Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carrier Characterization and Preparation
2.2. Bacterial Characterization and Plant Growth Promoting Properties
2.3. Biofertilizer Preparation
2.4. Survival Test of Strain L2 and A30 in Carrier Materials
2.5. Plant Development Assay
2.6. Statistical Analyses
3. Results
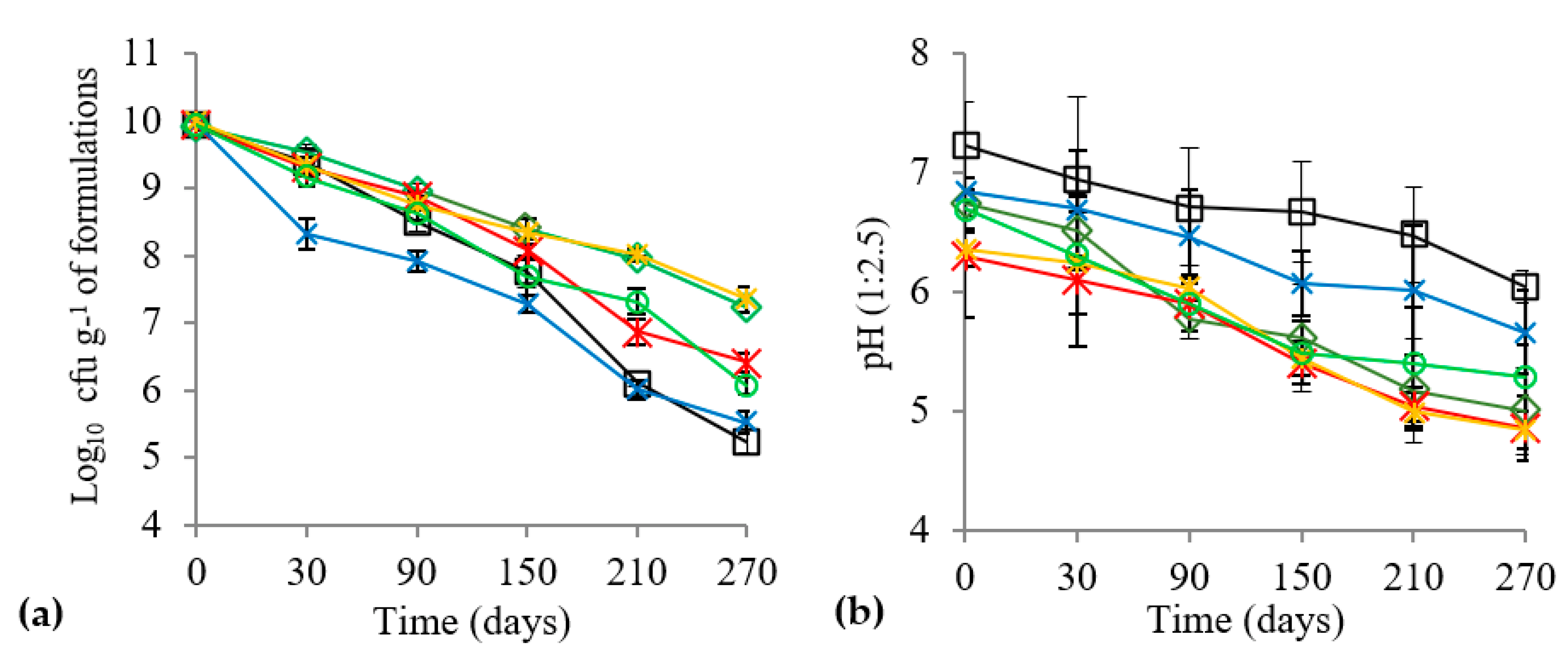
3.1. Carrier Characterization and Shelf Life, pH and MC of Biofertilizers
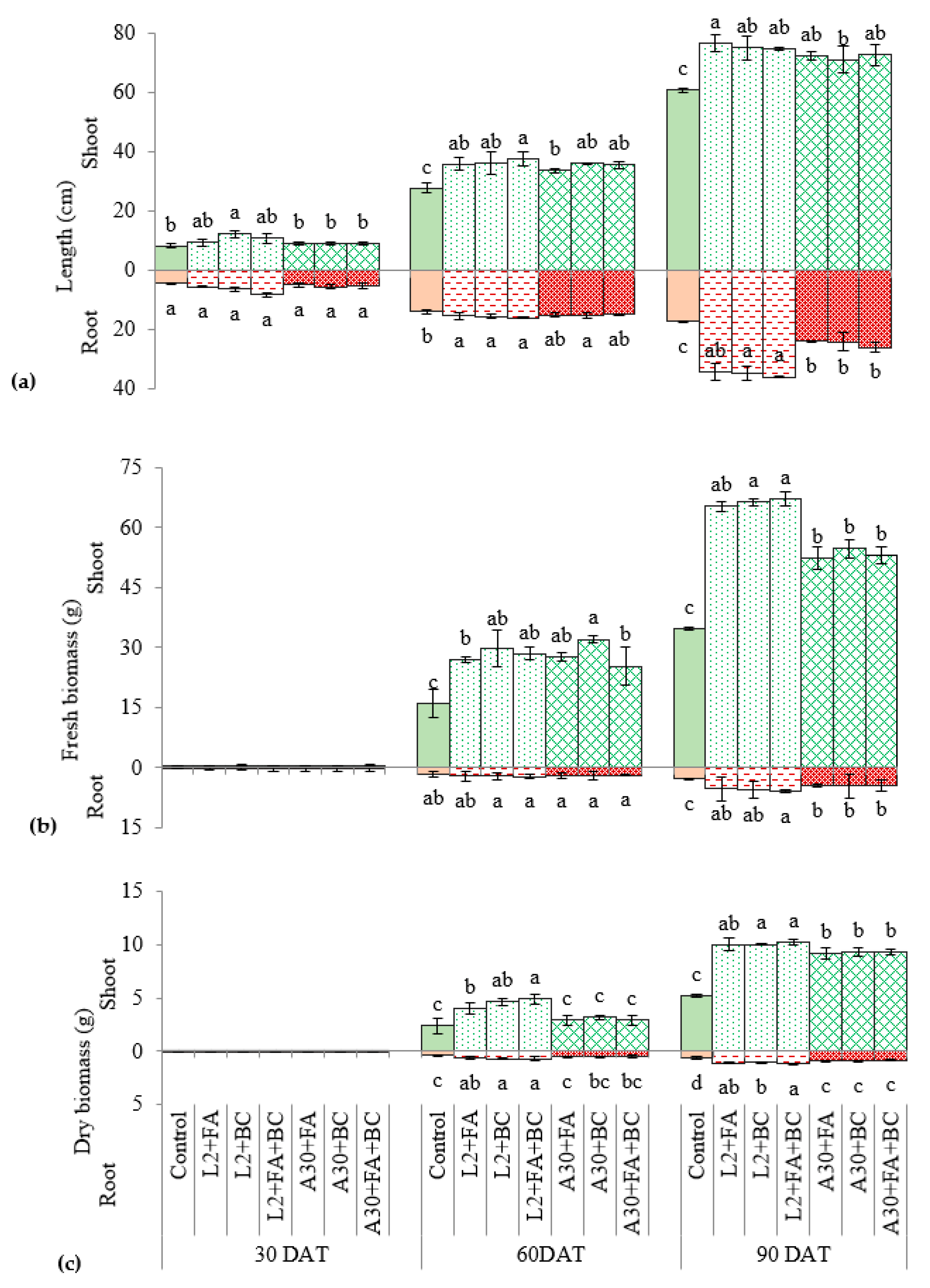
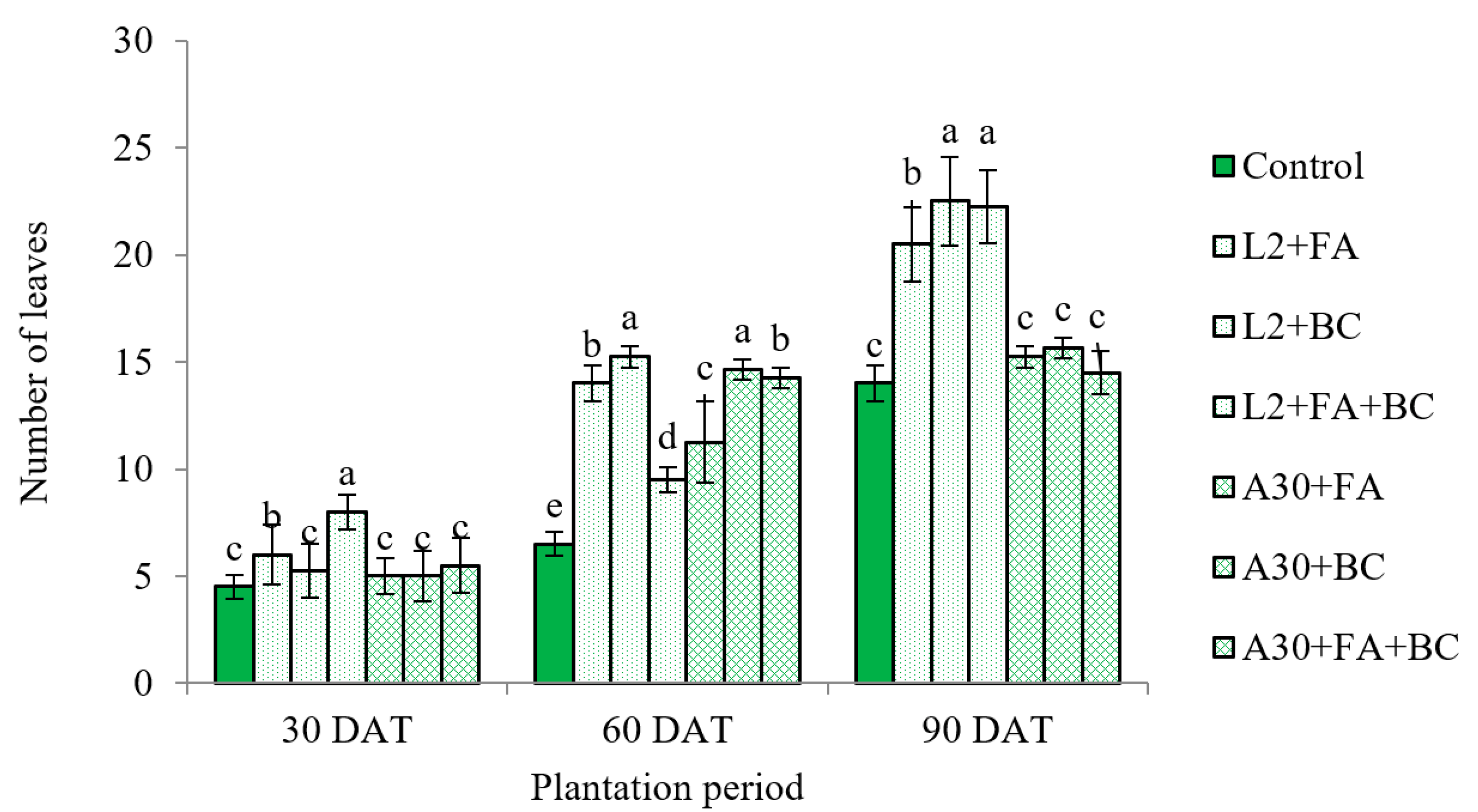
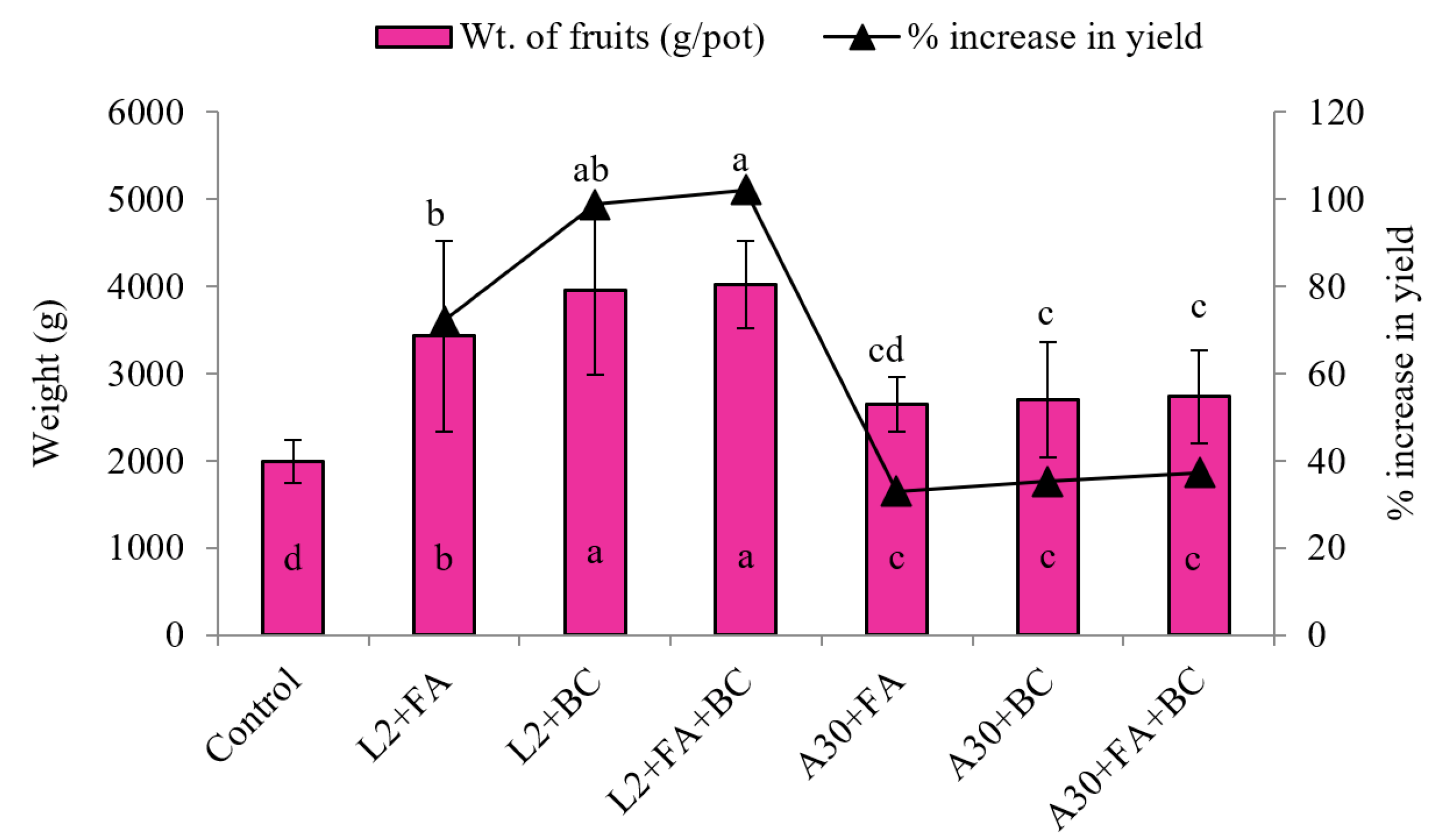
3.2. Biometric Growth Parameters
4. Discussion
4.1. Shelf Life of Biofertilizer
4.2. Plant Development and Fruit Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of Pesticides on Environmental and Human Health. In Toxicology Studies—Cells, Drugs and Environment; Andreazza, A.C., Scola, G., Eds.; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Tripti; Voropaeva, O.; Maleva, M.; Panikovskaya, K.; Borisova, G.; Rajkumar, M.; Bruno, L.B. Bioaugmentation with copper tolerant endophyte Pseudomonas lurida strain EOO26 for improved plant growth and copper phytoremediation by Helianthus annuus. Chemosphere 2021, 266, 128983. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Oliveira, R.S.; Nai, F.; Rajkumar, M.; Luo, Y.; Rocha, I.; Freitas, H. The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. J. Environ. Manag. 