Abstract
The application of exogenously applied salicylic acid plays important roles in improving the growth, yield, and bioactive compound compositions of different plant species. Curcuma longa is a medicinal plant that is commonly used as a spice and food additive, and has antioxidant potential. In this study, an innovative strategy for enhancing active compound production was investigated by applying a natural plant growth enhancer—namely, salicylic acid (SA)—to C. longa plants. The experiment was conducted using a complete randomized block design. The effects of SA on the growth, yield, and chemical compound contents of C. longa were recorded. Our findings demonstrated that SA significantly improved C. longa growth, yield, and curcuminoid content when compared to control treatment, with SA at 10−3 M having the greatest effect. The study also indicated that the increase in the curcuminoid content was accompanied by the overexpression of the curcumin synthase 1 (CURS1), 2 (CURS2), and 3 (CURS3) genes, as well as the diketide-CoA synthase (DCS) gene, which have been implicated in the synthesis of curcuminoids.
1. Introduction
Curcuma longa L. (Curcuma; turmeric) of the Zingiberaceae family is a widely cultivated plant that is grown in warm climates in many regions of the world [1]. It is a perennial herbaceous plant. The rhizome is the most valuable part of C. longa [2], containing a range of bioactive compounds in the form of non-volatile curcuminoids (curcumin and dimethoxy- and bisdemethoxycurcumin) and volatile oils (mono- and sesquiterpenoids) [3]. Turmeric has long been used as a spice and food additive to improve food’s palatability and storage stability because of its specific yellow color, taste, and antioxidant potential [4].
Curcuminoids are the most important bioactive constituents of turmeric [5]. Curcuminoids, especially curcumin, have valuable biological activities such as antioxidant, antitumor, anti-inflammatory, anti-acidogenic, radioprotective, and neuroprotective properties [6,7]. Analyses of turmeric by genomic and data-mining-based research have detected four type III polyketide synthase genes—namely, diketide-CoA synthase (DCS), curcumin synthase 1 (CURS1), curcumin synthase 2 (CURS2), and curcumin synthase 3 (CURS3)—which are involved in the curcuminoid biosynthesis pathway in various turmeric cultivars [8,9]. These genes are differentially associated with different curcuminoid contents [10,11]
Salicylic acid (SA) is a non-enzymatic antioxidant and plant hormone that influences a variety of physiological processes, including cell division and elongation, as well as initiating or boosting secondary chemical production in plants [12,13,14,15,16,17]. It is a member of the salicylate family of chemicals, which are phenolic compounds with an aromatic ring and a hydroxyl group that are generated by plants. Seed germination, vegetative growth, flowering, fruit output, senescence, stomatal closure, thermogenesis, photosynthesis, respiration, alterations in the alternate respiratory route, glycolysis, and the Krebs cycle are all affected by the application of SA [18].
Because SA is one of the elicitors involved in plants’ development and production, it may be applied exogenously to improve secondary metabolite synthesis; thus, the effects of SA on C. longa were investigated in this study. Salicylic acid was applied to C. longa plants as a foliar spray to explore its effects on plant development and yield, as well as the production of active compounds—in particular, that of curcuminoids. The purpose of this research was to find the most effective strategy to increase the levels of curcuminoids in the plant rhizomes by employing SA as an elicitor. In addition, the expression of curcuminoid synthesis genes in the rhizomes was investigated to relate the curcuminoid concentrations and curcuminoid gene expression levels.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The experiment was conducted under greenhouse conditions during two successive seasons in 2020 and 2021 at the Agriculture and Veterinary Research and Training Center, King Faisal University, Al-Ahsa, Saudi Arabia. During the experiment, the temperature ranged between 32 and 36 °C, relative humidity was 47–56%, and the average photoperiod was 14 h. Curcuma longa rhizomes provided by Sekem Company, Cairo, Egypt, were planted on 1 April and harvested on 30 November in both seasons.
