Thidiazuron Induced In Vitro Clonal Propagation of Lagerstroemia speciosa (L.) Pers.—An Important Avenue Tree
Abstract
:1. Introduction
2. Materials and Methods
2.1. Explants Source, Media and Culture Conditions
2.2. Shoot Induction
2.3. Shoot Multiplication
2.4. Ex Vitro Rooting and Acclimatization
2.5. Growth and Photosynthetic Traits
2.6. Genomic DNA Isolation and Molecular Screening
2.7. Statistical Analysis
3. Results and Discussion
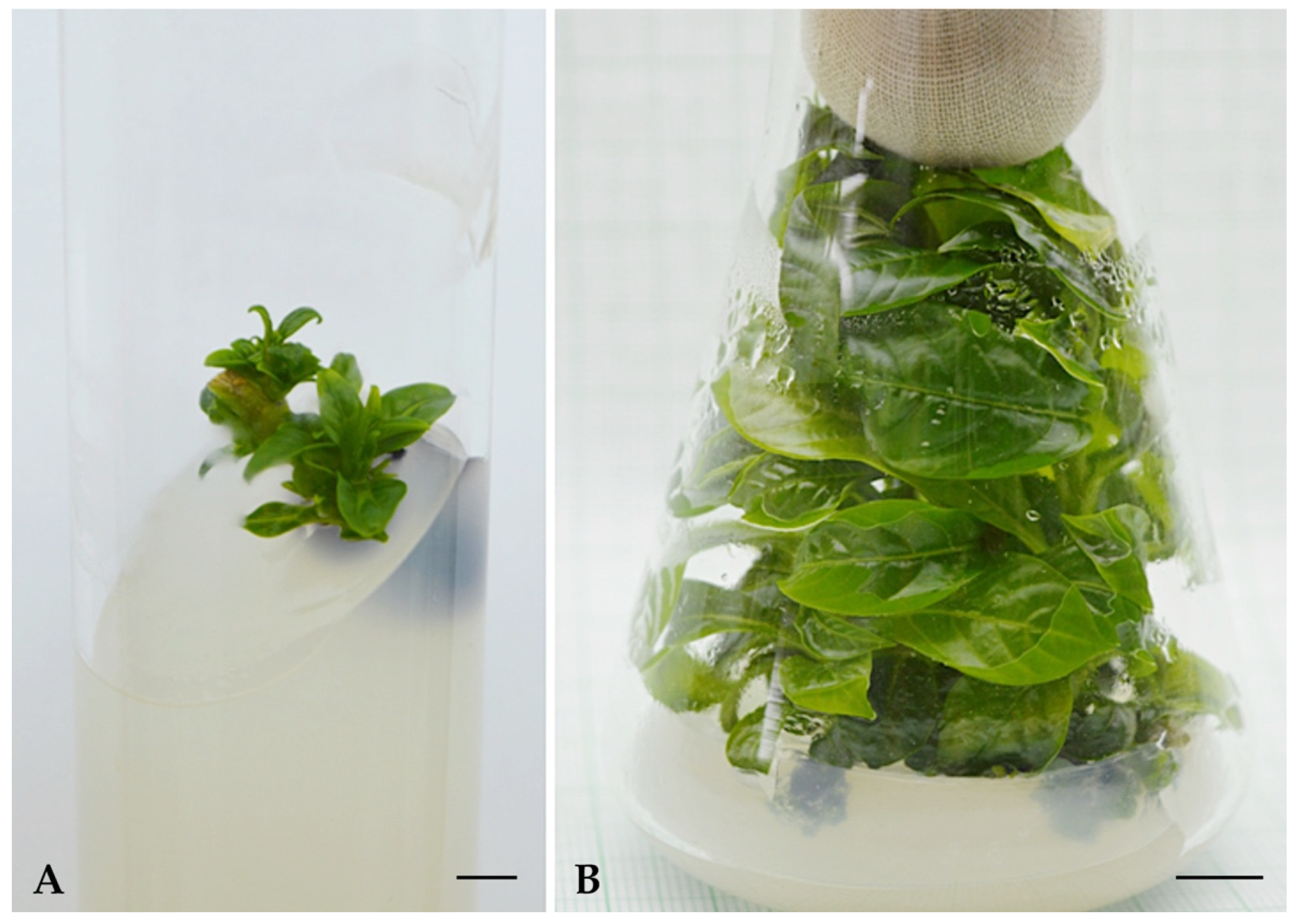
3.1. Shoot Induction
3.2. Shoot Multiplication
3.3. Ex Vitro Rooting and Acclimatization
3.4. Growth and Photosynthetic Traits
3.5. Molecular Screening
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, G.; Kim, J.; Himmeldirk, K.; Cao, Y.; Chen, X. Antidiabetes and Anti-obesity Activity of Lagerstroemia speciosa. Evid.-Based Complement. Altern. Med. 2007, 4, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, F.B.; Ahmed, S.; Noor, P.; Rahman, M.M.M.; Huq, S.M.A.; Akib, M.T.E.; Shohael, A.M. A comprehensive multi-directional exploration of phytochemicals and bioactivities of flower extracts from Delonix regia (Bojer ex Hook.) Raf., Cassia fistula L. and Lagerstroemia speciosa L. Biochem. Biophys. Rep. 2020, 24, 100805. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Roh, S.-G.; Li, C.-R.; Jin, C.-F.; Kim, A.; Choi, W.-C. Anti-diabetic effects of banaba leaf extracts (Lagerstroemia speciosa Pers.) through solvents. J. Life Sci. 2008, 18, 1305–1311. [Google Scholar] [CrossRef]
- Unno, T.; Sugimoto, A.; Kakuda, T. Xanthine oxidase inhibitors from the leaves of Lagerstroemia speciosa (L.) Pers. J. Ethnopharmacol. 2004, 93, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Unno, T.; Ushitani, M.; Hayashi, K.; Kakuda, T. Antiobesity Activity of Extracts from Lagerstroemia speciosa L. Leaves on Female KK-Ay Mice. J. Nutrf. Sci. Vitaminol. 1999, 45, 791–795. [Google Scholar] [CrossRef]
- Karsono, A.H.; Tandrasasmita, O.M.; Tjandrawinata, R.R. Bioactive fraction from Lagerstroemia speciosa leaves (DLBS3733) reduces fat droplet by inhibiting adipogenesis and lipogenesis. J. Exp. Pharm. 2019, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Garo, E.; Eldridge, G.R.; Goering, M.G.; DeLancey Pulcini, E.; Hamilton, M.A.; Costerton, J.W.; James, G.A. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob. Agents Chemother. 2007, 51, 1813–1817. [Google Scholar] [CrossRef] [Green Version]
- Thakur, R.S.; Devaraj, E. Lagerstroemia speciosa (L.) Pers. triggers oxidative stress mediated apoptosis via intrinsic mitochondrial pathway in HepG2 cells. Environ. Toxicol. 2020, 35, 1225–1233. [Google Scholar] [CrossRef]
- Rohit Singh, T.; Ezhilarasan, D. Ethanolic Extract of Lagerstroemia Speciosa (L.) Pers., Induces Apoptosis and Cell Cycle Arrest in HepG2 Cells. Nutr. Cancer 2020, 72, 146–156. [Google Scholar] [CrossRef]
