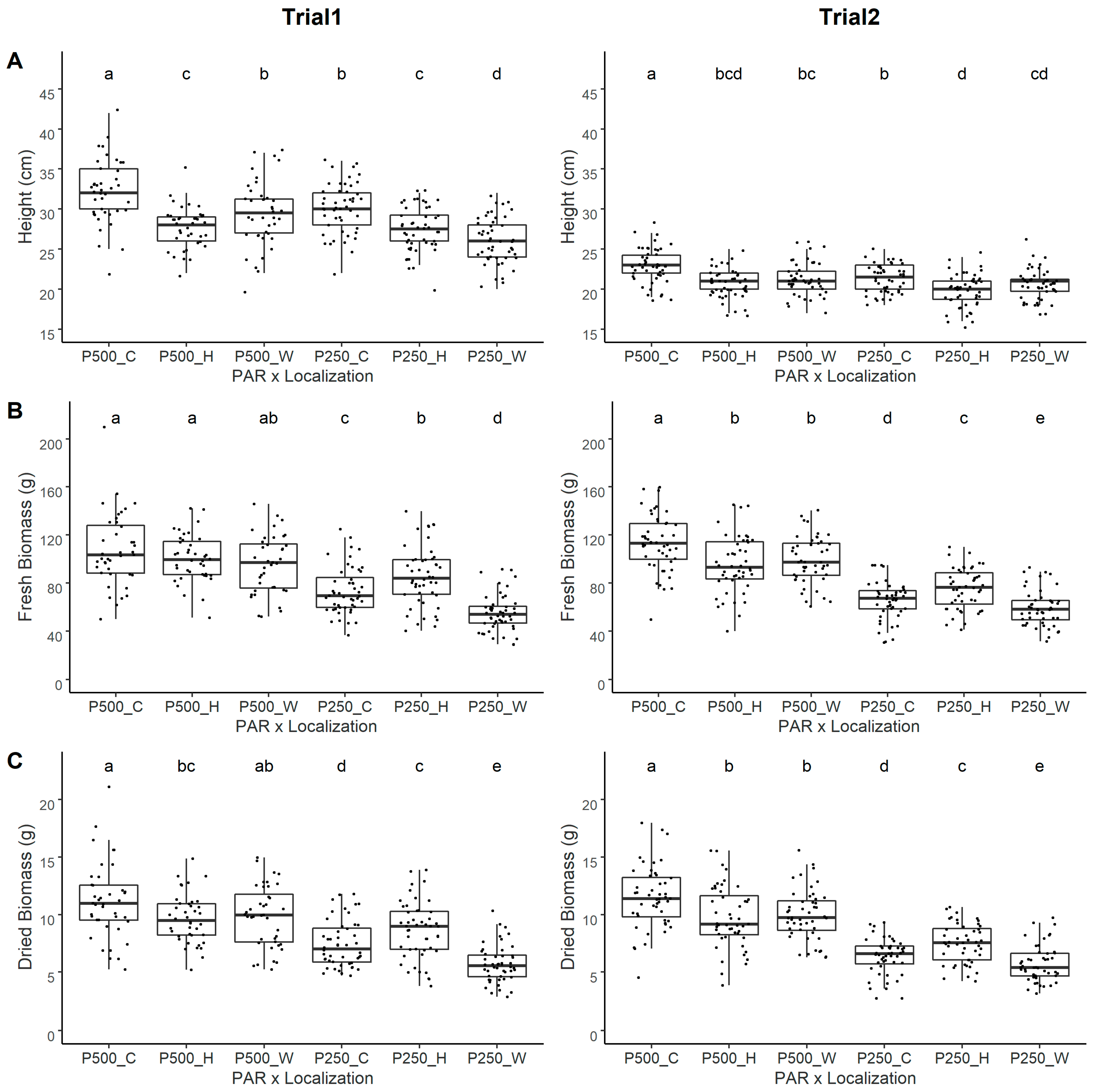
3.2. Lighting and Localization Experiment
3.3. Economic Evaluation of E. peplus Production
3.3.1. Economic Comparison of E. peplus and Romaine Lettuce Production
3.3.2. Economic Evaluation of the Production of Ingenol-Mebutate as a Raw Material
3.3.3. Economic Evaluation of Ingenol-Mebutate Production for Pharmaceutical Purposes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Feeding the Cities of the Future. Available online: https://www.fao.org/news/story/en/item/446763/icode/ (accessed on 31 January 2022).