The germination was carried out by planting in trays (depth of 1.0–2.0 cm) filled with a moist mixture of sand and peat moss (1:1 v:v). Turmeric seedlings, 5 cm tall and with three pairs of leaves, were transplanted into sandy soil (Table 1) and placed 40 × 40 cm2 from one another. Every two days, all of the plants were watered with groundwater (Table 2).

Table 1.
Physical and chemical properties of the experimental soil.

Table 2.
Chemical properties and compositions of the irrigation water.
The experiments were arranged in a completely randomized pattern. The plot area was 120 cm × 160 cm (19.20 m2), every plot had 20 plants, and there were 10 replicates of each treatment. The study employed distilled water as a control and two concentrations of SA (10−4 and 10−3 M) (Sigma-Aldrich, Chemie GmbH, Taufkirchen, Germany) as treatment groups, as described in [19,20]. SA solutions containing the surfactant (0.1% triton) were sprayed on the whole foliage three times in the morning (8–9 a.m.) at two-month intervals. Each time, the volume of sprayed solution was around 50 mL per plant. The control plants were sprayed with the same amount of deionized water plus 0.1% Triton.
The SA treatments began one month after the transplantation of seedlings from the germination trays to the soil. After eight months of cultivation, the whole plants were harvested, and the plant height (cm), number of leaves (n), number of roots and rhizomes/plant (n), dry weight of the leaves, roots, and rhizomes/plant (g), and rhizome diameter (mm) were recorded using 10 random plants from each treatment group.
2.2. Chemical Analysis
2.2.1. Measurement of Photosynthetic Pigments
The 3rd-bottom fresh leaf was excised from six randomly selected 8-month-old turmeric plants. The amounts of chlorophyll a and b and carotenoids were measured with 80% acetone, as described in [21]. The absorbance was measured using an Agilent 8453 UV–Visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA).
2.2.2. Mineral Composition
Plant leaf samples were dried for 48 h at 60 °C 270 days after planting. Dry leaf samples were crushed and digested with sulfuric acid [22]. The modified micro-Kjeldahl technique was used to determine the nitrogen content [23]. Calorimetry was used to measure the content of phosphorus, as reported in [24], and atomic absorption flame photometry was used to determine the potassium (K) content, as described in [25].
Analysis was performed on soil samples collected at the end of the experiment. Soil and water analyses were performed as described in [26].
2.3. GC/MS Analysis
One gram of the homogenized air-dried rhizome powder of three randomly selected turmeric plants from each treatment group (control and 10−3 and 10−4 M SA) planted in both seasons was added to a 28 mL stoppered culture tube and defatted with 30 mL of ethanol for one day, with shaking at 100 rpm on a rotary shaker. The extracts were filtered through a 0.2 μm syringe filter, and 2 µL was injected into the GC–MS system. Gas chromatography–mass spectrometry (GC–MS) was performed at the Department of Chemistry, Faculty of Science, King Faisal University, using a GC 1310-ISQ mass spectrometer (Thermo Scientific, Austin, TX, USA) with a TG–35MS direct capillary column (30 m × 0.25 mm × 0.25 µm film thickness). The separation conditions and method for identification of the separated components were as described in [27].
2.4. Determination of Curcumin, Bisdemethoxycurcumin, and Demethoxycurcumin Contents by High-Performance Liquid Chromatography (HPLC)
2.4.1. Instrumentation
The HPLC analysis was performed using a Waters 2690 Alliance HPLC system (Waters, Milford, MA, USA) equipped with a Waters 996 photodiode array detector (Waters, Milford, MA, USA).
2.4.2. Preparation of Curcumin, Bisdemethoxycurcumin, and Demethoxycurcumin Standard Curves
Authentic curcumin (≥99.5%), bisdemethoxycurcumin (≥98%), and demethoxycurcumin (≥98%) were purchased from Sigma-Aldrich. A stock solution of 1 mg/mL of three standards was prepared and diluted in methanol to obtain standard solutions of 10, 20, 30, 40, 50, and 60 μg/mL. All standard solutions were filtered using 0.22 μm syringe filters, and 10 μL of each standard solution was injected into the HPLC system per run.
