Physiological and Biochemical Adaptive Traits in Leaves of Four Citrus Species Grown in an Italian Charterhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Plant Materials
2.2. Experimental Design
2.3. Chlorophyll Fluorescence Measurements
2.4. Leaf Structural and Biochemical Traits
2.5. Statistical Analysis
3. Results
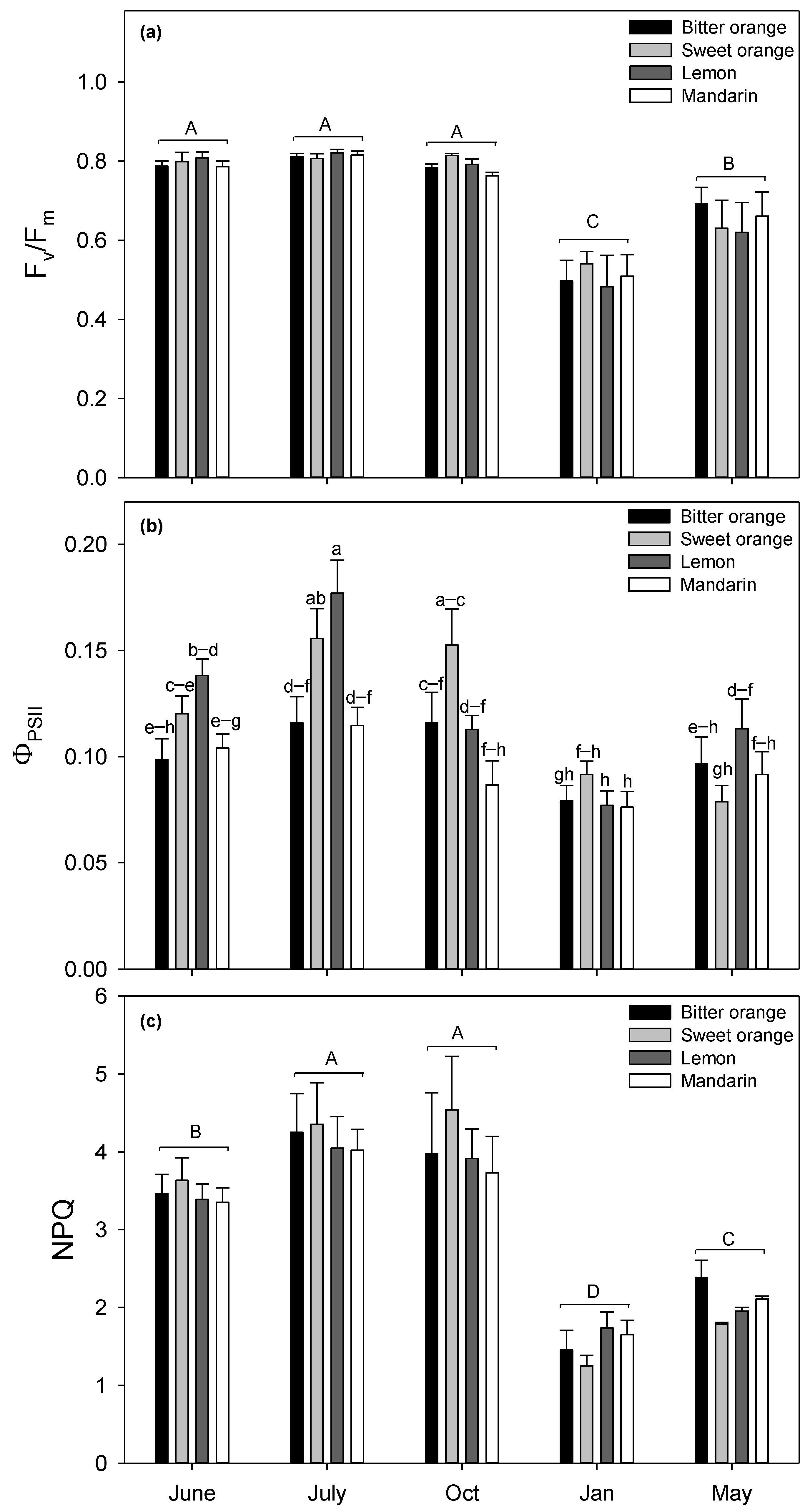
3.1. Chlorophyll Fluorescence Measurements
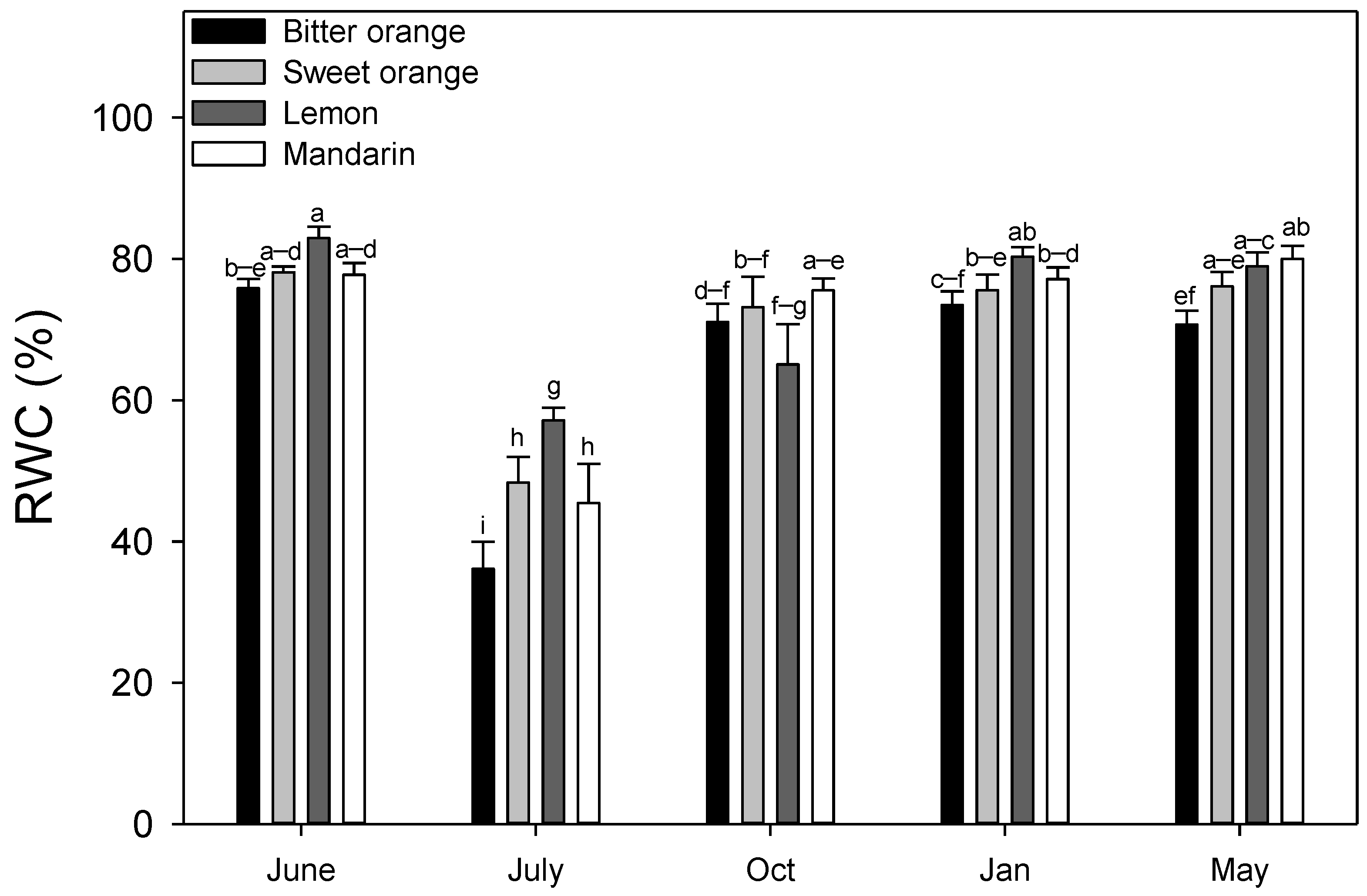