- Lotze-Campen, H.; Müller, C.; Bondeau, A.; Rost, S.; Popp, A.; Lucht, W. Global food demand, productivity growth, and the scarcity of land and water resources: A spatially explicit mathematical programming approach. Agric. Econ. 2008, 39, 325–338. [Google Scholar] [CrossRef]
- Davis, K. The origin and growth of urbanization in the world. Am. J. Sociol. 1955, 60, 429–437. [Google Scholar] [CrossRef]
- Agrilyst State of Indoor Farming. Available online: https://artemisag.com/wp-content/uploads/06/stateofindoorfarming-report-2017.pdf (accessed on 7 June 2021).
- Kozai, T.; Niu, G. Chapter 1—Introduction. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 3–6. ISBN 978-0-12-816691-8. [Google Scholar]
- Despommier, D. The rise of vertical farms. Sci. Am. 2009, 301, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Avgoustaki, D.D.; Xydis, G. How energy innovation in indoor vertical farming can improve food security, sustainability, and food safety? Adv. Food Secur. Sustain. 2020, 5, 1–51. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. Indoor vertical farming in the urban nexus context: Business growth and resource savings. Sustainability 2020, 12, 1965. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Banerjee, C.; Adenaeuer, L. Up, up and away! The economics of vertical farming. J. Agric. Stud. 2014, 2, 40–60. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Beacham, A.M.; Vickers, L.H.; Monaghan, J.M. Vertical farming: A summary of approaches to growing skywards. J. Hortic. Sci. Biotechnol. 2019, 94, 277–283. [Google Scholar] [CrossRef]
- Coyle, B.D.; Ellison, B. Will consumers find vertically farmed produce “out of reach”? Choices 2017, 32, 1–8. [Google Scholar]
- Cox, S.; Van Tassel, D. Vertical farming doesn’t stack up. Synth. Regen. 2010, 52. [Google Scholar]
- Benis, K.; Ferrao, P. Commercial farming within the urban built environment—Taking stock of an evolving field in northern countries. Glob. Food Secur. 2018, 17, 30–37. [Google Scholar] [CrossRef]
- Graamans, L.; Baeza, E.; van den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Sparks, R.E.; Stwalley, R.M., III. Design and testing of a modified hydroponic shipping container system for urban food production. Int. J. Appl. Agric. Sci. 2018, 4, 93–102. [Google Scholar]
- Debusschere, T.; Boekhout, R. When Will Vertical Farming Become Profitable? Available online: https://www.verticalfarmdaily.com/article/9321424/when-will-vertical-farming-become-profitable/ (accessed on 6 August 2021).
- VerticalFarmDaily Not Possible to Market Our Vertically Grown Vegetables in a Financially Attractive Way. Available online: https://www.verticalfarmdaily.com/article/9349889/not-possible-to-market-our-vertically-grown-vegetables-in-a-financially-attractive-way/ (accessed on 31 August 2021).