2.4.3. Preparation of C. longa Rhizome Methanolic Extracts
Five grams of the homogenized, air-dried rhizome powder of three randomly selected turmeric plants from each treatment group (control and 10−3 and 10−4 M SA) planted in the second season was combined with 50 mL of ethanol for four days using a maceration technique. All ethanol supernatants collected were combined and placed in a rotary evaporator (Büchi Labortechnik AC, Flawil, Switzerland) operated at 40 °C to remove the solvent completely. A known weight of the residue (ethanolic extract) was then dissolved in 5 mL of the mobile phase (0.1% trifluoroacetic acid in water: acetonitrile (50:50%)), and the solution was filtered using a 0.22 μm syringe filter. For each sample, 10 μL of filtrate was injected into the HPLC system.
2.4.4. HPLC Analysis Conditions
The HPLC separation and quantitation were performed at ambient temperature with a C18 Inertsil (4.6 mm × 250 mm, 5 µm) column. The mobile phase was prepared by mixing 0.1% trifluoroacetic acid in water with acetonitrile (50:50%). The flow rate was maintained at 1.5 mL/min. All determinations were performed at ambient temperature (25 °C), at a wavelength of 420 nm. The mobile phase was filtered using a 0.45 micrometer membrane filter (Millipore, Milford, MA, USA), and was degassed by vacuum prior to use.
2.5. Analysis of CURS1, -2, and -3 and DCS Gene Expression by Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR)
2.5.1. Total RNA Preparation and cDNA Synthesis
Rhizomes were collected from four randomly selected 8-month-old plants of each treatment group (control and 10−3 and 10−4 M SA) (second season) and stored at −80 °C until RNA extraction was performed. RNA was extracted from the frozen rhizome samples using an RNeasy Mini Kit (Qiagen Cat. No. 74104) according to the manufacturer’s protocol. The quantity and quality of the extracted total RNA from each sample were analyzed using a NanoDropTM 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Additionally, cDNA synthesis was performed using the ReadyScriptTM cDNA synthesis mix (Sigma-Aldrich Cat. No. RDRT) according to the manufacturer’s protocol. The reaction was incubated at 25 °C for 5 min, followed by 30 min of incubation at 42 °C, and a final heating to 85 °C for 5 min.
2.5.2. Real Time RT-PCR and Curcuminoid Gene Expression Analysis
A real-time PCR containing 2 µL of the cDNA reaction mixture, 0.3 µM concentrations (final concentrations) of each forward and reverse primer (Table 3), 12.5 µL of SYBR Green PCR Master Mix (QuantiTech SYBR Green PCR Kit, Qiagen Cat. No. 204143, Germantown, MD, USA), 1 µL of RNase inhibitor, and RNase-free water, at a final volume of 25 µL was set up. Primers for the target (CURS1, -2, and -3 and DCS) and housekeeping (Actin) genes were designed according to the gene sequences available at GenBank (Table 3). The real-time reaction was performed using an Applied Biosystems 7500 Real-Time PCR System under the following conditions: 95 °C initial heating for 10 min, followed by 45 cycles of 95 °C for 20 s and 60 °C for 1 min. PCR was performed in duplicate, in addition to the non-template control (NTC) and cDNA template negative. The specificity of the amplified product was verified by performing melting curve analyses at the end of the amplification cycles. The relative expression of the CURS1, -2, and -3 and DCS genes was quantified using the 2−ΔΔCT method [28], by normalizing the CURS1, -2, and -3 and DCS (target) gene expression with that of the Actin (reference) gene relative to the untreated control.

Table 3.
Sequences of forward and reverse primers for real-time RT-PCR.
2.6. Statistical Analysis
The experimental design was a complete randomized block design with 20 replicates. Data were statistically analyzed via ANOVA/MANOVA using Statistica 6 software (StatSoft) [29]. The significance of differences between means was assessed using the least significant difference test (LSD) at p = 0.05.