2015, 156, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, D.K.; Dubey, R.; Agarwal, M.; Dheeman, S.; Aeron, A.; Bajpai, V.K. Carrier based formulations of biocoenotic consortia of disease suppressive Pseudomonas aeruginosa KRP1 and Bacillus licheniformis KRB1. Ecol. Eng. 2015, 81, 272–277. [Google Scholar] [CrossRef]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Lowe, A.; Rafferty-McArdle, S.M.; Cassells, A.C. Effects of AMF- and PGPR-root inoculation and a foliar chitosan spray in single and combined treatments on powdery mildew disease in strawberry. Agric. Food Sci. 2012, 21, 28–38. [Google Scholar] [CrossRef]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Alam Khan, M.; Mohi-Ud-Din, M.; Miah, G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef] [Green Version]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.; Xiao, Y.; Wass, T.J.; Schenk, P. Identification of Soil Bacterial Isolates Suppressing Different Phytophthora spp. and Promoting Plant Growth. Front. Plant Sci. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A. Recovery of plant growth-promoting rhizobacteria from sodium alginate beads after 3 years following storage at 4 °C. J. Ind. Microbiol. Biotechnol. 2008, 35, 205–209. [Google Scholar] [CrossRef]
- Saharan, K.; Sarma, M.; Srivastava, R.; Sharma, A.; Johri, B.; Prakash, A.; Sahai, V.; Bisaria, V. Development of non-sterile inorganic carrier-based formulations of fluorescent pseudomonad R62 and R81 and evaluation of their efficacy on agricultural crops. Appl. Soil Ecol. 2010, 46, 251–258. [Google Scholar] [CrossRef]
- Sun, D.; Hale, L.; Crowley, D. Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol. Fertil. Soils 2016, 52, 515–522. [Google Scholar] [CrossRef]
- Danso, S.K.A.; Keya, S.O.; Alexander, M. Protozoa and the decline of Rhizobium populations added to soil. Can. J. Microbiol. 1975, 21, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Tamreihao, K.; Ningthoujam, D.S.; Nimaichand, S.; Singh, E.S.; Reena, P.; Singh, S.H.; Nongthomba, U. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol. Res. 2016, 192, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Trevors, J.T.; Jain, D.; Wolters, A.C.; Heijnen, C.E.; Van Overbeek, L.S. Survival of, and root colonization by, alginate-encapsulated Pseudomonas fluorescens cells following introduction into soil. Biol. Fertil. Soils 1992, 14, 14–22. [Google Scholar] [CrossRef]
- Nordin, N.; Abdullah, M.M.A.B.; Tahir, M.F.M.; Sandu, A.V.; Hussin, K. Utilization of Fly Ash Waste As Construction Material. Int. J. Conserv. Sci. 2016, 7, 161–166. [Google Scholar]
- Lokeshappa, B.; Dikshit, A.K. Disposal and management of flyash. In Proceedings of the 2011 International Conference on Life Science and Technology, Mumbai, India, 7–9 January 2011; Volume 3, pp. 11–14. [Google Scholar]
