Weather Conditions Influence on Lavandin Essential Oil and Hydrolate Quality
Abstract
:1. Introduction
2. Materials and Methods
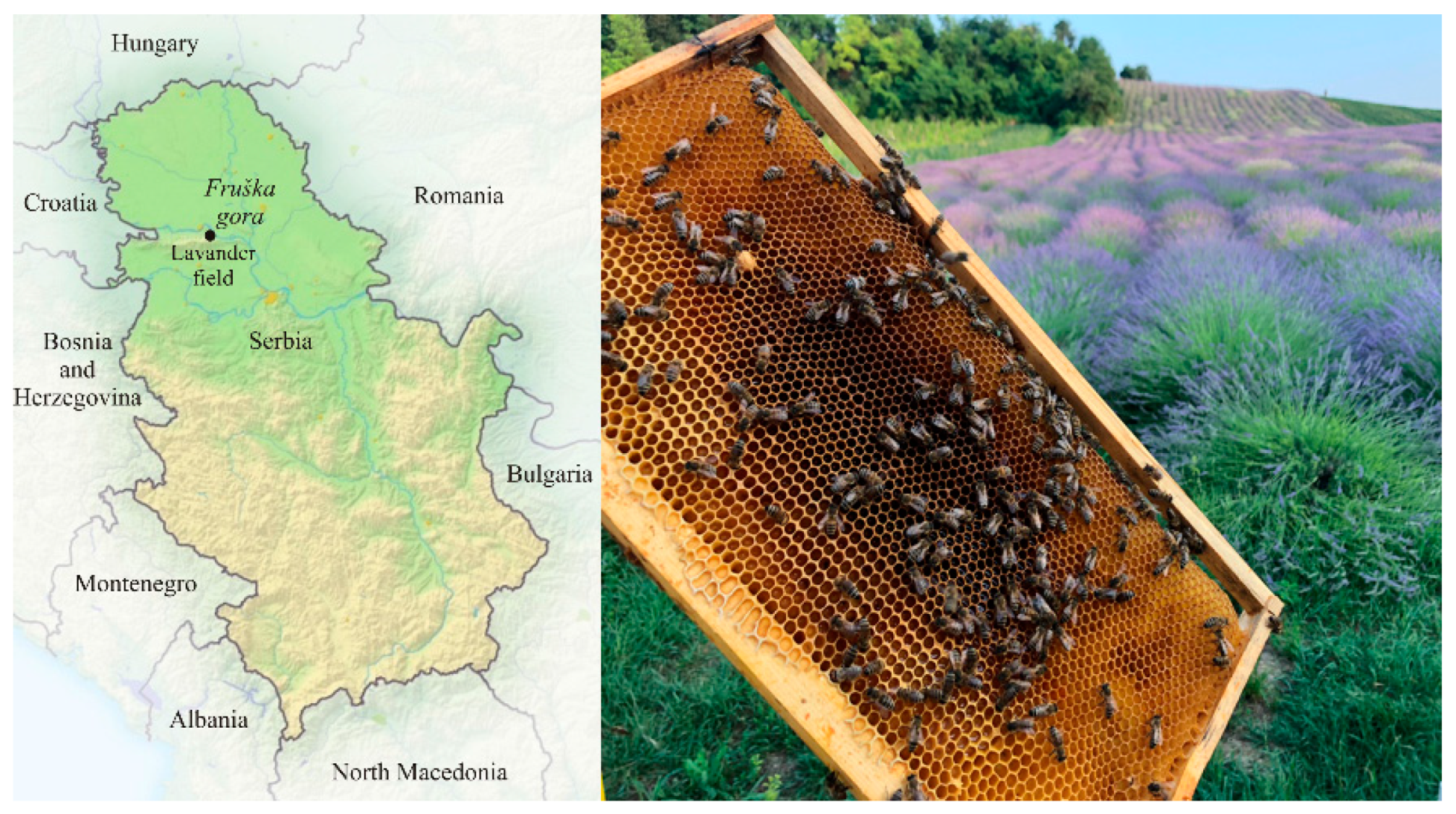
2.1. Plant Material
2.2. Soil Characteristics
2.3. Weather Conditions
2.4. Essential Oil and Hydrolate Extraction
2.5. Analysis of Volatile Compounds
2.6. Statistical Analysis
3. Results
3.1. Fresh Herb Yield and Essential Oil Content
3.2. Chemical Composition of Essential Oil
3.3. Chemical Composition of Hydrolate
3.4. Correlation between Chemical Compounds of Essential Oil and Hydrolate
4. Discussion
4.1. Essential Oil
4.2. Hydrolate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Szekely-Varga, Z.; Hitter, T.; Cantor, M. The healing power and the uses in landscape design of lavender (Lavandula angustifolia L.). Hop Med. Plants 2017, 25, 47–55. [Google Scholar]
- Pistelli, L.; Najar, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and phytochemical evaluation of lavandin and lavender cultivars cultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44. [Google Scholar] [CrossRef]
- Kivrak, S. Essential oil composition and antioxidant activities of eight cultivars of lavender and lavandin from western Anatolia. Ind. Crops Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Florina, G.E.; Camelia, M.; Teodor, G.V. Lavender-perfume and business in Romania. Agric. Res. Technol. 2019, 19, 140–144. [Google Scholar] [CrossRef]
- Detar, E.; Zambori-Nemeth, E.; Gosztola, B.; Harmath, A.; Ladanyi, M.; Pluhar, Z. Ontogenesis and harvest time are crucial for high quality lavender—Role of the flower development in essential oil properties. Ind. Crops Prod. 2021, 163, 113334. [Google Scholar] [CrossRef]
- Pecanha, D.A.; Freitas, M.S.M.; Vieira, M.E.; Cunha, J.M.; de Jesus, A.C. Phosphorus fertilization affects growth, essential oil yield and quality of true lavender in Brazil. Ind. Crops Prod. 2021, 170, 113803. [Google Scholar] [CrossRef]
- Stanković, I.; Vrandečić, K.; Ćosić, J.; Milojević, K.; Bulajić, A.; Krstić, B. The spreading of Alfalfa mosaic virus in lavandin in Croatia. Pestic. Phytomed. 2014, 29, 115–122. [Google Scholar] [CrossRef]
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical composition, antimicrobial and antioxidant activities of essential oils of Lavandula × intermedia ‘Budrovka’ and L. angustifolia cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Detar, E.; Zamborine Nemeth, E.; Gosztola, B.; Demjan, I.; Pluhar, Z. Effect of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula × intermedia Emeric ex Liosel.). Biochem. Syst. Ecol. 2020, 90, 104020. [Google Scholar] [CrossRef]
- Karapandzova, M.; Cvetkovikj, I.; Stefkov, G.; Stoimenov, V.; Crvenov, M.; Kulevanova, S. The influence of duration of the distillation of fresh and dried flowers on the essential oil composition of lavandin cultivated in Republic Macedonia. Maced. Pharm. Bull. 2012, 58, 31–38. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula × intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Prusinowska, R.; Śmigielsk, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Bajalan, I.; Pirbalouti, A.G. Variation in chemical composition of essential oil of populations of Lavandula × intermedia collected from Western Iran. Ind. Crops Prod. 2015, 69, 344–347. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Bialon, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical composition of the essential oil of the new cultivars of Lavandula angustifolia Mill. bread in Ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef] [PubMed]