3. Results
3.1. Effects of SA on Plant Development and Yield
The analyses of the SA foliar treatments’ effects on the growth parameters of C. longa during both the 2020 and 2021 planting seasons are presented in Table 4. During both planting seasons, a rise in SA concentration resulted in a significant increase in plant height when compared to the control. In both seasons, both SA concentrations (10−4 and 10−3 M) had a positive impact on leaves’ dry weight as compared to the control treatment. The 10−4 M SA treatment improved the number of leaves and the dry weight of the roots in both planting seasons. A further increase in the SA concentration (10−3 M) showed reduced enhancement effects on the abovementioned growth parameters compared to the control treatment in both seasons. When compared to other treatments, the presence of 10−3 M SA resulted in a significant reduction in the roots’ dry weight in both seasons. In the 2020 season, the 10−4 M SA treatment performed better than the 10−3 M SA treatment in terms of increasing the number of roots. In contrast, in the 2021 season, when compared to the control and 10−4 M SA treatments, the 10−3 SA treatment significantly increased the number of roots.

Table 4.
Effects of salicylic acid (SA) treatment on the plant height (cm), the number of leaves and roots (n), and the dry weight (g) of leaves and roots of C. longa.
In both seasons, the number of rhizomes, rhizome dry weight, and rhizome diameter were significantly higher in the SA-treated groups than in the control group (Table 5). Furthermore, the 10−3 M SA treatment had the highest rhizome number (34.33 and 19.0), rhizome dry weight (78.87 and 61.23 g), and rhizome diameter (18.25 and 20.15 mm) in the first and second seasons, respectively, while the control had the lowest rhizome number (16.67 and 10.7), rhizome dry weight (13.6 and 7.23 g), and rhizome diameter (16.3 and 14.47), respectively.

Table 5.
Effects of SA treatment on the number (n) of rhizomes, rhizome dry weight, and rhizome diameter (mm) of C. longa.
3.2. Photosynthetic Pigments and Mineral Contents
The data in Table 6 show that both (10−4 and 10−3 M) SA treatments positively and significantly affected the chlorophyll a and b and carotenoid contents compared with the control. The 10−3 M SA treatment yielded higher values compared to the 10−4 M SA treatment for all recorded parameters in both seasons (Table 6). The data in Table 7 show that both SA concentrations increased the nitrogen (N), phosphorus (P), and potassium (K) contents in the leaves of C. longa compared with the control plants. In comparison to the control and 10−4 M SA treatments, the 10−3 M treatment resulted in the highest N, P, and K contents in the C. longa leaves (Table 7).

Table 6.
Effects of SA treatment on the chlorophyll a (Chl a), b (Chl b), and carotenoid contents of C. longa leaves.

Table 7.
Effects of SA concentration on nitrogen (N), phosphorus (P), and potassium (K) percentages in C. longa leaves.
3.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis of Ethanolic Extracts
GC–MS was used to examine ethanolic extracts of C. longa rhizomes obtained from different SA concentration treatments as well as controls (Table 8 and Table 9). Following GC–MS analysis, at least 10 components were discovered in the ethanolic extracts of the C. longa rhizomes. Table 8 lists the names of the compounds and their retention times (RTs), peak areas (in percentage), molecular formulae, and molecular weights. The effects of SA on the composition of alpha-curcumene, (−)-zingiberene, beta-sesquiphellandrene, aromatic turmerone (ar-turmerone), curlone, beta-turmerone, and caryophyllene are displayed in Table 9. Substantial amounts of alpha-curcumene, (−)-zingiberene, beta-sesquiphellandrene, ar-turmerone, and curlone were present in the extracts of all treatments. These five components have been documented as the major components of C. longa essential oil. The application of 10−4 M SA resulted in an approximately 1.05-fold increase in alpha-curcumene in the first season; in the second season, 10−4 and 10−3 M SA resulted in 1.14- and 1.08-fold increases compared to the control, respectively, both of which were significant (Table 9). Both concentrations (10−4 and 10−3 M) of SA significantly increased the levels of (−)-zingiberene and beta-sesquiphellandrene, by about 1.3- and 1.1-fold, respectively, in the first season (Table 9). On the other hand, the 10−3 M SA treatment led to significantly lower amounts of beta-sesquiphellandrene compared with the control treatment and 10−4 M SA in the second season. Both SA treatments led to significantly increased levels of ar-turmerone and curlone in the second season. Beta-turmerone was found in the SA treatment groups in the second season, whereas caryophyllene was found in the control treatments in the second season, SA 10−3 in the first season, and 10−4 M in both seasons. When compared to the control, the SA treatments resulted in a significant decrease in caryophyllene content (Table 9).