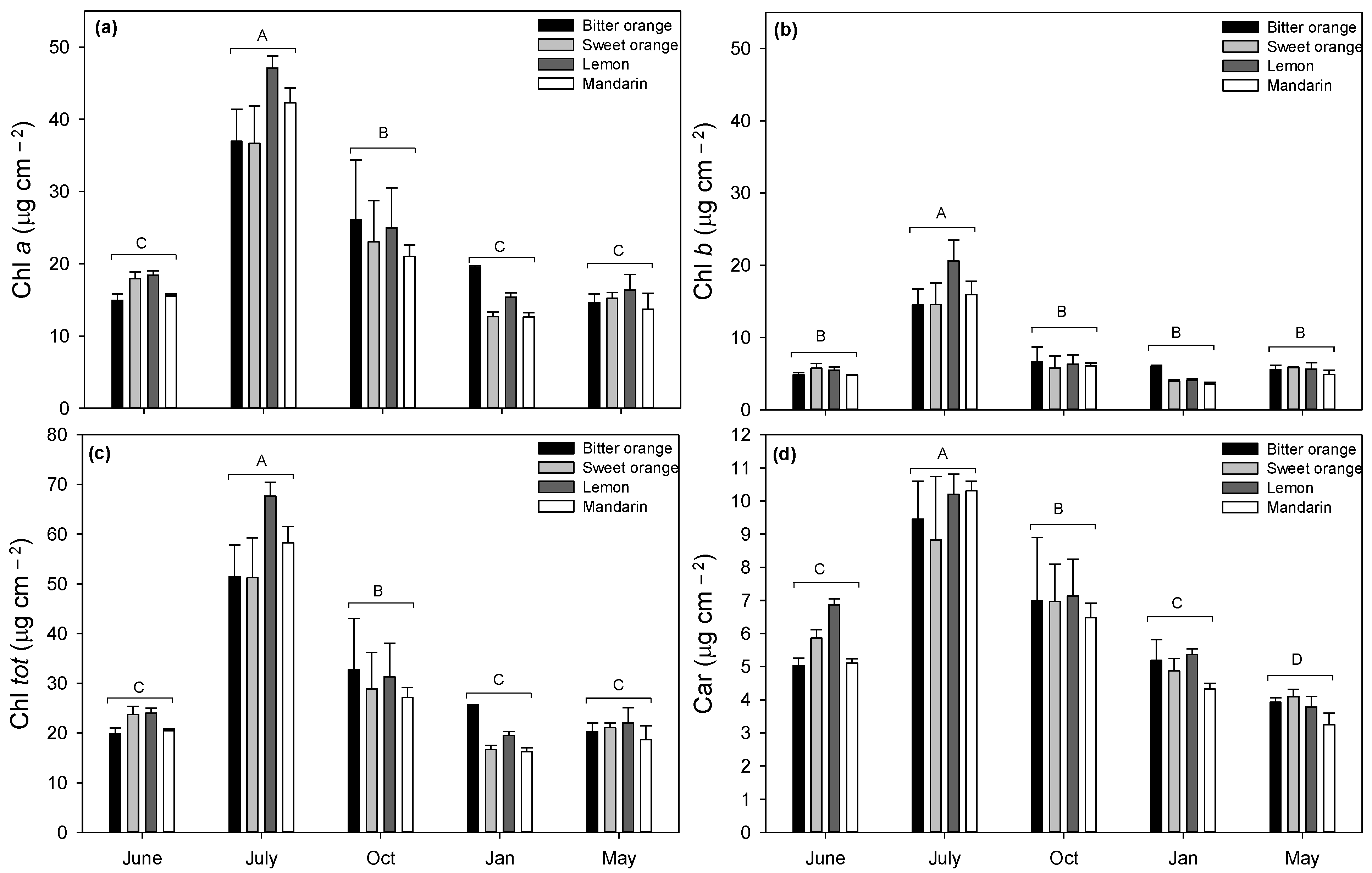
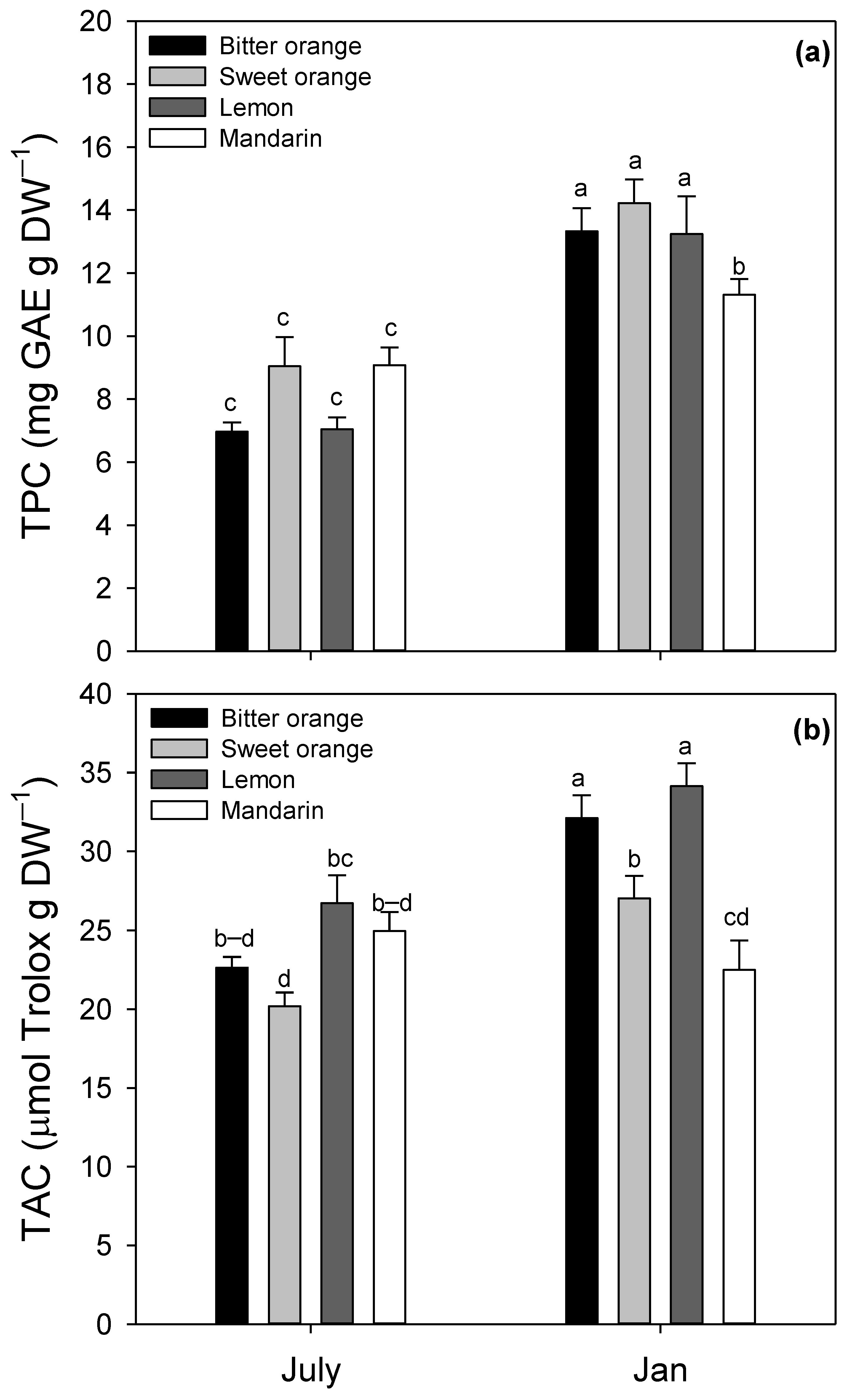
3.2. Leaf Structural and Biochemical Traits
4. Discussion
4.1. Seasonal Variation of Chlorophyll Fluorescence Parameters
4.2. Leaf Structural and Biochemical Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Sawi, S.A.; Ibrahim, M.E.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic potential of essential oils isolated from peels of three citrus species. Ann. Agric. Sci. 2019, 64, 89–94. [Google Scholar] [CrossRef]
- Wu, G.A.; Prochnik, S.; Jenkins, J.; Salse, J.; Hellsten, U.; Murat, F.; Perrier, X.; Ruiz, M.; Scalabrin, S.; Terol, J.; et al. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014, 32, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Curk, F.; Ancillo, G.; Ollitrault, F.; Perrier, X.; Jacquemoud-Collet, J.P.; Garcia-Lor, A.; Navarro, L.; Ollitrault, P. Nuclear species-diagnostic SNP markers mined from 454 amplicon sequencing reveal admixture genomic structure of modern Citrus varieties. PLoS ONE 2015, 10, e0125628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA (United States Department of Agriculture-Foreign Agricultural Service). USDA Crop Statistics. 2020. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/advQuery (accessed on 6 July 2021).