- Marincas, O.; Feher, I. A new cost-effective approach for lavender essential oils quality assessment. Ind. Crops Prod. 2018, 125, 241–247. [Google Scholar] [CrossRef]
- Giray, F.H. An analysis of world lavender oil markets and lessons for Turkey. J. Essent. Oil-Bear. Plants 2018, 21, 1612–1623. [Google Scholar] [CrossRef]
- Ozel, A. Determining leaf yield, some plant characters and leaf essential oil components of different cultivars of lavender and lavandin (Lavandula spp.) on the Harran plain ecological conditions. Appl. Ecol. Environ. Res. 2019, 17, 14087–14094. [Google Scholar] [CrossRef]
- Lane, A.W.; Mahmoud, S.S. Composition of essential oil from Lavandula angustifolia and L. intermedia varieties grown in British Columbia, Canada. Nat. Prod. Commun. 2008, 3, 1361–1366. [Google Scholar] [CrossRef] [Green Version]
- Stanojević, L.; Stanković, M.; Cakić, M.; Nikolić, V.; Nikolić, L.; Ilić, D.; Radulović, N. The effect of hydrodistillation techniques on yield, kinetics, composition and antimicrobial activity of essential oils from flowers of Lavandula officinalis L. Hem. Ind. 2011, 65, 455–463. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, Q.; Cheng, Q.; Khan, S.; Yu, X. Seasonal variation in chemical composition of essential oils extracted from lavandin flowers in the Yun-Gui plateau of China. Molecules 2021, 26, 5639. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. J. Evid.-Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariod, A.A. Chapter 13—Effect of essential oils on organoleptic (smell, taste, and texture) properties of food. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 131–137. [Google Scholar] [CrossRef]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates—By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant hydrolates—Antioxidant properties, chemical composition and potential applications. Biomed. Pharmacother. 2021, 142, 112033. [Google Scholar] [CrossRef]
- Pino-Otin, M.R.; Navarro, J.; Val, J.; Roig, F.; Mainar, A.M.; Ballestero, D. Spanish Satureja montana L. hydrolate: Ecotoxicological study in soil and water non-target organisms. Ind. Crops Prod. 2022, 178, 114553. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Sousa, A.C.; Caramelo, D.; Delgado, F.; de Oliveira, A.P.; Pastorinho, R. Chemical profile and eco-safety evaluation of essential oils and hydrolates from Cistus ladanifer, Helichrysum italicum, Ocimum basilicum and Thymbra capitata. Ind. Crops Prod. 2022, 175, 114232. [Google Scholar] [CrossRef]
- Prusinowska, R.; Smigielski, K.B. Hydrosols from lavender (Lavandula angustifoia)—Determination of the chemical composition using Dispersive Liquid-Liquid Microextraction (DLLME). J. Essent. Oil-Bear. Plants. 2015, 18, 519–528. [Google Scholar] [CrossRef]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Masi, V.L.; Tiezzi, A.; Trilli, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula × intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crops Prod. 2022, 145, 1126068. [Google Scholar] [CrossRef]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; De Leo, M. Reconsidering hydrosols as main products of aromatic plants manufactory: The lavandin (Lavandula × intermedia) case study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Masci, V.L.