Table 8.
Components identified by GC–MS analysis in the C. longa ethanolic rhizome extracts from different treatment groups. Each area (%) was calculated from the measurements obtained from the ethanolic extracts of 3 plantlets.

Table 9.
Comparison of curcumene, zingiberene, beta-sesquiphellandrene, ar-turmerone, curlone, beta-turmerone, and caryophyllene compositions in the ethanolic rhizome extracts from Curcuma longa plants grown under different concentrations of SA (M). Each mean value was calculated from the measurements obtained from the ethanolic extracts of 3 plantlets. ND: not detectable.
3.4. HPLC Results
The effects of different SA concentrations on the production of secondary metabolites in C. longa rhizomes were investigated (Table 10 and Figure 1). The amounts of bisdemethoxycurcumin, demethoxycurcumin, and curcumin present in the ethanolic extracts were quantified using high-performance liquid chromatography (HPLC) by comparing the standard curve prepared using a series of bisdemethoxycurcumin, demethoxycurcumin, and curcumin solutions with known concentrations. SA treatments significantly increased the bisdemethoxycurcumin, demethoxycurcumin, and curcumin levels compared to the control treatment (Table 10). As the SA concentration increased, the effect on all three compounds increased (Table 10). Foliar spraying with 10−3 M SA resulted in an increase in the bisdemethoxycurcumin, demethoxycurcumin, and curcumin levels by approximately 2.77-, 2.58-, and 2.57-fold, respectively, compared with the control treatment (Table 10 and Figure 1).

Table 10.
Effects of SA treatment on bisdemethoxycurcumin, demethoxycurcumin, and curcumin (ug/mL) accumulation in C. longa in the second season.
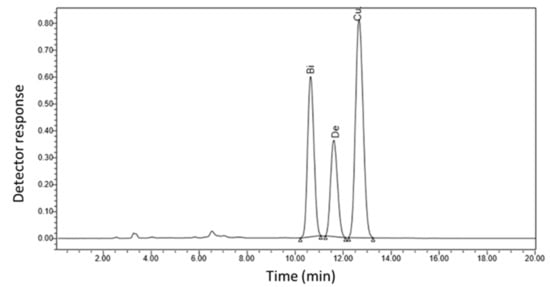
Figure 1.
HPLC chromatogram of organic extract of C. longa exposed to 10−3 M SA, showing the curcuminoid compounds bisdemethoxycurcumin (Bi), demethoxycurcumin (De), and curcumin (Cu).
3.5. Effect of SA on the Expression of Curcuminoid Biosynthesis Genes
Real-time PCR was used to assess the transcript levels of curcuminoid genes in the C. longa rhizomes eight months after planting in the SA and control treatments. The results showed that the expression of the curcuminoid genes was higher following foliar application of SA compared with the control treatment (Table 11). The results showed that the CURS2 and -3 genes had higher expression levels in the 10−3 M SA treatment compared with the CURS1 and DCS genes. The highest expression level was obtained for the CURS3 gene (a 36.0-fold increase). The CURS1 gene was upregulated in the SA treatments, with the highest expression (a 12.6-fold increase) obtained at 10−4 M, as shown in Table 11.

Table 11.
Differential expression profiling of the curcuminoid synthase genes, CURS1, CURS2, CURS3, and DCS, in C. longa rhizomes from control- and SA (10−4 and 10−3 M)-treated plants. Data were normalized using Actin as an internal reference gene.