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Menichini, F.; Loizzo, M.R.; Bonesi, M.; Conforti, F.; De Luca, D.; Statti, G.A.; de Cindio, B.; Menichini, F.; Tundis, R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem. Toxicol. 2011, 49, 1549–1555. [Google Scholar] [CrossRef]
- Lagha-Benamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crops Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.F.; Pistelli, L.; Zinnai, A. Nutraceutical oils produced by olives and Citrus peel of Tuscany varieties as sources of functional ingredients. Molecules 2019, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- Haraoui, N.; Allem, R.; Chaouche, T.M.; Belouazni, A. In-vitro antioxidant and antimicrobial activities of some varieties citrus grown in Algeria. Adv. Tradit. Med. 2020, 20, 23–34. [Google Scholar] [CrossRef]
- Inglese, P.; Bellavia, G.P. The Citrus in the Mediterranean Region. In Integrated Control of Citrus Pests in the Mediterranean Region; Vacante, V., Gerson, U., Eds.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2012; pp. 3–18. [Google Scholar] [CrossRef] [Green Version]
- Vu, J.C.V. Photosynthetic Responses of Citrus to Environmental Changes. In Handbook of Plant and Crop Stress, 2nd ed.; Pessarakli, M., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 947–962. [Google Scholar]
- Martínez-Alcántara, B.; Quiñones, A.; Forner-Giner, M.A.; Iglesias, D.J.; Primo-Millo, E.; Legaz, F. Impact of fertilizer-water management on nitrogen use efficiency and potential nitrate leaching in citrus trees. Soil. Sci. Plant Nutr. 2012, 58, 659–669. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C. Some aspects of Citrus ecophysiology in subtropical climates: Revisiting photosynthesis under natural conditions. Braz. J. Plant Physiol. 2007, 19, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Nebauer, S.G.; Renau-Morata, B.; Guardiola, J.L.; Molina, R.V. Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in Citrus. Tree Physiol. 2011, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nebauer, S.G.; Arenas, C.; Rodríguez-Gamir, J.; Bordón, Y.; Fortunato-Almeida, A.; Monerri, C.; Guardiola, J.L.; Molina, R.V. Crop load does not increase the photosynthetic rate in Citrus leaves under regular cropping conditions. A study throughout the year. Sci. Hortic. 2013, 160, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Bianchi, A.; Sgherri, C.; Quartacci, M.F.; De Leo, M.; Pistelli, L.; Palla, F.; et al. Bread fortified with cooked purple potato flour and Citrus albedo: An evaluation of its compositional and sensorial properties. Foods 2021, 10, 942. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Seager, R.; Osborn, T.J.; Kushnir, Y.; Simpson, I.R.; Nakamura, J.; Liu, H. Climate variability and change of Mediterranean-type climates. J. Clim. 2019, 32, 2887–2915. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of Citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 900, 87–92. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Scartazza, A.; Pompeiano, A.; Ciurli, A.; Lu, Y.; Guglielminetti, L.; Yamaguchi, J. Nitrate reductase modulation in response to changes in C/N balance and nitrogen source in Arabidopsis. Plant Cell Physiol. 2018, 59, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Das, B.; Choudhury, B.; Kar, M. Quantitative estimation of changes in biochemical constituents of mahua (Madhuca indica syn. Bassia latifolia) flowers during postharvest storage. J. Food Process. Preserv. 2010, 34, 831–844. [Google Scholar] [CrossRef]