- Eaves, J.; Eaves, S. Comparing the profitability of a greenhouse to a vertical farm in Quebec. Can. J. Agric. Econ. Can.. 2018, 66, 43–54. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Chong, C.; Wang, C.-H.; Wang, X. A decision support framework for the design and operation of sustainable urban farming systems. J. Clean. Prod. 2020, 268, 121928. [Google Scholar] [CrossRef]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Traditional medicine: Report by the secretariat. In Proceedings of the 56th World Health Assembly, Geneva, Switzerland, 19–28 May 2003. [Google Scholar]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Venditti, A.; Sciubba, F.; Tomai, P.; Antonetti, M.; Franceschin, M.; Di Cocco, M.E.; Gentili, A.; Delfini, M.; Serafini, M.; et al. Phytochemical profile of Euphorbia peplus L. collected in Central Italy and NMR semi-quantitative analysis of the diterpenoid fraction. J. Pharm. Biomed. Anal. 2018, 160, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Batanouny, K.H.; Stichler, W.; Ziegler, H. Photosynthetic pathways and ecological distribution of Euphorbia species in Egypt. Oecologia 1991, 87, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Berman, B. New developments in the treatment of actinic keratosis: Focus on ingenol mebutate gel. Clin. Cosmet. Investig. Dermatol. 2012, 5, 111–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G. Chapter 9—Plant responses to light. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 153–166. ISBN 978-0-12-816691-8. [Google Scholar]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Morphological and physiological responses in basil and brassica species to different proportions of red, blue, and green wavelengths in indoor vertical farming. J. Am. Soc. Hortic. Sci. 2020, 145, 267–278. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Bar-Tal, A. Substrates and their analysis. In Hydroponic Production of Vegetables and Ornamentals; Sawas, D., Passam, H., Eds.; Embrio Publications: Athens, Greece, 2002; pp. 25–105. [Google Scholar]
- Deepagoda, T.C.; Lopez, J.C.C.; Møldrup, P.; De Jonge, L.W.; Tuller, M. Integral parameters for characterizing water, energy, and aeration properties of soilless plant growth media. J. Hydrol. 2013, 502, 120–127. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant nutrition in future greenhouse production. In Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 393–403. ISBN 978-90-481-2532-6. [Google Scholar]
- Othman, Y.; Bataineh, K.; Al-Ajlouni, M.; Alsmairat, N.; Ayad, J.; Shiyab, S.; Al-Qarallah, B.; St Hilaire, R. Soilless culture: Management of growing substrate, water, nutrient, salinity, microorganism and product quality. Fresenius Environ. Bull. 2019, 28, 3249–3260. [Google Scholar]
- Alsmairat, N.G.; Al-Ajlouni, M.G.; Ayad, J.Y.; Othman, Y.A.; Hilaire, R.S. Composition of soilless substrates affect the physiology and fruit quality of two strawberry (Fragaria × ananassa Duch.) cultivars. J. Plant Nutr. 2018, 41, 2356–2364. [Google Scholar] [CrossRef]
- Al-Ajmi, A.; Al-Karaki, G.; Othman, Y. Effect of different substrates on fruit yield and quality of cherry tomato grown in a closed soilless system. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; pp. 491–494. [Google Scholar]
- Maloupa, E.; Gerasopoulos, D. Quality production of four cut gerberas in a hydroponic system of four substrates. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1999; pp. 433–438. [Google Scholar]
