Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality
Abstract
:1. Introduction
2. Materials and Methods
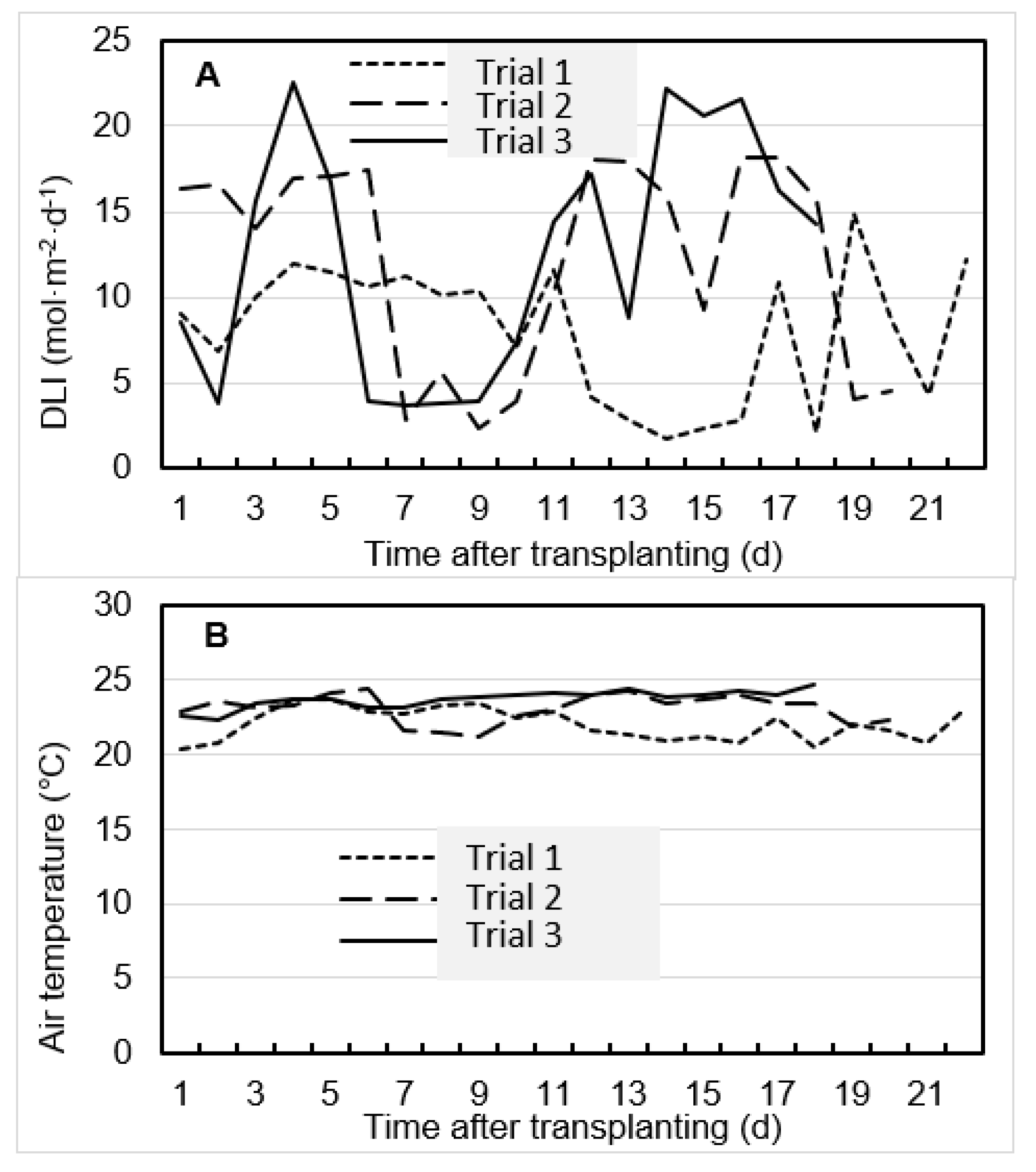
2.1. Experimental Sites and Environmental Conditions
2.2. Plant Materials and Maintenance
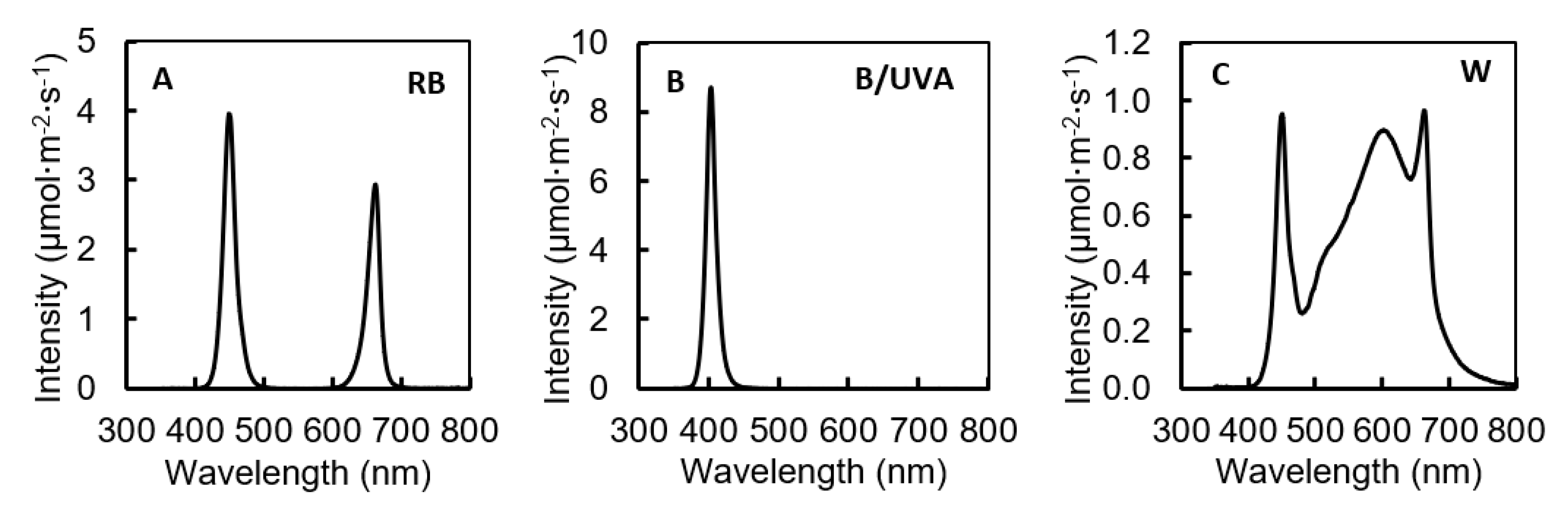
2.3. Light Treatments
2.4. Growth and Quality Measurements
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Shoot Biomass
3.2. Leaf Characteristics
3.3. Phytonutrients Concentrations
4. Discussion
4.1. Short-Term Pre-Harvest SL Improves Crop Quality Rather than Growth
4.2. Different LEDs Similarly Affect Lettuce Growth and Quality
4.3. Pre-Harvest SL at Nighttime Is Better than Daytime for Some Crop Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owen, W.G.; Lopez, R.G. End-of-production supplemental lighting with red and blue light-emitting diodes (LEDs) influences red pigmentation of four lettuce varieties. HortScience 2015, 50, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of pre-harvest supplemental UV-A/Blue and Red/Blue LED lighting on lettuce growth and nutritional quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Viršilė, A.; Duchovskis, P. Supplementary red-LED lighting and the changes in phytochemical content of two baby leaf lettuce varieties during three seasons. J. Food Agric. Environ. 2012, 10, 701–706. [Google Scholar]
- Zhang, M.; Whitman, C.M.; Runkle, E.S. Manipulating growth, color, and taste attributes of fresh cut lettuce by greenhouse supplemental lighting. Sci. Hortic. 2019, 252, 274–282. [Google Scholar] [CrossRef]
- Zukauskas, A.; Bliznikas, Z.; Breivė, K.; Novičkovas, A.; Samuolienė, G.; Urbonavičiūtė, A.; Brazaitytė, A.; Jankauskienė, J.; Duchovskis, P. Effect of supplementary pre-harvest LED lighting on the antioxidant properties of lettuce cultivars. Acta Hortic. 2011, 907, 87–90. [Google Scholar] [CrossRef]
