Relationship between Endogenous Ethylene Production and Firmness during the Ripening and Cold Storage of Raspberry (Rubus idaeus ‘Heritage’) Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Physiological and Quality Assessments during Ripening
2.3. Statistical Analysis
3. Results
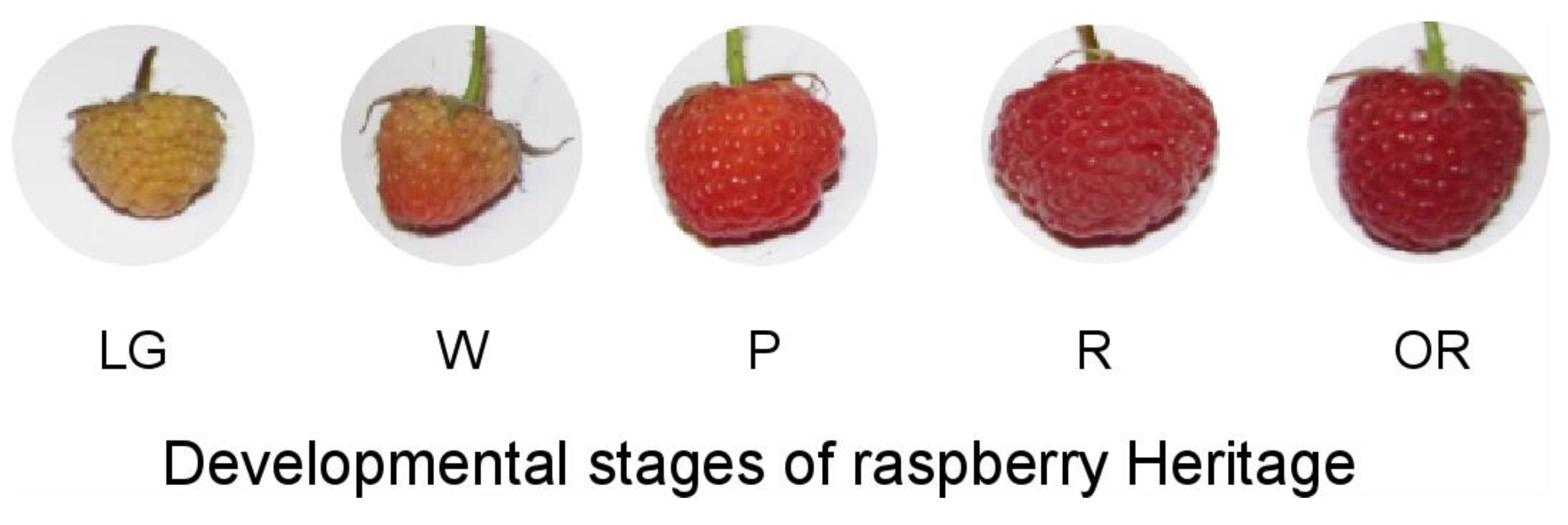
3.1. Physiological and Quality Parameters during the Development of Raspberry Fruit
3.2. Change in Physiological and Quality Parameters during Treatments
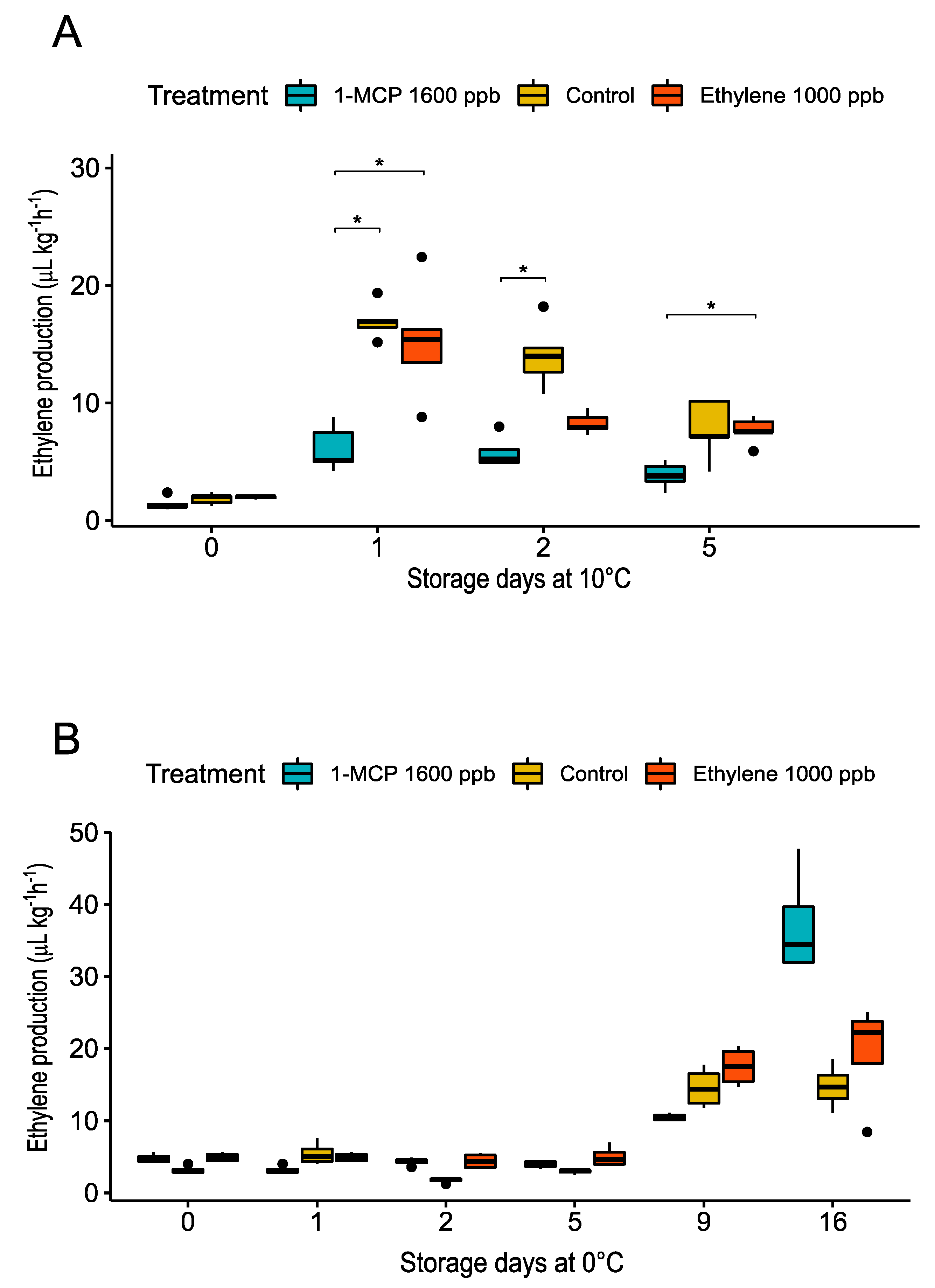
3.2.1. Physiological Changes during Treatments
3.2.2. Firmness Changes during Treatments
3.2.3. Total Soluble Solids (TSS) and Titratable Acidity (TA) Changes during Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Iannetta, P.P.M.; van den Berg, J.; Wheatley, R.E.; McNicol, R.J.; Davies, H.V. The role of ethylene and cell wall modifying enzymes in raspberry (Rubus idaeus) fruit ripening. Physiol. Plant. 1999, 105, 338–347. [Google Scholar] [CrossRef]
- Zheng, D.; Hrazdina, G. Cloning and characterization of an expansin gene, RiEXP1, and a 1-aminocyclopropane-1-carboxylic acid synthase gene, RiACS1 in ripening fruit of raspberry (Rubus idaeus L.). Plant Sci. 2010, 179, 133–139. [Google Scholar] [CrossRef]
- Iannetta, P.P.M.; Wyman, M.; Neelam, A.; Jones, C.; Davies, H.V.; Sexton, R. A causal role for ethylene and endo-b-1,4-glucanase in the abscission of red-raspberry (Rubus idaeus L.) drupelets. Physiol. Plant. 2000, 110, 535–543. [Google Scholar] [CrossRef]
- Sexton, R.; Palmer, J.M.; Whyte, N.; Littlejohns, S. Cellulase, fruit softening and abscission in red raspberry Rubus idaeus L. cv Glen Clova. Ann. Bot. 1997, 80, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, L.; Monsalve, L.; Morales-Quintana, L.; Valdenegro, M.; Martínez, J.P.; Defilippi, B.G.; González-Agüero, M. Differential expression of ethylene biosynthesis genes in drupelets and receptacle of raspberry (Rubus idaeus). J. Plant Physiol. 