- Béres, T.; Dragull, K.; Pospíšil, J.; Tarkowská, D.; Dančák, M.; Bíba, O.; Tarkowski, P.; Doležal, K.; Strnad, M. Quantitative analysis of ingenol in Euphorbia species via validated isotope dilution ultra-high performance liquid chromatography tandem mass spectrometry. Phytochem. Anal. 2018, 29, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental risk-based ranking of solvents using the combination of a multimedia model and multi-criteria decision analysis. Green Chem. 2017, 19, 1034–1042. [Google Scholar] [CrossRef]
- Appendino, G. Ingenane diterpenoids. Prog. Chem. Org. Nat. Prod. 2016, 102, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Nothias-Scaglia, L.-F.; Dumontet, V.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. LC-MS2-Based dereplication of Euphorbia extracts with anti-Chikungunya virus activity. Fitoterapia 2015, 105, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Han, W.; Wang, Z.; Shao, Y.; Wang, Y.; Zhang, Y.; Li, Z.; Xu, X.; Zhang, Y. Hepatocellular carcinoma growth is inhibited by Euphorbia helioscopia L. extract in nude mice xenografts. BioMed Res. Int. 2015, 2015, 601015. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.M.; Sandi, D. Extraction of coffee diterpenes and coffee oil using supercritical carbon dioxide. Food Chem. 2007, 101, 1087–1094. [Google Scholar] [CrossRef]
- Bar-Tal, A.; Saha, U.K.; Raviv, M.; Tuller, M. Chapter 7—Inorganic and synthetic organic components of soilless culture and potting mixtures. In Soilless Culture, 2nd ed.; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Elsevier: Boston, MA, USA, 2019; pp. 259–301. ISBN 978-0-444-63696-6. [Google Scholar]
- De Swaef, T.; Verbist, K.; Cornelis, W.; Steppe, K. Tomato sap flow, stem and fruit growth in relation to water availability in rockwool growing medium. Plant Soil 2012, 350, 237–252. [Google Scholar] [CrossRef]
- Deepagoda, T.C.; Moldrup, P.; Tuller, M.; Pedersen, M.; Lopez, J.C.C.; de Jonge, L.W.; Kawamoto, K.; Komatsu, T. Gas diffusivity-based design and characterization of greenhouse growth substrates. Vadose Zone J. 2013, 12, 1–13. [Google Scholar] [CrossRef]
- Raatz, L.; Bacchi, N.; Pirhofer Walzl, K.; Glemnitz, M.; Müller, M.E.H.; Joshi, J.; Scherber, C. How much do we really lose?—Yield losses in the proximity of natural landscape elements in agricultural landscapes. Ecol. Evol. 2019, 9, 7838–7848. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Nendel, C.; Funk, R.; Mitchell, M.G.E.; Lischeid, G. Modeling yields response to shading in the field-to-forest transition zones in heterogeneous landscapes. Agriculture 2019, 9, 6. [Google Scholar] [CrossRef]
- Board, J. Light interception efficiency and light quality affect yield compensation of soybean at low plant populations. Crop Sci. 2000, 40, 1285–1294. [Google Scholar] [CrossRef]
- Kasperbauer, M.J. Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol. 1987, 85, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience horts 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, morphology, yield, and phytochemical accumulation in basil plants influenced by substituting green light for partial red and/or blue light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.T.; Sharkey, T.D. Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ. 2001, 24, 929–936. [Google Scholar] [CrossRef]