- Zobayed, S.M.A. In vitro propagation of Lagerstroemia spp. from nodal explants and gaseous composition in the culture headspace. Environ. Control Biol. 2000, 38, 1–11. [Google Scholar] [CrossRef]
- Jayakumar, K.S.; Sajan, J.S.; Aswati Nair, R.; Padmesh Pillai, P.; Deepu, S.; Padmaja, R.; Agarwal, A.; Pandurangan, A.G. Corosolic acid content and SSR markers in Lagerstroemia speciosa (L.) Pers.: A comparative analysis among populations across the Southern Western Ghats of India. Phytochemistry 2014, 106, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hadiuzzaman, S.; Zobayed, S.; Roy, S.; Miah, M. In vitro cloning from seedling explants of Lagerstroemia speciosa Pers. and L. thorellii Gagnep. Bangladesh J. Bot. 1992, 21, 59–64. (In Bengali) [Google Scholar]
- Ahmad, A.; Ahmad, N.; Anis, M.; Alatar, A.A.; Abdel-Salam, E.M.; Qahtan, A.A.; Faisal, M. Gibberellic acid and thidiazuron promote micropropagation of an endangered woody tree (Pterocarpus marsupium Roxb.) using in vitro seedlings. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 449–462. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M.; Qahtan, A.A. Effects of 4-CPPU on in vitro multiplication and sustainable generation of Hibiscus rosa-sinensis L. ‘White Butterfly’. Saudi J. Biol. Sci. 2020, 27, 412–416. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Javed, S.B.; Alatar, A.A.; Anis, M.; El-Sheikh, M.A. In vitro regeneration of coral tree from three different explants using thidiazuron. HortTechnology 2019, 29, 946–951. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-Y. The use of thidiazuron in tissue culture. Vitr. Cell. Dev. Biol.-Plant 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Corredoira, E.; Ballester, A.; Vieitez, A.M. Thidiazuron-induced high-frequency plant regeneration from leaf explants of Paulownia tomentosa mature trees. Plant Cell Tissue Organ Cult. 2008, 95, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.-C.; Yu, X.M.; Shang, X.L.; Li, J.D.; Wu, Y.X. Factors influencing efficiency of shoot regeneration in Ziziphus jujuba Mill. ‘Huizao’. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 111–117. [Google Scholar] [CrossRef]
- Dangi, B.; Khurana-Kaul, V.; Kothari, S.L.; Kachhwaha, S. Micropropagtion of Terminalia bellerica from nodal explants of mature tree and assessment of genetic fidelity using ISSR and RAPD markers. Physiol. Mol. Biol. Plants 2014, 20, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Adhikari, S.; Dey, T.; Ghosh, P. RAPD and ISSR based evaluation of genetic stability of micropropagated plantlets of Morus alba L. variety S-1. Meta Gene 2015, 7, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Anis, M. Role of growth regulators on in vitro regeneration and histological analysis in Indian ginseng (Withania somnifera L.) Dunal. Physiol. Mol. Biol. Plants 2012, 18, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papafotiou, M.; Bertsouklis, K.F.; Trigka, M. Micropropagation of Arbutus unedo, A. andrachne, and their natural hybrid, A. x andrachnoides from seedling explants. J. Hortic. Sci. Biotechnol. 2013, 88, 768–775. [Google Scholar] [CrossRef]
- Husain, M.K.; Anis, M.; Shahzad, A. In Vitro Propagation of Indian Kino (Pterocarpus marsupium Roxb.) Using Thidiazuron. Vitr. Cell. Dev. Biol.-Plant 2007, 43, 59–64. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, N.; Ahmad, I.; Anis, M. Interactive effects of growth regulators, carbon sources, pH on plant regeneration and assessment of genetic fidelity using single primer amplification reaction (SPARS) techniques in Withania somnifera L. Appl. Biochem. Biotechnol. 2015, 177, 118–136. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, N.; Anis, M. Preconditioning of Nodal Explants in Thidiazuron-Supplemented Liquid Media Improves Shoot Multiplication in Pterocarpus marsupium (Roxb.). In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 175–187. [Google Scholar]
- Bertsouklis, K.F.; Papafotiou, M. Morphometric and Molecular Analysis of the Three Arbutus Species of Greece. Not. Bot. Horti Agrobot. 2016, 44, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Mishra, T.; Goyal, A.K.; Sen, A. Somatic Embryogenesis and Genetic Fidelity Study of the Micropropagated Medicinal Species, Canna indica. Horticulturae 2015, 1, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Saldaña, C.L.; Cancan, J.D.; Cruz, W.; Correa, M.Y.; Ramos, M.; Cuellar, E.; Arbizu, C.I. Genetic Diversity and Population Structure of Capirona (Calycophyllum spruceanum Benth.) from the Peruvian Amazon Revealed by RAPD Markers. Forests 2021, 12, 1125. [Google Scholar] [CrossRef]
- Salem, J.; Hassanein, A.; El-Wakil, D.A.; Loutfy, N. Interaction between Growth Regulators Controls In Vitro Shoot Multiplication in Paulownia and Selection of NaCl-Tolerant Variants. Plants 2022, 11, 498. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, h.k.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Dudley, S.A. Differing selection on plant physiological traits in response to environmental water availability: A test of adaptive hypotheses. Evolution 1996, 50, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Liu, Q.; Salih, S.; Hammerschlag, F. Etiolation of ‘Royal Gala’ apple (Malus × domestica Borkh.) shoots promotes high-frequency shoot organogenesis and enhanced, -glucuronidase expression from stem internodes. Plant Cell Rep. 1998, 18, 32–36. [Google Scholar] [CrossRef]