2015, 179, 100–105. [Google Scholar] [CrossRef]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M. Recent Advances in Hormonal Regulation and Cross-Talk during Non-Climacteric Fruit Development and Ripening. Horticulturae 2019, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Monsalve, L.; Ayala-Raso, A.; Bernales, M.; Valdenegro, M.; Defilippi, B.; González-Agüero, M.; Cherian, S.; Fuentes, L. Dataset on quality and physiological changes of raspberry fruit during their development and under auxin in-vitro assay. Data Brief 2018, 21, 1521–1525. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.M.; Nonnecke, G. Physiological changes during ripening of raspberry fruit. J. Hortic. Sci. 1992, 27, 331–333. [Google Scholar] [CrossRef]
- Bernales, M.; Monsalve, L.; Ayala-Raso, A.; Valdenegro, M.; Martínez, J.P.; Travisany, D.; Defilippi, B.; González-Agüero, M.; Cherian, S.; Fuentes, L. Expression of two indole-3-acetic acid (IAA)-amido synthetases (GH3) genes during fruit development and ripening of raspberry (Rubus idaeus Heritage). Sci. Hortic. 2019, 246, 168–175. [Google Scholar] [CrossRef]
- Stewart, D.; Iannetta, P.P.; Davies, H.V. Ripening-related changes in raspberry cell wall composition and structure. Phytochemistry 2001, 56, 423–428. [Google Scholar] [CrossRef]
- El-Kayal, W.E.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Phospholipase D inhibition by hexanal is associated with calcium signal transduction events in raspberry. Hortic. Res. 2017, 4, 17042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, C.; Hermosilla, A.; Contreras, E.; Naranjo, P.; Zoffoli, J.P.; Gambardella, M. Postharvest physiology and storage potential of new Chilean raspberry cultivars. Chil. J. Agric. Res. 2021, 81, 161–171. [Google Scholar] [CrossRef]