- Rosenfeld, H.J.; Aaby, K.; Lea, P. Influence of temperature and plant density on sensory quality and volatile terpenoids of carrot (Daucus carota L.) root. J. Sci. Food Agric. 2002, 82, 1384–1390. [Google Scholar] [CrossRef]
- Helmig, D.; Ortega, J.; Duhl, T.; Tanner, D.; Guenther, A.; Harley, P.; Wiedinmyer, C.; Milford, J.; Sakulyanontvittaya, T. Sesquiterpene emissions from pine trees—Identifications, emission rates and flux estimates for the contiguous United States. Environ. Sci. Technol. 2007, 41, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Mäenpää, M.; Hassinen, V.; Kontunen-Soppela, S.; Malec, L.; Rousi, M.; Pietikäinen, L.; Tervahauta, A.; Kärenlampi, S.; Holopainen, J.; et al. Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. J. Exp. Bot. 2010, 61, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Filella, I.; Wilkinson, M.J.; Llusià, J.; Hewitt, C.N.; Peñuelas, J. Volatile organic compounds emissions in Norway spruce (Picea abies) in response to temperature changes. Physiol. Plant. 2007, 130, 58–66. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Riikonen, J.; Ahonen, V.; Tervahauta, A.; Holopainen, T. Sensitivity of Norway spruce physiology and terpenoid emission dynamics to elevated ozone and elevated temperature under open-field exposure. Environ. Exp. Bot. 2013, 90, 32–42. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Tounekti, T.; Vadel, A.M.; Ennajeh, M.; Khemira, H.; Munné-Bosch, S. Ionic interactions and salinity affect monoterpene and phenolic diterpene composition in rosemary (Rosmarinus officinalis). J. Plant Nutr. Soil Sci. 2011, 174, 504–514. [Google Scholar] [CrossRef]
- Niessen, W.; Chanteux, A. Les Tableaux de Bord et Business Plan; Editions des Chambres de Commerce et d’Industrie de Wallonie, Ed.; Edipro: Seraing, Belgium, 2005. [Google Scholar]
- Hohmann, J.; Evanics, F.; Berta, L.; Bartók, T. Diterpenoids from Euphorbia peplus. Planta Med. 2000, 66, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Grue-Sørensen, G.; Petersen, A.K.; Högberg, T. Semisynthesis of ingenol 3-angelate (PEP005): Efficient stereoconservative angeloylation of alcohols. Synlett 2012, 23, 2647–2652. [Google Scholar] [CrossRef]
- Appendino, G.; Tron, G.C.; Cravotto, G.; Palmisano, G.; Annunziata, R.; Baj, G.; Surico, N. Synthesis of modified ingenol esters. Eur. J. Org. Chem. 1999, 1999, 3413–3420. [Google Scholar] [CrossRef]
- Winkler, J.D.; Rouse, M.B.; Greaney, M.F.; Harrison, S.J.; Jeon, Y.T. The first total synthesis of (±)-ingenol. J. Am. Chem. Soc. 2002, 124, 9726–9728. [Google Scholar] [CrossRef] [PubMed]
- Tanino, K.; Onuki, K.; Asano, K.; Miyashita, M.; Nakamura, T.; Takahashi, A.Y.; Kuwajima, I. Total synthesis of ingenol. J. Am. Chem. Soc. 2003, 125, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Maruyama, T.; Tang, H.; Murphy, P.D.; Greene, B.; Yusuff, A.N.; Wood, J.L. Total synthesis of ingenol. J. Am. Chem. Soc. 2004, 126, 16300–16301. [Google Scholar] [CrossRef] [PubMed]
- McKerrall, S.J.; Jørgensen, L.; Kuttruff, C.A.; Ungeheuer, F.; Baran, P.S. Development of a concise synthesis of (+)-ingenol. J. Am. Chem. Soc. 2014, 136, 5799–5810. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Grootendorst, P.; Lexchin, J.; Cunningham, C.; Greyson, D. The cost of drug development: A systematic review. Health Policy 2011, 100, 4–17. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Le Galès, C. Pourquoi les nouveaux médicaments sont-ils si chers? Med. Sci. 2018, 34, 354–361. [Google Scholar] [CrossRef] [PubMed]



| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| Substrate | Number of Aliquots | Total Ingenol | Number of Aliquots | Total Ingenol |
| Clay beads | 2 | 61.8 ± 1.57 a | 6 | 60.0 ± 12 a |
| Coco Fiber | 2 | 59.9 ± 3.84 a | 6 | 63.5 ± 8.84 a |
| Rockwool | 2 | 61.7 ± 1.41 a | 6 | 61.8 ± 5.17 a |
| Trial 1 | Trial 2 | ||||||
|---|---|---|---|---|---|---|---|
| Light Intensity | (μmolm−2 s−1) | 250 | 500 | 250 | 500 | ||
| Total Ingenol | (mg/kg) | 74.7 ± 25.4 a | 65.7 ± 19.6 a | 60.9 ± 2.61 a | 62.4 ± 3.08 a | ||
| Localization | Center | Alley | Wall | Center | Alley | Wall | |
| Total Ingenol | (mg/kg) | 71.5 ± 24.6 a | 72.4 ± 24.1 a | 68.0 ± 22.3 a | 62.8 ± 1.81 a | 61.3 ± 3.63 a | 60.9 ± 2.88 a |
| Crop | Euphorbia peplus | Romaine Lettuce | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Light | (μmolm−2 s−1) | 150 | 500 | 150 | |||||||
| Fresh Biomass per crop | g | 32.7 | 102 | 102 | |||||||
| R&D container | R&D+ container | R&D container | R&D+ container | Commercial container | |||||||
| Total Growing surface (sqm) | sqm | 30 | 40 | 30 | 40 | 50 | |||||
| Fresh Biomass (incl. 5% quality loss) | (kg/yr/sqm) | 6.4 | 6.34 | 19.97 | 19.39 | 34.04 | |||||
| OUTPUT | (kg/yr) | 192 | 254 | 599 | 776 | 1702 | |||||
| CAPEX | (EUR/sqm) | 3500 | 3000 | 3833.33 | 3375 | 3100 | |||||
| CAPEX (15-yr depreciation) | (EUR/yr) | 7000 | 8000 | 7667 | 9000 | 10,333 | |||||
| OPEX | |||||||||||
| Technical Staff at €210/day | (EUR/yr) | 12,023 | 13,395 | 12,023 | 13,395 | 19,467 | |||||
| Engineer staff at €310/day | (EUR/yr) | 4437 | 4394 | 4437 | 4394 | 1465 | |||||
| Director staff at €600/day | (EUR/yr) | 2147 | 2126 | 2147 | 2126 | 709 | |||||
| Electricity at €0.2/kW | (EUR/yr) | 6650 | 8845 | 9177 | 12,206 | 10,964 | |||||
| Water at €4.94/m3 | (EUR/yr) | 33 | 45 | 34 | 45 | 50 | |||||
| Seeds | (EUR/yr) | 0 | 0 | 0 | 0 | 75 | |||||
| Fertilizer | (EUR/yr) | 1001 | 1319 | 1001 | 1319 | 810 | |||||
| Substrates (rockwool) | (EUR/yr) | 1255 | 1657 | 1255 | 1657 | 934 | |||||
| pH adjustors | (EUR/yr) | 47 | 62 | 47 | 62 | 76 | |||||
| Container maintenance | (EUR/yr) | 1001 | 1301 | 1001 | 1301 | 1626 | |||||
| TOTAL | (EUR/yr) | 28,597 | 33,144 | 31,124 | 36,505 | 36,176 | |||||
| COST per kg of fresh biomass | |||||||||||
| CAPEX (15-yr depreciation) | (EUR/kg) | 36.44 | 20% | 31.54 | 19% | 12.80 | 20% | 11.60 | 20% | 6.07 | 22% |
| Labor Technical staff | (EUR/kg) | 62.59 | 34% | 52.81 | 34% | 20.07 | 31% | 17.27 | 29% | 11.44 | 42% |
| Labor Eng. staff | (EUR/kg) | 23.10 | 12% | 17.32 | 11% | 7.41 | 11% | 5.67 | 10% | 0.86 | 3% |
| Labor Director staff | (EUR/kg) | 11.18 | 6% | 8.38 | 5% | 3.58 | 6% | 2.74 | 5% | 0.42 | 2% |
| Electricity | (EUR/kg) | 34.62 | 19% | 34.87 | 21% | 15.32 | 24% | 15.74 | 27% | 6.44 | 24% |
| Water | (EUR/kg) | 0.18 | 0% | 0.18 | 0% | 0.06 | 0% | 0.06 | 0% | 0.03 | 0% |
| Seeds | (EUR/kg) | - | 0% | - | 0% | - | 0% | - | 0% | 0.04 | 0% |
| Fertilizer | (EUR/kg) | 5.21 | 3% | 5.20 | 3% | 1.67 | 3% | 1.70 | 3% | 0.48 | 2% |
| Substrates (rockwool) | (EUR/kg) | 6.53 | 4% | 6.53 | 4% | 2.09 | 3% | 2.14 | 4% | 0.55 | 2% |
| pH adjustors | (EUR/kg) | 0.24 | 0% | 0.24 | 0% | 0.08 | 0% | 0.08 | 0% | 0.04 | 0% |