- Matand, K.; Prakash, C.S. Evaluation of peanut genotypes for in vitro plant regeneration using thidiazuron. J. Biotechnol. 2007, 130, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. Molecular fate of thidiazuron and its effects on auxin transport in hypocotyls tissues of Pelargonium × hortorum Bailey. Plant Growth Regul. 2001, 35, 269–275. [Google Scholar] [CrossRef]
- Murthy, B.N.S.; Murch, S.J.; Saxena, P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. Vitr. Cell. Dev. Biol.-Plant 1998, 34, 267. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M. Synergetic effect of TDZ and BA on minimizing the post-exposure effects on axillary shoot proliferation and assessment of genetic fidelity in Rauvolfia tetraphylla (L.). Rend. Lincei Sci. Fis. Nat. 2018, 29, 109–115. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult. 2005, 80, 187–190. [Google Scholar] [CrossRef]
- Husain, M.K.; Anis, M. Rapid in vitro multiplication of Melia azedarach L. (a multipurpose woody tree). Acta Physiol. Plant. 2009, 31, 765–772. [Google Scholar] [CrossRef]
- Vengadesan, G.; Ganapathi, A.; Anand, R.P.; Selvaraj, N. In vitro propagation of Acacia sinuata (Lour.) Merr. from nodal segments of a 10-year-old tree. Vitr. Cell. Dev. Biol.-Plant 2003, 39, 409–414. [Google Scholar] [CrossRef]
- Thomas, T.D. Thidiazuron induced multiple shoot induction and plant regeneration from cotyledonary explants of mulberry. Biol. Plant. 2003, 46, 529–533. [Google Scholar] [CrossRef]
- Perveen, S.; Javed, S.B.; Anis, M.; Aref, I.M. Rapid in vitro multiplication and ex vitro establishment of Caribbean copper plant (Euphorbia cotinifolia L.): An important medicinal shrub. Acta Physiol. Plant. 2013, 35, 3391–3400. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M.; Hakeem, K.R. Development of an efficient micropropagation system for Tecoma stans (L.) Juss. ex Kunth using thidiazuron and effects on phytochemical constitution. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 442–453. [Google Scholar] [CrossRef]
- Siddique, I.; Anis, M. Direct plant regeneration from nodal explants of Balanites aegyptiaca L. (Del.): A valuable medicinal tree. New For. 2009, 37, 53–62. [Google Scholar] [CrossRef]
- Javed, S.; Anis, M.; Khan, P.; Aref, I. In vitro regeneration and multiplication for mass propagation of Acacia ehrenbergiana Hayne: A potential reclaiment of denude arid lands. Agrofor. Syst. 2013, 87, 621–629. [Google Scholar] [CrossRef]
- Ahmad, N.; Shahid, A.; Javed, S.B.; Khan, M.I.; Anis, M. Micropropagation of Vitex spp. through in vitro manipulation: Current status and future prospectives. J. Appl. Res. Med. Aromat. Plants 2015, 2, 114–123. [Google Scholar] [CrossRef]