- Giovanelli, G.; Limbo, S.; Buratti, S. Effects of new packaging solutions on physico-chemical, nutritional and aromatic characteristics of red raspberries (Rubus idaeus L.) in postharvest storage. Postharvest Biol. Technol. 2014, 98, 72–81. [Google Scholar] [CrossRef]
- Giongo, L.; Ajelli, M.; Poncetta, P.; Ramos-García, M.; Sambo, P.; Farneti, B. Raspberry texture mechanical profiling during fruit ripening and storage. Postharvest Biol. Technol. 2019, 149, 177–186. [Google Scholar] [CrossRef]
- Li, L.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Porat, R.; Lichter, A. The effects of 1-methylcyclopropane and ethylene on postharvest rachis browning in table grapes. Postharvest Biol. Technol. 2015, 107, 16–22. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Gudenschwager, O.; Chervin, C.; Campos-Vargas, R.; González-Agüero, M.; Defilippi, B. Study on differential expression of 1-aminocyclopropane-1-carboxylic acid oxidase genes in table grape cv. Thompson Seedless. Postharvest Biol. Technol. 2013, 76, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Correa, J.; Mamani, M.; Muñoz, C.; González, M.; Defilippi, B.; Campos, R.; Pinto, M.; Hinrichsen, P. New Stable QTLs for Berry Firmness in Table Grapes. Am. J. Enol. Vitic. 2016, 67, 212–217. [Google Scholar] [CrossRef]
- Lurie, S.; Ovadia, R.; Nissim-Levi, A.; Oren-Shamir, M.; Kaplunov, T.; Zutahy, Y.; Weksler, H.; Lichter, A. Abscisic acid improves colour development in ‘Crimson Seedless’ grapes in the vineyard and on detached berries. J. Hortic. Sci. 2009, 84, 639–644. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 23 February 2022).