4. Discussion
The effects of foliar spraying of C. longa plants with different concentrations of SA on the vegetative growth, yield, phytochemical composition, and expression patterns of curcuminoid genes were investigated. Our data indicate that foliar spraying with SA increased the plant growth parameters, rhizome dry weight, rhizome yield, photosynthetic pigments, and mineral contents in C. longa plants compared to the control treatment (sprayed with water). SA at 10−3 M had the most positive effects on plant height, number of roots, dry weight of leaves, and chlorophyll a, carotenoid, N, and K contents in the leaves in both seasons of cultivation, which were correlated with the highest rhizome numbers, rhizome dry weight, and rhizome diameter. These findings are consistent with previous findings [12,13,30] that stated that SA promotes plant development by increasing cell division and elongation as well as photosynthetic pigment levels, both of which are connected to increased nutrient intake. The effects of SA on plant development and productivity have been well established. Elwan and El-Hamahmy [19] reported that an SA-induced increase in pepper plant yield can be related to an increase in growth and photosynthesizing tissue, i.e., leaves. Many plants, including okra [31], chickpeas [20], fenugreek [32], peppermint [33], and sorghum [34], have increased chlorophyll levels as a result of SA treatments, resulting in considerable growth, photosynthesis, and production improvements. Due to their high abundance of chemically different metabolites, no single analytical approach has yet been able to detect the entire metabolome of higher plants—particularly medicinal and aromatic species [35]. We employed GC–MS and HPLC techniques to detect different chemical compounds in C. longa plants treated with different SA concentrations and control plants in this investigation. The bioactive components of the ethanolic rhizome extract of C. longa were determined by GC–MS. The results show that the SA treatments enhanced curcumene, (−)-zingiberene, ar-turmerone, and curlone levels, and that beta-turmerone was only found in the SA treatments. The application of SA has been implicated in the increased concentrations of effective (active) compounds in a variety of plants [36,37,38].
Polyphenolic curcuminoids—including bisdemethoxycurcumin, demethoxycurcumin, and curcumin—found in C. longa play significant roles in food, cosmetics, and medicinal compounds. Curcuminoids have extensive biological activity, with antioxidant, neuroprotective, antitumor, anti-inflammatory, anti-acidogenic, and radioprotective properties [39]. The HPLC examination of bisdemethoxycurcumin, demethoxycurcumin, and curcumin revealed that the SA treatments greatly enhanced these compounds compared to the control, with the effect of SA at 10−3 M on height resulting in the largest amounts of the three compounds mentioned. The SA’s elicitor action on the synthesis of secondary metabolites in medicinal plants has been previously assessed [13,14,15,16,17]. A correlation between the expression of curcuminoid genes and curcumin biosynthesis in C. longa was found through RT-PCR amplification and HPLC analysis. The bisdemethoxycurcumin, demethoxycurcumin, and curcumin contents appear to correspond to the expression levels of curcuminoid genes. These results are also similar to those of several previous studies. The accumulation of major products of secondary metabolism in plants has been correlated with the expression of coordinate genes under the effect of suitable elicitors [16,40,41].
5. Conclusions
Our findings show that foliar spraying of C. longa plants with 10−4 and 10−3 M SA three times during vegetative growth (beginning at 60 days post-planting of rhizomes, at two-month intervals) under greenhouse conditions in Al-Ahsa, Saudi Arabia, enhanced plant growth and rhizome yield after 60 days of C. longa plant culture.
The addition of SA to the foliar spray improved the plant growth, photosynthetic pigment content, and N, P, and K contents. The SA foliar spray also enhanced the curcuminoid content (bisdemethoxycurcumin, demethoxycurcumin, and curcumin), which corresponded with the curcuminoid gene expression levels. The treatment with 10−3 M SA yielded significantly better results in almost all agronomic parameters compared to the control, while the treatment with 10−4 M SA indicated better results, but only in some cases was this improvement significant, so the application of 10−3 M SA would be more recommendable.