| Container maintenance | (EUR/kg) | 5.21 | 3% | 5.13 | 3% | 1.67 | 3% | 1.68 | 3% | 0.96 | 3% |
| TOTAL | (EUR/kg) | 185.31 | 162.22 | 64.74 | 58.67 | 27.33 | |||||
| Retail Sales Prices | Retail Purchase Prices (at 50% Gross Margin) | ||
|---|---|---|---|
| Europe-Belgium | (EUR/kg) | 5.00 | 2.50 |
| USA-New York | (EUR/kg) | 22.00 | 11.00 |
| Asia-Singapore | (EUR/kg) | 26.00 | 13.0 |
| EXTRACTION COSTS | ||||
|---|---|---|---|---|
| Extraction Method | ||||
| Ethyl Acetate 120 °C | Ethyl Acetate Room Temp. | Supercritical CO2 | ||
| OUTPUT | ||||
| Dried biomass R&D+ container | (kg/yr) | 85 | 85 | 85 |
| Dried biomass R&D container | (kg/yr) | 66 | 66 | 66 |
| Ingenol-mebutate per dried kg | (mg/kg) | 43.76 | 23.94 | 32.17 |
| CAPEX | ||||
| Drying equipment | (EUR) | 15,000 | 15,000 | 15,000 |
| Grinding equipment | (EUR) | 20,000 | 20,000 | 20,000 |
| Extraction pilot | (EUR) | 500,000 | 100,000 | 800,000 |
| Evaporation equipment | (EUR) | 30,000 | 30,000 | 30,000 |
| Purification equipment | (EUR) | 500,000 | 500,000 | 500,000 |
| Occupation Rate | (%) | 10 | 10 | 10 |
| TOTAL CAPEX (20-yr depreciation) | (EUR/yr) | 5325 | 3325 | 6835 |
| OPEX | ||||
| Technical Staff at €210/day | (EUR/batch) | 840 | 840 | 840 |
| Engineer staff at €310/day | (EUR/batch) | 620 | 310 | 620 |
| Director staff at €600/day | (EUR/batch) | 600 | 300 | 600 |
| Electricity at €0.2/kW | (EUR/batch) | 4000 | 800 | 4000 |
| Water at €4.94/m3 | (EUR/batch) | 49.4 | 49.4 | 0 |
| Solvent (CO2, EtOAc) | (EUR/batch) | 228.10 | 228.10 | 199.3 |
| Filtration/Evaporation/Concentration | (EUR/batch) | 2500 | 3500 | 500 |
| Purification consumables | (EUR/batch) | 1500 | 1500 | 1500 |
| Purification solvents | (EUR/batch) | 1500 | 1500 | 1500 |
| Equipment maintenance | (EUR/batch) | 200 | 200 | 500 |
| Total OPEX costs/batch | (EUR/batch) | 12,037.5 | 9,227.5 | 10,259.30 |
| Total OPEX costs/year | (EUR/yr) | 90,281.25 | 69,206.22 | 76,944.75 |
| CAPEX + OPEX | (EUR/yr) | 95,606.25 | 72,531.22 | 83,769.75 |
| Extraction Method | ||||
|---|---|---|---|---|
| Ethyl Acetate 120 °C | Ethyl Acetate Room Temp. | Supercritical CO2 | ||
| OUTPUT | ||||
| Ingenol-mebutate per year in R&D container | (g/yr) | 2.88 | 1.58 | 2.12 |
| Ingenol-mebutate per year in R&D+ container | (g/yr) | 3.73 | 2.04 | 2.75 |
| CULTIVATION COST | ||||
| R&D container | (EUR/yr) | 38,790 | 38,790 | 38,790 |
| R&D+ container | (EUR/yr) | 45,505 | 45,505 | 45,505 |
| EXTRACTION COST | (EUR/yr) | 95,606 | 72,531 | 83,770 |
| CULTIVATION & EXTRACTION COST | ||||
| R&D container | (EUR/yr) | 134,397 | 111,322 | 122,560 |
| R&D+ container | (EUR/yr) | 141,111 | 118,036 | 129,275 |
| Production cost of Ingenol-mebutate | ||||
| R&D container | (EUR/mg) | 46.6 | 70.5 | 57.8 |
| R&D+ container | (EUR/mg) | 37.8 | 57.8 | 47 |
| Cost price of Ingenol-mebutate | ||||
| R&D container | (EUR/mg) | 49 | 73 | 60 |
| R&D+ container | (EUR/mg) | 40 | 60 | 50 |
| Market Price/Unit | Potential Gross Margin (EUR/yr/Container) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R&D Container | R&D+ Container | ||||||||
| 1 mg | 5 mg | 10 mg | 1 mg | 5 mg | 10 mg | 1 mg | 5 mg | 10 mg | |
| Supplier 1 | 67 | 213 | 352 | 51,913 | 5162 | - | 100,803 | 48,535 | - |
| Supplier 2 | 123.4 | 246.8 | - | 214,720 | - | - | 311,557 | 174,689 | - |