- Pistelli, L.; D’Angiolillo, F.; Morelli, E.; Basso, B.; Rosellini, I.; Posarelli, M.; Barbafieri, M. Response of spontaneous plants from an ex-mining site of Elba island (Tuscany, Italy) to metal(loid)s contamination. Environ. Sci. Pollut. R. 2017, 24, 7809–7820. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rana, G.; Katerji, N. Measurement and estimation of actual evapotranspiration in the field under Mediterranean climate: A review. Europ. J. Agron. 2000, 13, 125–153. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Yelenosky, G. Water deficit and associated changes in some photosynthetic parameters in leaves of ‘Valencia’ orange (Citrus sinensis L. Osbeck). Plant Physiol. 1988, 88, 375–378. Available online: https://www.jstor.org/stable/4271580 (accessed on 14 March 2022). [CrossRef] [Green Version]
- Jifon, J.L.; Syvertsen, J.P. Moderate shade can increase net gas exchange and reduce photoinhibition in Citrus leaves. Tree Physiol. 2003, 23, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.P.; Zhou, H.F.; Zhang, L.C. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci. Hortic. 2006, 108, 260–267. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 2009, 47, 215–222. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Seasonal and diurnal changes in photosynthetic limitation of young sweet orange trees. Environ. Exper. Bot. 2009, 66, 203–211. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Espinoza-Núñez, E.; Ramos, R.A.; Machado, D.F.S.P. Moderate warm temperature improves shoot growth, affects carbohydrate status and stimulates photosynthesis of sweet orange plants. Braz. J. Plant Physiol. 2012, 24, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Santini, J.; Giannettini, J.; Pailly, O.; Herbette, S.; Ollitrault, P.; Berti, L.; Luro, F. Comparison of photosynthesis and antioxidant performance of several Citrus and Fortunella species (Rutaceae) under natural chilling stress. Trees 2013, 27, 71–83. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Oliveira, R.F. Temperature response of photosynthesis and its interaction with light intensity in sweet orange leaf discs under nonphotorespiratory condition. Ciênc. Agrotecnol. 2006, 30, 670–678. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Tajvar, Y.; Fotouhi Ghazvini, R.; Hamidoghli, Y.; Sajedi, R. Antioxidant changes of Thomson navel orange (Citrus sinensis) on three rootstocks under low temperature stress. Hort. Environ. Biotechnol. 2011, 52, 576–580. [Google Scholar] [CrossRef]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Santos, C.M.A.; Ribeiro, R.V.; Magalhães Filho, J.R.; Machado, D.F.S.P.; Machado, E.C. Low substrate temperature imposes higher limitation to photosynthesis of orange plants as compared to atmospheric chilling. Photosynthetica 2011, 49, 546–554. [Google Scholar] [CrossRef]
- Jiang, J.; Hou, R.; Yang, N.; Li, L.; Deng, J.; Qin, G.; Ding, D. Physiological and TMT-labeled proteomic analyses reveal important roles of sugar and secondary metabolism in Citrus junos under cold stress. J. Proteom. 2021, 237, 104145. [Google Scholar] [CrossRef]
- Keutgen, N.; Chen, K. Responses of citrus leaf photosynthesis, chlorophyll fluorescence, macronutrient and carbohydrate contents to elevated CO2. J. Plant Physiol. 2001, 158, 1307–1316. [Google Scholar] [CrossRef]