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/GC-MS analysis and investigation of antibacterial, antioxidant and cytotoxic activity of essential oils and hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods 2021, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Vujičić, M.D.; Vasiljević, D.A.; Markovi, S.B.; Hose, T.A.; Lukić, T.; Hadžić, O.; Janićević, S. Preliminary geosite assessment model (GAM) and its application on Fruška Gora Mountain, potential geotourism destination of Serbia. Acta Geogr. Slov. 2011, 51, 361–376. [Google Scholar] [CrossRef]
- Renaud, E.; Charles, D. Essential oil quality and composition from 10 cultivars of organically grown lavender and lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Evangelopoulos, V.; Gougoilias, N.; Wogiatzi, E. Lavender organic cultivation yield and essential oil can be improved by using bio-stimulants. Acta Agric. Scand. Sect. B 2020, 70, 648–656. [Google Scholar] [CrossRef]
- Seidler-Lozykowska, K.; Mordalski, R.; Kucharski, W.; Kedzia, B.; Bocianowski, J. Yielding and quality of lavender flowers (Lavandula angustifolia Mill.) from organic cultivation. Acta Sci. Pol. Hortorum Cultus 2014, 13, 173–183. [Google Scholar]
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S.; et al. Plants of the genus Lavandula: From farm to pharmacy. Nat. Prod. Commun. 2018, 13, 1385–1402. [Google Scholar] [CrossRef] [Green Version]
- Blažeković, B.; Stanic, G.; Pepeljnjak, S.; Vladimir-Knezevic, S. In vitro antibacterial and antifungal activity of Lavandula × intermedia Emeric ex Loisel. ‘Budrovka’. Molecules 2011, 16, 4241–4253. [Google Scholar] [CrossRef]
- Vrandečić, K.; Ćosić, J.; Jurković, D.; Ilić, J. Bolesti lavande u istočnom delu Hrvatske. Glas. Biljn. Zaštite 2013, 5, 391–396. (In Croatian) [Google Scholar]
- Aćimović, M.; Pezo, L.; Zeremski, T.; Lončar, B.; Marjanović Jeromela, A.; Stanković Jeremić, J.; Cvetković, M.; Sikora, V.; Ignjatov, M. Weather conditions influence on hyssop essential oil quality. Processes 2021, 9, 1152. [Google Scholar] [CrossRef]
- Mészáros, M.; Markovlc, S.B.; Mijovkć, D.; Jovanovlć, M. Physical geographic characteristics and geo-heritage of Fruska gora mountain (Vojvodina, Serbia). Acta Geogr. Szeged. 2004, 38, 148–157. [Google Scholar]
- Aćimović, M.; Cvetković, M.; Stanković Jeremić, J.; Pezo, L.; Varga, A.; Čabarkapa, I.; Kiprovski, B. Biological activity and profiling of Salvia sclarea essential oil obtained by steam and hydrodistillation extraction methods via chemometrics tools. Flavour Fragr. J. 2022, 37, 20–32. [Google Scholar] [CrossRef]
- Aćimović, M.; Ivanović, S.; Simić, K.; Pezo, L.; Zeremski, T.; Ovuka, J.; Sikora, V. Chemical Characterization of Marrubium vulgare Volatiles from Serbia. Plants 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental effects on yield and composition of essential oil in wild populations of spike lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Salata, A.; Buczkowska, H.; Nurzynska-Wierdak, R. Yield, essential oil content, and quality performance of Lavandula angustifolia leaves, as affected by supplementary irrigation and drying methods. Agriculture 2020, 10, 590. [Google Scholar] [CrossRef]
- Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scario, V. Cultivation substrate composition influences morphology, volatilome and essential oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Porter, N.G.; Shaw, M.L.; Hurndell, L.C. Preliminary studies of lavender as an essential oil crop for New Zealand. N. Z. J. Agric. Res. 1982, 25, 389–394. [Google Scholar] [CrossRef]