Author Contributions
Conceptualization, F.E.S. and S.K.; methodology and formal analysis, F.E.S.; data curation, S.K. and F.E.S.; writing—original draft preparation, M.A.A. and F.E.S.; writing—review and editing, F.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research, King Faisal University, for funding this research through grant number “AN000620”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The greenhouse personnel at King Faisal University’s Agriculture and Veterinary Research and Training Centre provided invaluable support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Setzer, W.N.; Duong, L.; Poudel, A.; Mentreddy, S.R. Variation in the chemical composition of five varieties of Curcuma longa rhizome essential oils cultivated in north alabama. Foods 2021, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.; Padmaja, G.; Remani, P. Antitumour Effects of Isocurcumenol Isolated from Curcuma zedoaria Rhizomes on Human and Murine Cancer Cells. Int. J. Med. Chem. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). Ref. Ser. Phytochem. 2021, 297–318. [Google Scholar]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H. Curcumin and its derivatives: A review of their biological activities. Syst. Rev. Pharm. 2020, 11, 472–481. [Google Scholar]
- Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J. Biol. Chem. 2009, 284, 11160–11170. [Google Scholar] [CrossRef] [Green Version]
- Katsuyama, Y.; Kita, T.; Horinouchi, S. Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett. 2009, 583, 2799–2803. [Google Scholar] [CrossRef] [Green Version]
- Sandeep, I.S.; Das, S.; Nasim, N.; Mishra, A.; Acharya, L.; Joshi, R.K.; Nayak, S.; Mohanty, S. Differential expression of CURS gene during various growth stages, climatic condition and soil nutrients in turmeric (Curcuma longa): Towards site specific cultivation for high curcumin yield. Plant Physiol. Biochem. 2017, 118, 348–355. [Google Scholar] [CrossRef]
- Ayer, D.K.; Modha, K.; Parekh, V.; Patel, R.; Vadodariya, G.; Ramtekey, V.; Bhuriya, A. Associating gene expressions with curcuminoid biosynthesis in turmeric. J. Genet. Eng. Biotechnol. 2020, 18, 83. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Alyemeni, M.N.; Ahmad, A. Salicylic acid mediated changes in growth, photosynthesis, nitrogen metabolism and antioxidant defense system in Cicer arietinum L. Plant Soil Environ. 2012, 58, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Pedroso, R.C.N.; Branquinho, N.A.D.Á.; Hara, A.C.B.D.A.M.; Silva, F.G.; Kellner Filho, L.C.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Januario, A.H. Effect of salicylic acid and silver nitrate on rutin production by hyptis marrubioides cultured in vitro. Cienc. Rural. 2019, 49. [Google Scholar] [CrossRef] [Green Version]
- Pedro, H.G.; Ana, C.A.U.P. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Afr. J. Biotechnol. 2016, 15, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, S.M.; Naghavi, M.R.; Ghorbani, M.; Priyanatha, C.; Zandi, P. Effects of abiotic elicitors on expression and accumulation of three candidate benzophenanthridine alkaloids in cultured greater celandine cells. Molecules 2021, 26, 1395. [Google Scholar] [CrossRef] [PubMed]
- Kandoudi, W.; Radácsi, P.; Gosztola, B.; Németh, É.Z. Elicitation of medicinal plants in vivo—Is it a realistic tool? The effect of methyl jasmonate and salicylic acid on lamiaceae species. Horticulturae 2022, 8, 5. [Google Scholar] [CrossRef]
- Lan, T.T.P.; Huy, N.D.; Luong, N.N.; Quang, H.T.; Tan, T.H.; Thu, L.T.A.; Loc, N.H. Effect of salicylic acid and yeast extract on curcuminoids biosynthesis gene expression and curcumin accumulation in cells of Curcuma zedoaria. J. Plant Biotechnol. 2019, 46, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Elwan, M.W.M.; El-Hamahmy, M.A.M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009, 122, 521–526. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Hayat, Q.; Wijaya, L.; Hayat, S. Effect of salicylic acid on the growth, photosynthetic efficiency and enzyme activities of leguminous plant under cadmium stress. Not. Bot. Hortic. Agrobot. 2014, 42, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists. Official Method of Analysis of the Association of Officail Chemist. In Official Methods of Analysis, 14th ed.; Howitz, Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1984. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Hans Publishers: Delhi, India, 1942. [Google Scholar]
- Jackson, M.L. Soil Chemica Analysis; Prentice Hall: New Delhi, India, 1967. [Google Scholar]
- Murphy, J.; Riley, J.P. Modified single-solution method for the determination of phosphorus in natural water. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mazumdar, B.; Majumder, K.; Mazumdar, B.C.; Majumder, K. Methods of Physiochemical Analysis of Fruits; Daya Publications: Delhi, India, 2003. [Google Scholar]
- Page, A.L. Methods of Soil Analysis—Part 2: Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; Volume 9, pp. 421–422. [Google Scholar]
- El Sherif, F.; Albotnoor, N.; Yap, Y.K.; Meligy, A.; Khattab, S. Enhanced bioactive compounds composition in Lavandula officinalis in-vitro plantlets using NaCl and Moringa oleifera, Aloe vera and Spirulina platensis extracts. Ind. Crops Prod. 2020, 157, 112890. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- StatSoft STATISTICA for Windows, Version 6; StatSoft Inc.: Tulsa, OK, USA, 2001.