- Elmongy, M.S.; Cao, Y.; Zhou, H.; Xia, Y. Root development enhanced by using indole-3-butyric acid and naphthalene acetic acid and associated biochemical changes of in vitro azalea microshoots. J. Plant Growth Regul. 2018, 37, 813–825. [Google Scholar] [CrossRef]
- Ahmad, A.; Anis, M. Meta-topolin improves in vitro morphogenesis, rhizogenesis and biochemical analysis in Pterocarpus marsupium Roxb.: A potential drug-yielding tree. J. Plant Growth Regul. 2019, 38, 1007–1016. [Google Scholar] [CrossRef]
- Ahmad, N.; Anis, M. Rapid clonal multiplication of a woody tree, Vitex negundo L. through axillary shoots proliferation. Agrofor. Syst. 2007, 71, 195–200. [Google Scholar] [CrossRef]
- Faisal, M.; Singh, S.; Anis, M. In vitro regeneration and plant establishment of Tylophora indica (Burm. F.) Merrill: Petiole callus culture. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 511–515. [Google Scholar] [CrossRef]
- Shahzad, A.; Faisal, M.; Anis, M. Micropropagation through excised root culture of Clitoria ternatea and comparison between in vitro—Regenerated plants and seedlings. Ann. Appl. Biol. 2007, 150, 341–349. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.; Kahl, G. Molecular marker technologies for plant improvement. World J. Microbiol. Biotechnol. 1995, 11, 438–448. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Ahmad, N.; Anis, M.; Hegazy, A.K. An Efficient and Reproducible Method for in vitro Clonal Multiplication of Rauvolfia tetraphylla L. and Evaluation of Genetic Stability using DNA-Based Markers. Appl. Biochem. Biotechnol. 2012, 168, 1739–1752. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Ruta chalepensis L. Plants 2021, 10, 2820. [Google Scholar] [CrossRef]
- Yusuf, A.; Nikhilesh, S.; Rao, P.S. Micropropagation of Vitex negundo L. and Random Amplified Polymorphic DNA Analysis of Micropropagated Plants. J. Sustain. For. 2012, 31, 267–282. [Google Scholar] [CrossRef]
- Purohit, S.; Jugran, A.K.; Bhatt, I.D.; Palni, L.M.S.; Bhatt, A.; Nandi, S.K. In vitro approaches for conservation and reducing juvenility of Zanthoxylum armatum DC: An endangered medicinal plant of Himalayan region. Trees 2017, 31, 1101–1108. [Google Scholar] [CrossRef]
- Thu, H.T.M.; Naing, A.H.; Jeong, H.Y.; Kim, C.K. Regeneration of Genetically Stable Plants from In Vitro Vitrified Leaves of Different Carnation Cultivars. Plants 2020, 9, 950. [Google Scholar] [CrossRef]
- Clapa, D.; Hârța, M. Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy 2021, 11, 2268. [Google Scholar] [CrossRef]


| TDZ (µM) | Percent (%) Response | No. of Shoots per Explant Mean ± SE | Shoot Length (cm) Mean ± SE |
|---|---|---|---|
| 0.0 (C) | 25 | 1.00 ± 0.00 e | 1.58 ± 0.05 c |