- Blanke, M.M. Photoinhibition in Citrus. In Proceedings of the International Society of Citriculture IX Congress, ISC2000, Orlando, FL, USA, 3–7 December 2000; pp. 619–622. [Google Scholar]
- Pimentel, C.; Bernacchi, C.; Long, S. Limitations to photosynthesis at different temperatures in the leaves of Citrus limon. Braz. J. Plant Physiol. 2007, 19, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Insausti, P.; PLoSchuk, E.L.; Izaguirre, M.M.; Podworny, M. The effect of sunlight interception by sooty mold on chlorophyll content and photosynthesis in orange leaves (Citrus sinensis L.). Eur. J. Plant Pathol. 2015, 143, 559–565. [Google Scholar] [CrossRef]
- Mullan, D.; Pietragalla, J. Leaf Relative Water Content. In Physiological Breeding II: A Field Guide to Wheat Phenotyping; Pask, A.J.D., Pietragalla, J., Mullan, D.M., Reynolds, M.P., Eds.; CIMMYT: Mexico City, Mexico, 2012; pp. 25–27. [Google Scholar]
- Norman, S.M.; Maier, V.P.; Pon, D.L. Abscisic acid accumulation and carotenoid and chlorophyll content in relation to water stress and leaf age of different types of Citrus. J. Agric. Food Chem. 1990, 38, 1326–1334. [Google Scholar] [CrossRef]
- Turrell, F.M.; Weber, J.R.; Austin, S.W. Chlorophyll content and reflection spectra of Citrus leaves. Bot. Gaz. 1961, 123, 10–15. [Google Scholar] [CrossRef]
- Han, S.; Chen, L.; Jiang, H.; Smith, B.R.; Yang, L.; Xie, C. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Melgar, J.C.; Syvertsen, J.P.; Martínez, V.; García-Sánchez, F. Leaf gas exchange, water relations, nutrient content and growth in citrus and olive seedlings under salinity. Biol. Plant. 2008, 52, 385–390. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Dong, X.; Han, Y.; Ma, Q.; Ding, Y.; Zhao, F.; Zhang, J.; Chen, H.; Xu, Q.; et al. Carotenoid accumulation affects redox status, starch metabolism, and flavonoid/anthocyanin accumulation in citrus. BMC Plant Biol. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, D.J.; Lliso, I.; Tadeo, F.R.; Talon, M. Regulation of photosynthesis through source: Sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol. Plant. 2002, 116, 563–572. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G. Leaf temperature in sweet orange plants under field conditions: Influence of meteorological elements. Rev. Bras. Agrometeorol. 2005, 13, 353–368. [Google Scholar]
- Mataa, M.; Tominaga, S.; Kozaki, I. Seasonal changes of carbohydrate constituents in Ponkan (Citrus reticulata Blanco). J. Jpn. Soc. Hort. Sci. 1996, 65, 513–523. [Google Scholar] [CrossRef]
- Crifò, T.; Puglisi, I.; Petrone, G.; Reforgiato Recupero, G.; Lo Piero, A.R. Expression analysis in response to low temperature stress in blood oranges: Implication of the flavonoid biosynthetic pathway. Gene 2011, 476, 1–9. [Google Scholar] [CrossRef]
- Garcia-Luis, A.; Fornes, F.; Guardiola, J.L. Leaf carbohydrates and flower formation in Citrus. J. Am. Soc. Hortic. Sci. 1995, 120, 222–227. [Google Scholar] [CrossRef]