- Desautels, A.; Biswas, K.; Lane, A.; Boeckelmann, A.; Mahmoud, S.S. Suppression of linalool acetate production in Lavandula × intermedia. Nat. Prod. Commun. 2009, 4, 1533–1536. [Google Scholar] [CrossRef] [Green Version]
- Baydar, H.; Kineci, S. Scent composition of essential oil, concrete, absolute and hydrosol from lavandin (Lavandula × intermedia Emeric ex Loisel.). J. Essent. Oil-Bear. Plants. 2009, 12, 131–136. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H. Determination of lavender and lavandin cultivars (Lavandula sp.) containing high quality essential oil in Isparta, Turkey. Turk. J. Field Crop. 2013, 18, 58–65. [Google Scholar]
- Karaman, R.; Erbas, S.; Baydar, H.; Kaya, M. Allelopathic effect of lavandin (Lavandula × intermedia Emeric ex Loilsel. Var Super A) oil on germination and seedling development of some weed and field crops. Harran Tarım ve Gıda Bilimleri Derg. 2014, 18, 35–41. [Google Scholar]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Liquid and vapour phase of lavandin (Lavandula × intermedia) essential oil: Chemical composition and antimicrobial activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pljevljakušić, D.; Drinić, Z. GC/MS chemical analysis of lavandin (Lavandula × intermedia) hydrolat: Sucessive extraction fraction. Lek. Sirovine 2020, 40, 33–39. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Argentieri, M.P.; Bellardi, M.G.; Avato, P. Neatocidal activity of essential oil from lavandin (Lavandula × intermedia Emeric ex Loisel.) as related to chemical profile. Molecules 2021, 26, 6448. [Google Scholar] [CrossRef] [PubMed]
- Kunicka-Styczynska, A.; Smigielski, K.; Prusinowska, R.; Rajkowska, K.; Kusmider, B.; Sikora, M. Preservative activity of lavender hydrosols in moisturizing body gels. Lett. Appl. Microbiol. 2014, 60, 27–32. [Google Scholar] [CrossRef]
- Silha, D.; Švarcova, K.; Bajer, T.; Kralovec, K.; Tesarova, E.; Mouckova, M.; Bajerova, P. Chemical composition of natural hydrolates and their antimicrobial activity on Arcobacter-like cells in comparison with other microorganisms. Molecules 2020, 25, 5654. [Google Scholar] [CrossRef]
- Azza, S.; Lyoussi, B.; Miguel, M. Antioxidant activity of some Morrocan hydrosols. J. Med. Plants Res. 2011, 5, 6688–6696. [Google Scholar] [CrossRef]
- Prusinowska, R.; Smigielski, K.; Kunicka-Styczynska, A. Hydrolates from lavender (Lavandula angustifolia)—Chemical composition, antimicrobial and antioxidant properties. PhD Interdiscip. J. 2013, 3, 33–39. [Google Scholar]





| pH | CaCO3 | Humus | Total Nitrogen | P2O5 | K2O | ||
|---|---|---|---|---|---|---|---|
| 1MKCl | H2O | (%) | (mg·100 g−1 Soil) | ||||
| Hill | 7.13 | 8.22 | 11.27 | 1.03 | 0.051 | 3.6 | 8.0 |
| Middle | 7.19 | 8.34 | 8.10 | 0.56 | 0.028 | 7.4 | 8.0 |
| Valley | 7.41 | 8.31 | 7.89 | 0.96 | 0.048 | 7.4 | 8.0 |
| 2019 | 2020 | 2021 | |
|---|---|---|---|
| Date of harvest | 11th–17th July | 27th June–2nd July | 9th–15th July |
| Fresh herb yield (kg/ha) | 4.840 | 5.040 | 5.670 |
| Essential oil content (%) | 1.26 | 1.19 | 1.03 |
| No | Compound | RI | 2019 | 2020 | 2021 | Temp. Coeff. | Prec. Coeff. |
|---|---|---|---|---|---|---|---|
| EO19 | EO20 | EO21 | |||||
| 6 | n-hexanol O | 861 | 0.2 | - | - | 0.70 | −0.25 |
| 7 | α-thujene MT | 924 | 0.1 | 0.1 | 0.1 | 0.70 | −0.25 |
| 8 | α-pinene MT | 931 | 0.9 | 0.6 | 1.4 | 0.71 | −0.23 |