| 1.0 | 45 | 3.48 ± 0.08 c | 2.63 ± 0.06 a |
| 5.0 | 75 | 14.52 ± 0.38 a | 2.17 ± 0.13 b |
| 10.0 | 55 | 6.05 ± 0.14 b | 0.86 ± 0.08 d |
| 15.0 | 30 | 2.08 ± 0.12 d | 0.40 ± 0.06 e |
| 20.0 | 00 | 0.00 ± 0.00 f | 0.00 ± 0.00 f |
| TDZ (µM) | Percent (%) Response | No. of Shoots per Explant Mean ± SE | Shoot Length (cm) Mean ± SE |
|---|---|---|---|
| 0.0 (C) | 25 | 1.00 ± 0.00 g | 1.58 ± 0.06 d |
| 3.5 | 50 | 6.25 ± 0.18 f | 2.86 ± 0.09 a |
| 4.0 | 60 | 9.10 ± 0.33 e | 2.51 ± 0.09 b |
| 4.5 | 70 | 12.83 ± 0.15 b | 2.24 ± 0.05 bc |
| 5.0 | 75 | 14.52 ± 0.38 a | 2.17 ± 0.13 c |
| 5.5 | 65 | 11.58 ± 0.32 c | 2.05 ± 0.08 c |
| 6.0 | 60 | 10.14 ± 0.11 d | 1.49 ± 0.06 d |
| 6.5 | 55 | 8.69 ± 0.21 e | 1.06 ± 0.17 e |
| Cytokinins (µM) | Percent (%) Response | No. of Shoots per Explant Mean ± SE | Shoot Length (cm) Mean ± SE | ||
|---|---|---|---|---|---|
| BA | Kin | 2iP | |||
| 0.0 (C) | 0.0 | 0.0 | 60 | 12.06 ± 0.47 i | 2.43 ± 0.18 h |
| 0.25 | 70 | 15.16 ± 0.28 fg | 4.32 ± 0.24 cd | ||
| 0.5 | 70 | 15.85 ± 0.24 cde | 4.94 ± 0.31 b | ||
| 1.0 | 80 | 18.62 ± 0.75 a | 5.87 ± 0.23 a | ||
| 2.0 | 75 | 16.26 ± 0.31 bc | 5.12 ± 0.28 b | ||
| 4.0 | 70 | 15.06 ± 0.37 fgh | 4.56 ± 0.19 c | ||
| 0.25 | 55 | 14.68 ± 0.16 gh | 3.85 ± 0.21 e | ||
| 0.5 | 60 | 15.82 ± 0.41 cde | 4.08 ± 0.21 de | ||
| 1.0 | 70 | 16.56 ± 0.44 b | 5.26 ± 0.20 b | ||
| 2.0 | 65 | 16.04 ± 0.26 bcd | 4.52 ± 0.22 c | ||
| 4.0 | 60 | 15.48 ± 0.21 def | 4.36 ± 0.21 cd | ||
| 0.25 | 50 | 14.62 ± 0.11 gh | 3.04 ± 0.11 g | ||
| 0.5 | 55 | 15.22 ± 0.32 efg | 3.43 ± 0.17 f | ||
| 1.0 | 60 | 15.95 ± 0.38 bcd | 3.74 ± 0.20 ef | ||
| 2.0 | 55 | 14.98 ± 0.41 fgh | 3.02 ± 0.23 g | ||
| 4.0 | 50 | 14.43 ± 0.12 h | 2.90 ± 0.21 g | ||
| Auxin (µM) | Percent (%) Response | No. of Shoots per Explant Mean ± SE | Shoot Length (cm) Mean ± SE | ||
|---|---|---|---|---|---|
| IAA | IBA | NAA | |||
| 0.10 | 75 | 19.45 ± 0.40 de | 5.68 ± 0.09 c | ||
| 0.25 | 80 | 20.38 ± 0.48 cd | 6.04 ± 0.08 b | ||
| 0.50 | 85 | 18.62 ± 0.42 ef | 5.27 ± 0.15 de | ||
| 0.10 | 80 | 17.36 ± 0.28 g | 5.05 ± 0.06 ef | ||
| 0.25 | 80 | 17.55 ± 0.22 fg | 5.34 ± 0.07 de | ||
| 0.50 | 75 | 16.93 ± 0.34 g | 4.76 ± 0.15 f | ||
| 0.10 | 85 | 22.08 ± 0.22 b | 6.25 ± 0.10 b | ||
| 0.25 | 90 | 24.53 ± 0.61 a | 7.16 ± 0.12 a | ||
| 0.50 | 80 | 20.85 ± 0.32 c | 5.50 ± 0.09 cd | ||
| PGRs (µM) | Percent (%) Rooting | No. of Roots per Microshoot Mean ± SE | Root Length (cm) Mean ± SE | ||
|---|---|---|---|---|---|
| IAA | IBA | NAA | |||
| 0.0 (C) | 0 | 0.00 ± 0.00 i | 0.00 ± 0.00 h | ||
| 200 | 60 | 3.62 ± 0.07 f | 2.10 ± 0.10 de | ||
| 400 | 75 | 5.84 ± 0.12 c | 2.65 ± 0.09 b | ||
| 600 | 65 | 4.78 ± 0.13 e | 2.37 ± 0.07 cd | ||
| 800 | 55 | 2.93 ± 0.09 g | 1.76 ± 0.09 f | ||