- Gulen, H.; Cansev, A.; Eris, A. Cold hardiness of olive (Olea europaea L.) cultivars in cold-acclimated and non-acclimated stages: Seasonal alteration of soluble sugars and phospholipids. J. Agric. Sci. 2009, 147, 459–467. [Google Scholar] [CrossRef]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L.; Huber, J.L.A.; Huber, S.C. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol. 1992, 100, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Muthiah, P.L.; Umamaheswari, M.; Asokkumar, K. In vitro antioxidant activities of leaves, fruits and peel extracts of Citrus. Int. J. Phytopharm. 2012, 2, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Ordoñez-Gómez, E.S.; Reátegui-Díaz, D.; Villanueva-Tiburcio, J.E. Polifenoles totales y capacidad antioxidante en cáscara y hojas de doce cítricos. Total polyphenols and antioxidant capacity of peel and leaves in twelve Citrus. Sci. Agropecu. 2018, 9, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Doerfler, H.; Lyon, D.; Nägele, T.; Sun, X.; Fragner, L.; Hadacek, F.; Egelhofer, V.; Weckwerth, W. Granger causality in integrated GC–MS and LC–MS metabolomics data reveals the interface of primary and secondary metabolism. Metabolomics 2013, 9, 564–574. [Google Scholar] [CrossRef] [Green Version]

| Months | Tair (°C) | Tmin (°C) | Tmax (°C) | RH (%) | Wind Speed (km/h) | Precipitations Days | Monthly Mean Precipitations (mm) |
|---|---|---|---|---|---|---|---|
| June | 21.3 | 15.8 | 26.2 | 75.8 | 11 | 7 | 37.4 |
| July | 24.2 | 18.7 | 29.2 | 79 | 10.3 | 8 | 31 |
| October | 18.1 | 13.4 | 23.6 | 76.1 | 12.3 | 12 | 80 |
| January | 5.3 | 0.8 | 10.6 | 78.7 | 10.2 | 10 | 56.8 |
| May | 14.2 | 10.2 | 18.1 | 81.3 | 11.5 | 14 | 173.1 |
| Date of Leaf Samplings | Daily Mean Tair (°C) | Tmin (°C) | Tmax (°C) | RH (%) | Wind Speed (km/h) | Daily Precipitations (mm) |
|---|---|---|---|---|---|---|
| 27 June 2018 | 21.0 | 14.0 | 26.0 | 71.0 | 12 | 0.4 |
| 26 July 2018 | 25.0 | 19.0 | 31.0 | 81.0 | 9 | 0.0 |
| 24 October 2018 | 15.0 | 8.0 | 22.0 | 88.0 | 8 | 0.0 |
| 9 January 2019 | 3.0 | –2.0 | 10.0 | 86.0 | 6 | 0.2 |
| 15 May 2019 | 12.0 | 9.0 | 15.0 | 59.0 | 16 | 0.0 |
| Measurements | Species p | Month p | Species × Month p |
|---|---|---|---|
| RWC | 0.002 | <0.001 | <0.001 |
| Chl a | 0.408 | <0.001 | 0.611 |
| Chl b | 0.762 | <0.001 | 0.816 |
| Chl tot | 0.453 | <0.001 | 0.585 |
| Chl a/b | 0.570 | <0.001 | 0.107 |
| Car | 0.291 | <0.001 | 0.739 |
| Car/Chl tot | 0.602 | <0.001 | 0.429 |
| Fv/Fm | 0.987 | <0.001 | 0.998 |
| ΦPSII | <0.001 | <0.001 | 0.001 |
| NPQ | 0.849 | <0.001 | 0.820 |
| TPC | 0.197 | <0.001 | 0.013 |
| TAC | <0.001 | <0.001 | <0.001 |
| Soluble sugars | 0.002 | <0.001 | 0.149 |
| Starch | 0.139 | 0.003 | 0.987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curadi, M.; Marchioni, I.; Mancino, M.; Pistelli, L.; Pistelli, L.; Scartazza, A. Physiological and Biochemical Adaptive Traits in Leaves of Four Citrus Species Grown in an Italian Charterhouse. Horticulturae 2022, 8, 324. https://doi.org/10.3390/horticulturae8040324
Curadi M, Marchioni I, Mancino M, Pistelli L, Pistelli L, Scartazza A. Physiological and Biochemical Adaptive Traits in Leaves of Four Citrus Species Grown in an Italian Charterhouse. Horticulturae. 2022; 8(4):324. https://doi.org/10.3390/horticulturae8040324
Chicago/Turabian StyleCuradi, Maurizio, Ilaria Marchioni, Matteo Mancino, Luisa Pistelli, Laura Pistelli, and Andrea Scartazza. 2022. "Physiological and Biochemical Adaptive Traits in Leaves of Four Citrus Species Grown in an Italian Charterhouse" Horticulturae 8, no. 4: 324. https://doi.org/10.3390/horticulturae8040324
APA StyleCuradi, M., Marchioni, I., Mancino, M., Pistelli, L., Pistelli, L., & Scartazza, A. (2022). Physiological and Biochemical Adaptive Traits in Leaves of Four Citrus Species Grown in an Italian Charterhouse. Horticulturae, 8(4), 324. https://doi.org/10.3390/horticulturae8040324