| 9 | camphene MT | 945 | 0.4 | 0.7 | 0.5 | 0.71 | −0.24 |
| 12 | sabinene MT | 970 | 0.3 | 0.1 | 0.4 | 0.70 | −0.24 |
| 14 | β-pinene MT | 974 | 1.6 | 0.9 | 2.1 | 0.71 | −0.21 |
| 15 | 3-octanone O | 984 | 0.1 | - | - | 0.70 | −0.25 |
| 16 | dehydro-1,8-cineole OMT | 989 | - | 0.1 | - | 0.70 | −0.25 |
| 17 | myrcene MT | 990 | 0.5 | 0.1 | 0.7 | 0.70 | −0.24 |
| 18 | δ-3-carene MT | 1008 | 0.3 | 0.1 | 0.4 | 0.70 | −0.24 |
| 19 | hexyl acetate O | 1010 | 0.1 | - | - | 0.70 | −0.25 |
| 20 | α-terpinene MT | 1013 | - | - | 0.1 | 0.70 | −0.25 |
| 22 | p-cymene MT | 1022 | 0.4 | 0.8 | 0.3 | 0.71 | −0.24 |
| 23 | limonene MT | 1025 | 1.1 | 0.7 | 1.1 | 0.71 | −0.23 |
| 24 | 1,8-cineole OMT | 1028 | 12.9 | 16.4 | 19.0 | 0.82 | 0.11 |
| 25 | cis-β-ocimene MT | 1033 | 1.9 | - | 3.6 | 0.72 | −0.21 |
| 27 | trans-β-ocimene MT | 1044 | 0.2 | - | 0.4 | 0.70 | −0.24 |
| 28 | γ-terpinene MT | 1055 | 0.1 | - | 0.2 | 0.70 | −0.25 |
| 29 | cis-sabinene hydrate (IPP vs OH) OMT | 1063 | 0.2 | 0.2 | - | 0.70 | −0.25 |
| 30 | cis-linalool oxide (furanoid) OMT | 1069 | 0.1 | 2.4 | - | 0.71 | −0.23 |
| 32 | trans-linalool oxide (furanoid) OMT | 1085 | - | 2.1 | - | 0.71 | −0.23 |
| 33 | terpinolene MT | 1086 | 0.2 | - | 0.3 | 0.70 | −0.24 |
| 34 | linalool OMT | 1101 | 41.8 | 33.3 | 42.0 | 0.98 | 0.59 |
| 36 | allo-ocimene MT | 1126 | 0.3 | - | 0.6 | 0.70 | −0.24 |
| 38 | trans-pinocarveol OMT | 1134 | - | 0.3 | - | 0.70 | −0.25 |
| 40 | camphor OMT | 1141 | 3.7 | 4.6 | 3.9 | 0.73 | −0.16 |
| 41 | hexyl isobutanoate O | 1145 | 0.1 | 0.1 | - | 0.70 | −0.25 |
| 43 | borneol OMT | 1164 | 11.4 | 16.1 | 8.1 | 0.79 | 0.01 |
| 45 | trans-linalool oxide (pyranoid) OMT | 1168 | - | 0.2 | - | 0.70 | −0.25 |
| 46 | terpinen-4-ol OMT | 1175 | 6.6 | 3.5 | 5.3 | 0.74 | −0.14 |
| 48 | cryptone O | 1180 | - | 0.3 | - | 0.70 | −0.25 |
| 49 | α-terpineol OMT | 1188 | 0.8 | 0.5 | - | 0.70 | −0.24 |
| 50 | hexyl butanoate O | 1189 | 0.4 | 0.4 | 0.5 | 0.70 | −0.24 |
| 51 | myrtenal OMT | 1190 | - | 0.2 | - | 0.70 | −0.25 |
| 55 | hexyl 2-methyl butanoate O | 1234 | 0.2 | 0.3 | 0.1 | 0.70 | −0.24 |
| 56 | cumin aldehyde O | 1236 | - | 0.1 | - | 0.70 | −0.25 |
| 57 | carvone OMT | 1238 | - | 0.1 | - | 0.70 | −0.25 |
| 58 | hexyl isovalerate O | 1239 | 0.1 | - | - | 0.70 | −0.25 |
| 61 | linalyl acetate OMT | 1254 | 6.5 | 8.8 | 5.3 | 0.75 | −0.10 |
| 63 | bornyl acetate OMT | 1284 | 0.1 | 0.2 | - | 0.70 | −0.25 |
| 64 | lavandulyl acetate OMT | 1289 | 0.6 | 1.2 | 0.3 | 0.71 | −0.23 |
| 65 | neryl acetate OMT | 1363 | 0.1 | - | - | 0.70 | −0.25 |
| 66 | daucene ST | 1377 | 0.1 | - | - | 0.70 | −0.25 |
| 67 | geranyl acetate OMT | 1382 | 0.2 | - | - | 0.70 | −0.25 |
| 68 | hexyl hexanoate O | 1384 | 0.2 | 0.2 | - | 0.70 | −0.25 |
| 69 | 7-epi-sesquithujene ST | 1388 | 0.1 | - | - | 0.70 | −0.25 |
| 70 | sesquithujene ST | 1403 | 0.1 | - | - | 0.70 | −0.25 |
| 71 | α-santalene ST | 1414 | - | 0.3 | - | 0.70 | −0.25 |
| 72 | trans-caryophyllene ST | 1417 | 0.9 | - | 0.6 | 0.70 | −0.24 |
| 73 | trans-α-bergamotene ST | 1433 | 0.1 | - | - | 0.70 | −0.25 |
| 74 | trans-β-farnesene ST | 1455 | 2.0 | 0.3 | 1.1 | 0.71 | −0.23 |
| 75 | germacrene D ST | 1480 | 0.3 | - | 0.1 | 0.70 | −0.25 |
| 76 | lavandulyl isovalerate OST | 1508 | 0.3 | 0.4 | - | 0.70 | −0.24 |
| 77 | γ-cadinene ST | 1513 | tr | 0.1 | - | 0.70 | −0.25 |
| 79 | caryophyllene oxide OST | 1580 | 0.2 | 0.8 | - | 0.70 | −0.24 |