- Iannetta, P.P.M.; Laarhovenb, L.J.; Medina-Escobar, N.; James, E.K.; McManus, M.T.; Davies, H.V.; Harren, F.J.M. Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiol. Plant. 2006, 127, 247–259. [Google Scholar] [CrossRef]
- Bower, J.H.; Biasi, W.V.; Mitcham, E.J. Effects of ethylene and 1-MCP on the quality and storage life of strawberries. Postharvest Biol. Technol. 2003, 28, 417–423. [Google Scholar] [CrossRef]
- Cara, B.; Giovannoni, J. Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci. 2008, 175, 106–113. [Google Scholar] [CrossRef]
- Chervin, C.; El-Kereamy, A.; Roustan, J.-P.; Latché, A.; Lamon, J.; Bouzayen, M. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 2004, 167, 1301–1305. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, M.; Ren, J.; Qi, J.; Zhang, G.; Leng, P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010, 10, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.W.; Gunaseelan, K.; Pidakala, P.; Wang, M.; Schaffer, R.J. Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J. Exp. Bot. 2009, 60, 2689–2699. [Google Scholar] [CrossRef] [Green Version]
- Sjulin, T.M.; Robbins, J. Effects of maturity, harvest date and storage time on postharvest quality of red raspberry fruit. J. Am. Soc. Hortic. Sci. 1987, 112, 481–487. [Google Scholar]
- Silva, P.A.; de Abreu, C.M.P.; de Queiroz, E.R.; Corrêa, A.D.; Santos, C.D. Storage of Strawberries (Fragaria ananassa L.) Cv. ‘Oso Grande’, Subjected to 1-MCP. Acta Sci. Technol. 2012, 34, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, N.M.; Bustamante, C.A.; Civello, P.M.; Martínez, G.A. Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J. Sci. Food Agric. 2010, 90, 683–689. [Google Scholar] [CrossRef]
- Ku, V.V.V.; Wills, R.B.H.; Ben-Yehoshua, S. 1-Methylcyclopropene can differentially affect the postharvest life of strawberries exposed to ethylene. HortScience 1999, 34, 119–120. [Google Scholar] [CrossRef] [Green Version]
- Kano, Y.; Asahira, T. Roles of cytokinin and abscisic acid in the maturing of strawberry fruits. J. JPN Soc. Hortic. Sci. 1981, 50, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic acid is a major regulator of grape berry ripening onset: New insights into ABA signaling network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [Green Version]
- Gagné, S.; Cluzet, S.; Mérillon, J.M.; Gény, L. ABA initiates anthocyanin production in grape cell cultures. J. Plant Growth Regul. 2011, 30, 1–10. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2013, 65, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Kühn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kereamy, A.; Chervin, C.; Roustan, J.P.; Cheynier, V.; Souquet, J.M.; Moutounet, M.; Raynal, J.; Ford, C.; Latch’e, A.; Pech, J.C.; et al. Exogenous ethylene stimulates the longterm expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003, 119, 175–182. [Google Scholar] [CrossRef]
- Figueroa, N.E.; Gatica-Meléndez, C.; Figueroa, C.R. Ethylene application at the immature stage of Fragaria chiloensis fruit represses the anthocyanin biosynthesis with a concomitant accumulation of lignin. Food Chem. 2021, 358, 129913. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-H.; Luo, J.-J.; Tian, L.; Li, C.-L.; Xing, Y.; Shen, Y.-Y. New evidence for the role of ethylene in strawberry fruit ripening. J. Plant Growth Regul. 2013, 32, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cui, J.; Chen, J.; Yao, J.; Hao, Y.; Fan, Y.; Liu, Y. Evaluation of physicochemical properties in three raspberries (Rubus idaeus) at five ripening stages in northern China. Sci. Hortic. 2020, 263, 109–146. [Google Scholar] [CrossRef]
- Tian, M.; Prakash, S.; Elgar, H.; Young, H.; Burmeister, D.M.; Ross, G.S. Responses of strawberry fruit to 1-Methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul. 2000, 32, 83–90. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monsalve, L.; Bernales, M.; Ayala-Raso, A.; Álvarez, F.; Valdenegro, M.; Alvaro, J.-E.; Figueroa, C.R.; Defilippi, B.G.; Fuentes, L. Relationship between Endogenous Ethylene Production and Firmness during the Ripening and Cold Storage of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Horticulturae 2022, 8, 262. https://doi.org/10.3390/horticulturae8030262
Monsalve L, Bernales M, Ayala-Raso A, Álvarez F, Valdenegro M, Alvaro J-E, Figueroa CR, Defilippi BG, Fuentes L. Relationship between Endogenous Ethylene Production and Firmness during the Ripening and Cold Storage of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Horticulturae. 2022; 8(3):262. https://doi.org/10.3390/horticulturae8030262
Chicago/Turabian StyleMonsalve, Liliam, Maricarmen Bernales, Aníbal Ayala-Raso, Fernanda Álvarez, Mónika Valdenegro, Juan-Eugenio Alvaro, Carlos Rodrigo Figueroa, Bruno Giorgio Defilippi, and Lida Fuentes. 2022. "Relationship between Endogenous Ethylene Production and Firmness during the Ripening and Cold Storage of Raspberry (Rubus idaeus ‘Heritage’) Fruit" Horticulturae 8, no. 3: 262. https://doi.org/10.3390/horticulturae8030262
APA StyleMonsalve, L., Bernales, M., Ayala-Raso, A., Álvarez, F., Valdenegro, M., Alvaro, J.-E., Figueroa, C. R., Defilippi, B. G., & Fuentes, L. (2022). Relationship between Endogenous Ethylene Production and Firmness during the Ripening and Cold Storage of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Horticulturae, 8(3), 262. https://doi.org/10.3390/horticulturae8030262