| 200 | 70 | 5.35 ± 0.07 d | 2.50 ± 0.08 bc | ||
| 400 | 85 | 8.72 ± 0.20 a | 3.48 ± 0.10 a | ||
| 600 | 75 | 6.45 ± 0.11 b | 2.63 ± 0.11 b | ||
| 800 | 60 | 4.84 ± 0.12 e | 2.35 ± 0.06 cd | ||
| 200 | 35 | 2.92 ± 0.14 g | 1.85 ± 0.11 ef | ||
| 400 | 50 | 4.65 ± 0.05 e | 2.32 ± 0.12 cd | ||
| 600 | 40 | 3.47 ± 0.08 f | 2.05 ± 0.05 e | ||
| 800 | 30 | 2.25 ± 0.4 h | 1.50 ± 0.06 g | ||
| Plants | Plant Height | Branches per Plant | Chl a (mg g−1 Fresh Mass) | Chl b (mg g−1 Fresh Mass) | Car (mg g−1 Fresh Mass) | Pn (µmol m−2 s−1) | gs (mol m−2 s−1) | WUE (mol m−2 s−1) | E (mmol m−2 s−1) |
|---|---|---|---|---|---|---|---|---|---|
| In vitro | 8.5 ± 0.52 | 4.10 ± 0.20 | 0.83 ± 0.05 | 0.64 ± 0.07 | 0.49 ± 0.03 | 5.3 ± 0.21 | 0.43 ± 0.03 | 30.1 ± 1.15 | 0.43 ± 0.03 |
| Ex vitro | 9.5 ± 0.65 | 4.52 ± 0.17 | 0.90 ± 0.03 | 0.57 ± 0.02 | 0.41 ± 0.02 | 5.7 ± 0.17 | 0.47 ± 0.07 | 28.3 ± 0.88 | 0.57 ± 0.05 |
| S. No. | Primers | Sequence (5′-3′) | No. of Bands |
|---|---|---|---|
| 1 | OPB01 | GTTTCGCTCG | 10 |
| 2 | OPB02 | TGATCCCTGG | 0 |
| 3 | OPB03 | CATCCCCCTG | 6 |
| 4 | OPB04 | GGACTGGAGT | 0 |
| 5 | OPB05 | TGCGCCCTTC | 6 |
| 6 | OPB06 | TGCTCTGCCC | 9 |
| 7 | OPB07 | GGTGACGCAG | 3 |
| 8 | OPB08 | GTCCACACGG | 0 |
| 9 | OPB09 | TGGGGGACTC | 0 |
| 10 | OPB10 | CTGCTGGGAC | 10 |
| 11 | OPB11 | GTAGACCCGT | 0 |
| 12 | OPB12 | CCTTGACGCA | 7 |
| 13 | OPB13 | TTCCCCCGCT | 2 |
| 14 | OPB14 | TCCGCTCTGG | 5 |
| 15 | OPB15 | GGAGGGTGTT | 0 |
| 16 | OPB16 | TTTGCCCGGA | 10 |
| 17 | OPB17 | AGGGAACGAG | 0 |
| 18 | OPB18 | CCACAGCAGT | 0 |
| 19 | OPB19 | ACCCCCGAAG | 2 |
| 20 | OPB20 | GGACCCTTAC | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Faisal, M.; Ahmad, A.; Alatar, A.A.; Qahtan, A.A.; Alok, A. Thidiazuron Induced In Vitro Clonal Propagation of Lagerstroemia speciosa (L.) Pers.—An Important Avenue Tree. Horticulturae 2022, 8, 359. https://doi.org/10.3390/horticulturae8050359
Ahmad N, Faisal M, Ahmad A, Alatar AA, Qahtan AA, Alok A. Thidiazuron Induced In Vitro Clonal Propagation of Lagerstroemia speciosa (L.) Pers.—An Important Avenue Tree. Horticulturae. 2022; 8(5):359. https://doi.org/10.3390/horticulturae8050359
Chicago/Turabian StyleAhmad, Naseem, Mohammad Faisal, Anees Ahmad, Abdulrahman A. Alatar, Ahmed A. Qahtan, and Anshu Alok. 2022. "Thidiazuron Induced In Vitro Clonal Propagation of Lagerstroemia speciosa (L.) Pers.—An Important Avenue Tree" Horticulturae 8, no. 5: 359. https://doi.org/10.3390/horticulturae8050359
APA StyleAhmad, N., Faisal, M., Ahmad, A., Alatar, A. A., Qahtan, A. A., & Alok, A. (2022). Thidiazuron Induced In Vitro Clonal Propagation of Lagerstroemia speciosa (L.) Pers.—An Important Avenue Tree. Horticulturae, 8(5), 359. https://doi.org/10.3390/horticulturae8050359