| 80 | epi-α-bisabolol OST | 1682 | 0.1 | - | - | 0.70 | −0.25 |
| Monoterpene hydrocarbons (MT) | 8.3 | 4.1 | 12.2 | ||||
| Oxygenated monoterpenes (OMT) | 85.0 | 90.2 | 83.9 | ||||
| Sesquiterpene hydrocarbons (ST) | 3.6 | 0.7 | 1.8 | ||||
| Oxygenated sesquiterpens (OST) | 0.6 | 1.2 | - | ||||
| Other (O) | 1.4 | 1.4 | 0.6 | ||||
| Total identified | 98.9 | 97.6 | 98.5 | ||||
| No | Compound | RI | 2019 | 2020 | 2021 | Temp. Coeff. | Prec. Coeff. |
|---|---|---|---|---|---|---|---|
| EO19 | EO20 | EO21 | |||||
| 1 | 3-methyl-2-butenal O | 772 | 0.1 | - | 0.4 | 0.70 | −0.24 |
| 2 | hexanal O | 798 | - | - | 0.2 | 0.70 | −0.25 |
| 3 | 2,2-dimethyl-3(2H)-furanone O | 829 | - | - | 0.1 | 0.70 | −0.25 |
| 4 | furfural O | 832 | - | - | 0.1 | 0.70 | −0.25 |
| 5 | cis-3-hexenol O | 847 | - | - | 0.3 | 0.70 | −0.25 |
| 6 | n-hexanol O | 858 | 1.0 | 0.6 | 2.2 | 0.71 | −0.22 |
| 10 | 4-methyl pent-2-enolide (impure) O | 949 | 0.1 | - | 0.3 | 0.70 | −0.25 |
| 11 | 2-ethenyltetrahydro-2,6,6-trimethyl-2H-pyran O | 968 | 0.1 | 0.1 | - | 0.70 | −0.25 |
| 13 | 1-octen-3-ol O | 974 | 0.9 | 0.6 | 0.9 | 0.71 | −0.23 |
| 15 | 3-octanone O | 982 | - | - | 0.1 | 0.70 | −0.25 |
| 16 | dehydro-1,8-cineole OMT | 990 | tr | - | 0.1 | 0.70 | −0.25 |
| 21 | 1,4-cineole OMT | 1014 | 0.1 | - | - | 0.70 | −0.25 |
| 24 | 1,8-cineole OMT | 1030 | 12.7 | 14.4 | 26.2 | 0.84 | 0.15 |
| 26 | lavender lactone O | 1036 | - | 0.2 | 0.1 | 0.70 | −0.25 |
| 30 | cis-linalool oxide (furanoid) OMT | 1071 | 1.4 | 11.5 | 2.7 | 0.74 | −0.13 |
| 31 | camphenilone NOMT | 1080 | - | - | 0.1 | 0.70 | −0.25 |
| 32 | trans-linalool oxide (furanoid) OMT | 1088 | 1.3 | 10.9 | 2.4 | 0.74 | −0.14 |
| 34 | linalool OMT | 1102 | 26.0 | 21.9 | 32.1 | 0.90 | 0.33 |
| 35 | hotrienol OMT | 1102 | - | 1.0 | - | 0.70 | −0.24 |
| 37 | nopinone NOMT | 1136 | 0.2 | 0.2 | 0.2 | 0.70 | −0.24 |
| 38 | trans-pinocarveol OMT | 1134 | - | 0.2 | 0.1 | 0.70 | −0.25 |
| 39 | trans-sabinol (trans for OH vs. IPP) OMT | 1136 | 0.1 | - | - | 0.70 | −0.25 |
| 40 | camphor OMT | 1143 | 7.1 | 4.5 | 6.3 | 0.74 | −0.12 |
| 42 | neoiso-3-thujanol OMT | 1146 | - | 0.2 | - | 0.70 | −0.25 |
| 43 | borneol OMT | 1166 | 24.4 | 16.8 | 10.6 | 0.81 | 0.10 |
| 44 | cis-linalool oxide (pyanoid) OMT | 1168 | 0.6 | 1.0 | 0.5 | 0.71 | −0.23 |
| 45 | trans-linalool oxide (pyanoid) OMT | 1173 | 0.4 | 0.8 | 0.4 | 0.71 | −0.24 |
| 46 | terpinen-4-ol OMT | 1177 | 12.2 | 6.4 | 9.7 | 0.77 | −0.05 |
| 47 | p-cymen-8-ol O | 1179 | - | 0.2 | 0.2 | 0.70 | −0.24 |
| 48 | cryptone O | 1180 | - | 1.0 | 0.3 | 0.71 | −0.24 |
| 49 | α-terpineol OMT | 1190 | 6.0 | 4.5 | 1.4 | 0.73 | −0.17 |
| 51 | myrtenol OMT | 1191 | - | 0.1 | - | 0.70 | −0.25 |
| 52 | verbenone OMT | 1208 | 0.2 | 0.3 | 0.1 | 0.70 | −0.24 |
| 53 | trans-carveol OMT | 1216 | - | 0.1 | - | 0.70 | −0.25 |
| 54 | nerol OMT | 1222 | - | 0.3 | - | 0.70 | −0.25 |
| 57 | carvone OMT | 1243 | 0.1 | 0.1 | - | 0.70 | −0.25 |
| 60 | geraniol OMT | 1248 | - | 0.9 | - | 0.70 | −0.24 |
| 61 | linalyl acetate OMT | 1248 | - | - | 0.2 | 0.70 | −0.25 |
| 64 | lavandulyl acetate OMT | 1286 | - | 0.1 | - | 0.70 | −0.25 |
| 78 | cis-nerolidol OST | 1526 | - | - | 0.2 | 0.70 | −0.25 |
| Nor oxygenated monoterpenes (NOMT) | 0.2 | 0.2 | 0.3 | ||||
| Oxygenated monoterpenes (OMT) | 92.6 | 96.0 | 92.8 | ||||
| Sesquiterpene hydrocarbons (ST) | - | - | - | ||||
| Oxygenated sesquiterpens (OST) | - | - | 0.2 | ||||
| Other (O) | 2.2 | 2.7 | 5.2 | ||||