| Characteristics of Picato® gel | ||||||
|---|---|---|---|---|---|---|
| A: Distributor Price/unit | (EUR/3 gel) | 36.9 | ||||
| B: Distributor Price/unit | (EUR/2 gel) | 36.9 | ||||
| Volume per gel tube | (g) | 0.47 | ||||
| A: 0.015% IngMeb | (µg IngMeb/gel) | 70.4 | ||||
| B: 0.05% IngMeb | (µg IngMeb/gel) | 235 | ||||
| Production cost of Picato® gel | R&D container | R&D+ container | R&D container | R&D+ container | (R&D+ container): 10 units | |
| Cultivation yield (fresh biomass) | (kg/yr) | 192 | 254 | 599 | 776 | 7756.4 |
| Extraction yield at 0.0043758% | (g IngMeb/yr) | 0.92 | 1.22 | 2.88 | 3.73 | 37.3 |
| OUTPUT | ||||||
| A: 0.015% IngMeb | (gel/yr) | 13,064 | 17,336 | 40,954 | 53,015 | 530,152 |
| B: 0.05% IngMeb | (gel/yr) | 3934 | 5195 | 12,273 | 15,887 | 158,871 |
| 100% A- 3 gels/unit | (EUR/yr) | 161,492 | 213,232 | 503,737 | 652,087 | 6,520,870 |
| 100% B- 2 gels/unit | (EUR/yr) | 72,592 | 95,849 | 226,432 | 293,117 | 2,931,166 |
| 75% A-25%B | (EUR/yr) | 139,267 | 183,887 | 434,411 | 562,344 | 5,623,444 |
| CAPEX | ||||||
| GMP gel production | (EUR) | 200,000 | 200,000 | 200,000 | 200,000 | 400,000 |
| Capex (30 yr depreciation) | (EUR/yr) | 6667 | 6667 | 6667 | 6667 | 13,333 |
| OPEX | ||||||
| Development costs | (EUR) | 300,000,000 | 300,000,000 | 300,000,000 | 300,000,000 | 300,000,000 |
| Development cost (20-yr depreciation) | (EUR/yr) | 15,000,000 | 15,000,000 | 15,000,000 | 15,000,000 | 15,000,000 |
| Cultivation cot | (EUR/yr) | 35,597 | 41,144 | 38,790 | 45,505 | 455,050 |
| Extraction cost—EtOAc 120 °C | (EUR/yr) | 92,944 | 92,944 | 95,606 | 95,606 | 143,531 |
| Gel production cost (75% A-25%B) | (EUR/yr) | 8123 | 10,725 | 25,338 | 32,800 | 327,999 |
| Flat fees | (EUR/yr) | 45,000 | 45,000 | 90,000 | 90,000 | 300,000 |
| CAPEX + OPEX | (EUR/yr) | 15,188,330 | 15,196,480 | 15,256,401 | 15,270,577 | 16,239,914 |
| Return on investment—100% A | (yr) | 94 | 71 | 30 | 23 | 3 |
| Return on investment—100% B | (yr) | 209 | 158 | 67 | 52 | 5 |
| Return on investment—75%A-25%B | (yr) | 109 | 83 | 35 | 27 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bafort, F.; Kohnen, S.; Maron, E.; Bouhadada, A.; Ancion, N.; Crutzen, N.; Jijakli, M.H. The Agro-Economic Feasibility of Growing the Medicinal Plant Euphorbia peplus in a Modified Vertical Hydroponic Shipping Container. Horticulturae 2022, 8, 256. https://doi.org/10.3390/horticulturae8030256
Bafort F, Kohnen S, Maron E, Bouhadada A, Ancion N, Crutzen N, Jijakli MH. The Agro-Economic Feasibility of Growing the Medicinal Plant Euphorbia peplus in a Modified Vertical Hydroponic Shipping Container. Horticulturae. 2022; 8(3):256. https://doi.org/10.3390/horticulturae8030256
Chicago/Turabian StyleBafort, Françoise, Stephan Kohnen, Etienne Maron, Ayoub Bouhadada, Nicolas Ancion, Nathalie Crutzen, and M. Haïssam Jijakli. 2022. "The Agro-Economic Feasibility of Growing the Medicinal Plant Euphorbia peplus in a Modified Vertical Hydroponic Shipping Container" Horticulturae 8, no. 3: 256. https://doi.org/10.3390/horticulturae8030256
APA StyleBafort, F., Kohnen, S., Maron, E., Bouhadada, A., Ancion, N., Crutzen, N., & Jijakli, M. H. (2022). The Agro-Economic Feasibility of Growing the Medicinal Plant Euphorbia peplus in a Modified Vertical Hydroponic Shipping Container. Horticulturae, 8(3), 256. https://doi.org/10.3390/horticulturae8030256