- He, D.; Kozai, T.; Niu, G.; Zhang, X. Light-emitting diodes for horticulture. In Light-Emitting Diodes—Materials, Processes, Devices and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 513–547. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Heo, J.W.; Kang, D.H.; Bang, H.S.; Hong, S.G.; Chun, C.; Kang, K.K. Early growth, pigmentation, protein content, and phenylalanine ammonia-lyase activity of red curled lettuces grown under different lighting conditions. Korean J. Hort. Sci. Technol. 2012, 30, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Cashmore, A.R. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 1997, 11, 421–427. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E. LEDs on Lettuce: White Light versus Red + Blue Light. Produce Grower. 2021. Available online: https://www.producegrower.com/article/production-leds-on-lettuce-white-light-versus-red-blue-light/#:~:text=%C2%B7s%E2%80%931.-Results,red%20%2B%2056%25%20blue%20light (accessed on 4 February 2022).
- Dou, H.; Niu, G.; Gu, M. Pre-harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.; Kong, Y.; Zheng, Y. Overnight supplemental blue, rather than far-red, light improves microgreen yield and appearance quality without compromising nutritional quality during winter greenhouse production. HortScience 2020, 55, 1468–1474. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Silva, S.; Costa, E.; Calhau, C.; Morais, R.; Pintado, M. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Dorais, M. The use of supplemental lighting for vegetable crop production: Light intensity, crop response, nutrition, crop management, cultural practices. In Proceedings of the Canadian Greenhouse Conference, Toronto, ON, Canada, 9–10 October 2003; Available online: https://www.agrireseau.net/legumesdeserre/documents/cgc-dorais2003fin2.pdf (accessed on 4 February 2022).