| Total identified | 95.0 | 98.9 | 98.5 | ||||
| EO20 | EO21 | H19 | H20 | H21 | |
|---|---|---|---|---|---|
| EO19 | 0.967 | 0.987 | 0.855 | 0.795 | 0.908 |
| EO20 | 0.959 | 0.896 | 0.868 | 0.928 | |
| EO21 | 0.820 | 0.788 | 0.940 | ||
| H19 | 0.893 | 0.874 | |||
| H20 | 0.870 |
| No. | Cultivar | Origin | Limonene | 1,8-cineole | Cis-Β-Ocimene | Trans-Β-Ocimene | Camphor | Linalool | Linalyl Acetate | Terpinene-4-Ol | Lavandulyl Acetate | Lavandulol | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hidcote Giant | Canada | 1.4 | 17.1 | 7.8 | 12.2 | 13.3 | 23.8 | 0.3 | 4.1 | 0.0 | 1.6 | [19] |
| 2 | Grosso | Canada | 2.0 | 10.7 | 3.4 | 4.4 | 10.8 | 30.6 | 8.3 | 3.3 | 3.7 | 0.0 | [19] |
| 3 | Super | Canada | 2.7 | 13.1 | 3.5 | 6.2 | 5.5 | 28.3 | 10.0 | 0.0 | 3.4 | 0.0 | [19] |
| 4 | OK-Farms Super | Canada | 2.4 | 4.8 | 3.1 | 8.0 | 2.1 | 35.2 | 11.6 | 0.0 | 1.8 | 0.0 | [19] |
| 5 | French Super | Canada | 2.5 | 5.2 | 4.0 | 9.8 | 4.8 | 25.2 | 12.2 | 0.5 | 2.7 | 0.0 | [19] |
| 6 | Super | Turkey | 0.0 | 2.6 | 0.0 | 0.0 | 4.8 | 34.0 | 47.7 | 0.0 | 0.0 | 0.0 | [49] |
| 7 | n.s. | Macedonia | 0.0 | 6.7 | 2.3 | 0.8 | 6.6 | 39.0 | 2.1 | 5.1 | 1.9 | 1.7 | [11] |
| 8 | Dutch | Turkey | 1.1 | 7.6 | 0.0 | 0.0 | 11.3 | 44.8 | 8.2 | 1.5 | 0.0 | 0.1 | [50] |
| 9 | Giant Hidcote | Turkey | 1.3 | 12.0 | 0.0 | 0.0 | 6.6 | 41.6 | 3.9 | 1.5 | 0.0 | 0.2 | [50] |
| 10 | Super A | Turkey | 0.8 | 2.1 | 0.0 | 0.0 | 7.5 | 38.1 | 36.2 | 0.5 | 0.0 | 0.0 | [50] |
| 11 | Super A | Turkey | 0.4 | 3.2 | 1.5 | 6.6 | 43.7 | 24.6 | 5.4 | 0.0 | 0.0 | [51] | |
| 12 | Sumiens | Italy | 0.7 | 12.0 | 1.9 | 1.0 | 7.1 | 40.4 | 9.9 | 0.2 | 0.3 | 0.0 | [3] |
| 13 | Super A | Italy | 0.6 | 6.9 | 1.3 | 0.7 | 6.6 | 36.2 | 18.4 | 3.3 | 4.5 | 0.1 | [3] |
| 14 | Grosso | Italy | 0.5 | 8.1 | 1.2 | 0.8 | 8.1 | 38.4 | 15.7 | 3.6 | 4.1 | 0.1 | [3] |
| 15 | Super | Turkey | 0.7 | 0.0 | 2.6 | 1.5 | 5.3 | 36.8 | 33.1 | 0.0 | 1.2 | 0.1 | [4] |
| 16 | Grey Hedge | Turkey | 2.3 | 0.0 | 1.8 | 5.0 | 6.4 | 28.5 | 4.6 | 6.9 | 0.8 | 0.5 | [4] |
| 17 | Budrovka | Croatia | 4.0 | 8.4 | 0.0 | 0.4 | 0.1 | 57.1 | 9.8 | 2.3 | 0.2 | 1.1 | [9] |
| 18 | Grosso | Turkey | 2.5 | 33.1 | 0.0 | 0.0 | 21.8 | 0.2 | 0.0 | 1.0 | 0.2 | 0.4 | [18] |
| 19 | Dutch | Turkey | 2.9 | 35.8 | 0.0 | 0.0 | 22.2 | 0.2 | 0.0 | 0.8 | 0.1 | 0.2 | [18] |
| 20 | Abriel | Turkey | 2.5 | 35.9 | 0.0 | 0.0 | 22.8 | 0.1 | 0.0 | 1.0 | 0.2 | 0.1 | [18] |
| 21 | Grosso | Italy | 1.0 | 5.2 | 0.9 | 0.7 | 6.0 | 41.6 | 23.0 | 4.8 | 3.2 | 0.0 | [52] |
| 22 | n.s. | Serbia | 0.4 | 14.6 | 0.4 | 0.0 | 16.3 | 23.1 | 10.0 | 0.7 | 2.6 | 0.9 | [53] |
| 23 | Grappenhall | Hungary | 1.4 | 9.0 | 9.3 | 3.4 | 2.8 | 46.8 | 3.3 | 3.0 | 0.9 | 1.2 | [10] |
| 24 | Grosso | Hungary | 0.7 | 3.0 | 4.1 | 1.9 | 14.8 | 55.2 | 4.4 | 0.9 | 1.2 | 1.4 | [10] |
| 25 | n.s. | China | 0.0 | 44.8 | 0.0 | 0.5 | 12.2 | 7.6 | 0.0 | 0.6 | 0.0 | 0.0 | [21] |
| 26 | Abrialis | Italy | 0.5 | 7.0 | 3.0 | 8.3 | 9.4 | 40.3 | 18.4 | 0.6 | 1.4 | 0.8 | [54] |
| 27 | Rinaldi Cerioni | Italy | 0.4 | 10.0 | 0.0 | 0.0 | 11.5 | 65.8 | 0.5 | 2.9 | 0.0 | 0.6 | [54] |
| 28 | Sumiens | Italy | 0.7 | 12.1 | 3.0 | 0.4 | 6.8 | 48.0 | 14.9 | 0.3 | 0.0 | 0.0 | [54] |
| 29 | Budrovka | Serbia | 1.0 | 16.1 | 1.8 | 0.2 | 4.1 | 39.0 | 6.9 | 5.1 | 0.7 | 0.0 | TS |
| AVERAGE | 1.3 | 12.0 | 2.0 | 2.4 | 9.1 | 34.1 | 11.6 | 2.1 | 1.2 | 0.4 | |||
| RANGE | ≤4.0 | ≤44.8 | ≤9.3 | ≤12.2 | 0.1–22.8 | 0.1–65.8 | ≤47.7 | ≤6.9 | ≤4.5 | ≤1.7 | |||