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Youssef, S.M.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus cv. Red Balady). Agronomy 2022, 12, 168. [Google Scholar] [CrossRef]
- Mohammad, F.; Wajid, M.A.; Bhat, M.A. Effect of Salicylic Acid Sprays on the Performance of Fenugreek Grown with Graded Levels of Salinity. Haya Saudi J. Life Sci. 2019, 4, 346–354. [Google Scholar] [CrossRef]
- Gonçalves, F.C.D.M.; Parreiras, N.D.S.; Campos, F.G.; Mantoan, L.P.B.; Boaro, C.S.F. Exogenous salicylic acid modifies gas exchange and biomass production of Mentha x piperita L. Aust. J. Crop Sci. 2020, 14, 98–107. [Google Scholar] [CrossRef]
- Dehnavi, A.R.; Zahedi, M.; Ludwiczak, A.; Piernik, A. Foliar Application of Salicylic Acid Improves Salt Tolerance of Sorghum (Sorghum bicolor (L.) Moench). Plants 2022, 11, 368. [Google Scholar] [CrossRef]
- Liu, R.; Bao, Z.X.; Zhao, P.J.; Li, G.H. Advances in the study of metabolomics and metabolites in some species interactions. Molecules 2021, 26, 3311. [Google Scholar] [CrossRef]
- Bekinbo, M.; Tariah, F.S.A.; Dapper, D. Comparative GC-MS determination of bioactive constituents of the methanolic extracts of Curcuma longa rhizome and Spondias mombin leaves Bekinbo MT, Amah-Tariah FS and Dapper DV. J. Med. Plants Stud. 2020, 8, 1–6. Available online: http://www.plantsjournal.com (accessed on 22 April 2022).
- Arivoli, S.; Tennyson, S.; Divya, S.; Rani, S.; Marin, G. GC-MS analysis of bioactive compounds of Curcuma longa Linnaeus (Zingiberaceae) rhizome extract. J. Pharmacogn. Phytochem. 2019, 8, 49–52. Available online: http://www.phytojournal.com (accessed on 22 April 2022).
- Kulyal, P.; Acharya, S.; Ankari, A.B.; Kokkiripati, P.K.; Tetali, S.D.; Raghavendra, A.S. Variable Secondary Metabolite Profiles Across Cultivars of Curcuma longa L. and C. aromatica Salisb. Front. Pharmacol. 2021, 12, 659546. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.T.; Arasu, M.V.; Al-Dhabi, N.A.; Yeo, S.K.; Jeon, J.; Park, J.S.; Lee, S.Y.; Park, S.U. Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in agastache rugosa cell culture. Molecules 2016, 21, 426. [Google Scholar] [CrossRef]
- Yap, Y.K.; El-Sherif, F.; Habib, E.S.; Khattab, S. Moringa oleifera leaf extract enhanced growth, yield and silybin content while mitigating salt-induced adverse effects on the growth of silybum marianum. Agronomy 2021, 11, 2500. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).