- Gaudreau, L.; Charbonneau, J.; Vézina, L.-P.; Gosselin, A. Photoperiod and photosynthetic photon flux influence growth and quality of greenhouse-grown lettuce. HortScience 1994, 29, 1285–1289. [Google Scholar] [CrossRef] [Green Version]
- Cernusak, L.A. Gas exchange and water-use efficiency in plant canopies. Plant Biol. 2020, 22, 52–67. [Google Scholar] [CrossRef]
- Lamalakshmi Devi, E.; Kumar, S.; Basanta Singh, T.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A. Adaptation strategies and defence mechanisms of plants during environmental stress. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 359–413. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Sims, D.A. Photosynthetic acclimation to changing light environments: Scaling from the leaf to the whole plant. In Exploitation of Environmental Heterogeneity by Plants. Ecophysiological Processes Above-and Belowground; Academic Press: Cambridge, MA, USA, 1994; pp. 145–174. [Google Scholar] [CrossRef]
- Kong, Y.; Ratner, K.; Avraham, L.; Shahak, Y. Pearl netting improves photosynthetic light use associated with modification of structural and physiochemical traits of sweet pepper leaves. Acta Hortic. 2015, 1170, 337–344. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, S.; Wang, Z.; Liu, Z.; Yao, Y. Low light stress on the growth, development and photosynthetic characters of peach tree. Chin. Agric. Sci. Bull. 2009, 25, 139–142. (In Chinese) [Google Scholar]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.; Visser, R.G.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L. The power of plants: How fruit and vegetables work as source of nutraceuticals and supplements. Int. J. Food Sci. Nutr. 2021, 72, 660–664. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Bergstrand, K.; Asp, H.; Schüssler, H. Development and acclimatisation of horticultural plants subjected to narrow-band lighting. Eur. J. Hortic. Sci 2014, 79, 45–51. [Google Scholar]
- Kong, Y.; Llewellyn, D.; Zheng, Y. Response of growth, yield, and quality of pea shoots to supplemental light-emitting diode lighting during winter greenhouse production. Can. J. Plant Sci. 2018, 98, 732–740. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Response of growth, yield, and quality of edible-podded snow peas to supplemental LED lighting during winter greenhouse production. Can. J. Plant Sci. 2019, 99, 676–687. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily light integral: A research review and high-resolution maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition—A historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Lanoue, J.; Thibodeau, A.; Little, C.; Zheng, J.; Grodzinski, B.; Hao, X. Light Spectra and Root Stocks Affect Response of Greenhouse Tomatoes to Long Photoperiod of Supplemental Lighting. Plants 2021, 10, 1674. [Google Scholar] [CrossRef] [PubMed]
- Lanoue, J.; Zheng, J.; Little, C.; Grodzinski, B.; Hao, X. Continuous light does not compromise growth and yield in mini-cucumber greenhouse production with supplemental LED light. Plants 2021, 10, 378. [Google Scholar] [CrossRef]



| Macroelement (mM) | Microelement (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | Fe | B | Mn | Zn | Cu | Mo |
| 10.7 | 1.13 | 5.38 | 3.25 | 1.44 | 1.44 | 53.7 | 27.8 | 6.0 | 1.83 | 1.10 | 0.63 |
| LED for Pre-Harvest SL | Total PFD (μmol·m−2·s−1) | PFD Proportion (%) in Different Wavebands | ||||
|---|---|---|---|---|---|---|
| 340–399 (nm) | 400–499 (nm) | 500–599 (nm) | 600–699 (nm) | 700–799 (nm) | ||
| RB 1 | 167.2 | 0 | 57 | 0 | 43 | 0 |
| B/UVA | 167.4 | 27 | 73 | 0 | 0 | 0 |
| W | 167.8 | 0 | 19 | 38 | 40 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality. Horticulturae 2022, 8, 435. https://doi.org/10.3390/horticulturae8050435
Hooks T, Sun L, Kong Y, Masabni J, Niu G. Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality. Horticulturae. 2022; 8(5):435. https://doi.org/10.3390/horticulturae8050435
Chicago/Turabian StyleHooks, Triston, Ling Sun, Yun Kong, Joseph Masabni, and Genhua Niu. 2022. "Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality" Horticulturae 8, no. 5: 435. https://doi.org/10.3390/horticulturae8050435
APA StyleHooks, T., Sun, L., Kong, Y., Masabni, J., & Niu, G. (2022). Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality. Horticulturae, 8(5), 435. https://doi.org/10.3390/horticulturae8050435