| ISO STANDARD | 0.5–1.5 | 4.0–7.0 | 0.5–1.5 | tr-1.0 | 6.0–8.0 | 24.0–35.0 | 28.0–38.0 | 1.5–5.0 | 1.5–3.0 | 0.2–0.8 |
| No. | Species/ Variety/ Cultivar | Origin | Extraction Technique/ Plant Material | Linalool | 1,8-cineole | Linalool Oxides | α-terpineol | Camphor | Borneol | Terpinen-4-Ol | Geraniol | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LI cv “Super” | Turkey | f.f., SD | 55.6 | 9.8 | 6.0 | 0.0 | 13.4 | 13.5 | 0.0 | 1.6 | [49] |
| 2 | LO | Morocco | n.s. | 45.0 | 14.8 | 0.4 | 11.8 | 15.7 | 11.3 | 0.0 | 0.0 | [57] |
| 3 | LA | Poland | HV400 | 39.2 | 0.0 | 19.2 | 7.1 | 1.3 | 4.8 | 4.6 | 2.9 | [58] |
| 4 | LA | Poland | HV3200 | 29.0 | 0.0 | 19.1 | 12.7 | 3.0 | 9.3 | 6.9 | 0.0 | [58] |
| 5 | LA | Poland | f.h. | 53.0 | 2.9 | 0.4 | 8.3 | 0.9 | 5.3 | 3.9 | 4.0 | [55] |
| 6 | LA | Poland | d.h. | 48.0 | 2.7 | 0.6 | 8.8 | 1.5 | 5.8 | 6.6 | 5.0 | [55] |
| 7 | LA | Poland | f.f. | 43.6 | 4.4 | 1.0 | 7.5 | 0.0 | 6.6 | 5.6 | 3.4 | [55] |
| 8 | LA | Poland | d.f. | 39.2 | 0.0 | 19.2 | 7.0 | 1.3 | 4.8 | 4.6 | 2.9 | [55] |
| 9 | LA | Poland | d.f., VH400 | 43.6 | 4.4 | 0.0 | 7.5 | 0.0 | 6.6 | 5.6 | 0.0 | [28] |
| 10 | LA | Poland | d.f., VH800 | 44.9 | 2.0 | 0.0 | 8.5 | 1.3 | 5.2 | 5.4 | 3.4 | [28] |
| 11 | LA | Poland | d.f., VH1200 | 43.9 | 4.0 | 1.2 | 5.8 | 2.7 | 4.0 | 3.9 | 4.5 | [28] |
| 12 | LA | Poland | d.f., VH1600 | 25.7 | 2.7 | 1.4 | 4.1 | 1.0 | 4.6 | 3.7 | 1.1 | [28] |
| 13 | LI | Italy | f.f. | 43.8 | 25.4 | 0.1 | 1.8 | 12.8 | 4.3 | 4.5 | 0.0 | [30] |
| 14 | LI | Italy | f.s. | 34.4 | 28.9 | 0.0 | 2.2 | 15.4 | 4.0 | 2.7 | 0.2 | [30] |
| 15 | LI | Serbia | d.f. | 7.7 | 6.8 | 67.3 | 2.7 | 7.2 | 0.0 | 0.4 | 0.0 | [53] |
| 16 | LA | Croatia | d.f., SD | 7.9 | 20.6 | 21.0 | 10.4 | 0.4 | 0.0 | 1.1 | 1.0 | [56] |
| 17 | LA | Croatia | d.f., HD | 23.2 | 19.5 | 13.8 | 13.0 | 0.5 | 0.0 | 1.2 | 2.3 | [56] |
| 18 | LI cv „Grosso“ | Italy | n.s. | 12.6 | 52.9 | 0.7 | 4.8 | 19.6 | 3.0 | 5.4 | 0.0 | [29] |
| 19 | LA | Italy | f., SD | 42.9 | 11.8 | 0.1 | 12.6 | 18.4 | 5.8 | 8.4 | 0.0 | [31] |
| 20 | LI cv „Budrovka“ | Serbia | f.f., SD | 26.7 | 17.8 | 11.3 | 4.0 | 6.0 | 17.3 | 9.4 | 0.3 | TS |
| AVERAGE | 35.5 | 11.6 | 9.1 | 7.0 | 6.1 | 5.8 | 4.2 | 1.6 | ||||
| RANGE | 7.7–55.6 | ≤52.9 | ≤67.3 | ≤13.0 | ≤19.6 | ≤17.3 | ≤9.4 | ≤5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aćimović, M.; Lončar, B.; Stanković Jeremić, J.; Cvetković, M.; Pezo, L.; Pezo, M.; Todosijević, M.; Tešević, V. Weather Conditions Influence on Lavandin Essential Oil and Hydrolate Quality. Horticulturae 2022, 8, 281. https://doi.org/10.3390/horticulturae8040281
Aćimović M, Lončar B, Stanković Jeremić J, Cvetković M, Pezo L, Pezo M, Todosijević M, Tešević V. Weather Conditions Influence on Lavandin Essential Oil and Hydrolate Quality. Horticulturae. 2022; 8(4):281. https://doi.org/10.3390/horticulturae8040281
Chicago/Turabian StyleAćimović, Milica, Biljana Lončar, Jovana Stanković Jeremić, Mirjana Cvetković, Lato Pezo, Milada Pezo, Marina Todosijević, and Vele Tešević. 2022. "Weather Conditions Influence on Lavandin Essential Oil and Hydrolate Quality" Horticulturae 8, no. 4: 281. https://doi.org/10.3390/horticulturae8040281
APA StyleAćimović, M., Lončar, B., Stanković Jeremić, J., Cvetković, M., Pezo, L., Pezo, M., Todosijević, M., & Tešević, V. (2022). Weather Conditions Influence on Lavandin Essential Oil and Hydrolate Quality. Horticulturae, 8(4), 281. https://doi.org/10.3390/horticulturae8040281