- Dhadse, S.; Kumari, P.; Bhagia, L.J. Fly ash characterization, utilization and Government initiatives in India-A review. J. Sci. Ind. Res. 2008, 67, 11–18. [Google Scholar]
- Naval, A.; Gajre, G.; Mishra, S.; Dixit, A.D. Fly ash: A Suitable Material for Solid Waste Management through Vermicomposting. In Waste to Resource Material; Indian Institute of Environment Management: Maharashtra, India, 2014. [Google Scholar]
- Sanou, I.; Seynou, M.; Zerbo, L.; Ouedraogo, R. Mineralogy, Physical and Mechanical Properties of Adobes Stabilized with Cement and Rice Husk Ash. Sci. J. Chem. 2019, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Ahirwal, J.; Maiti, S.K.; Das, R. An assessment of metal in fly ash and their translocation and bioaccumulation in perennial grasses growing at the reclaimed opencast mines. Int. J. Environ. Res. 2015, 9, 1089–1096. [Google Scholar]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements—A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef] [Green Version]
- Zafar-Ul-Hye, M.; Tahzeeb-Ul-Hassan, M.; Abid, M.; Fahad, S.; Brtnicky, M.; Dokulilova, T.; Datta, R.; Danish, S. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020, 10, 12159. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-Ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, H.; Wu, H. Biochar as a Fuel: 1. Properties and Grindability of Biochars Produced from the Pyrolysis of Mallee Wood under Slow-Heating Conditions. Energy Fuels 2009, 23, 4174–4181. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Lehmann, J.; Engelhard, M. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Erratum: Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, S.A.; Doyle, R.; Bowman, J. Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- De La Rosa, J.M.; Paneque, M.; Hilber, I.; Blum, F.; Knicker, H.E.; Bucheli, T.D. Assessment of polycyclic aromatic hydrocarbons in biochar and biochar-amended agricultural soil from Southern Spain. J. Soils Sediments 2016, 16, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Jambhulkar, H.P.; Shaikh, S.M.S.; Kumar, M.S. Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: A review. Chemosphere 2018, 213, 333–344. [Google Scholar] [CrossRef]
- Feng, L.; Roughley, R.J.; Copeland, L. Morphological Changes of Rhizobia in Peat Cultures. Appl. Environ. Microbiol. 2002, 68, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Tripti; Kumar, A.; Usmani, Z.; Kumar, V.; Anshumali. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J. Environ. Manag. 2017, 190, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wijewardana, C.; Reddy, K.R.; Krutz, L.J.; Gao, W.; Bellaloui, N. Drought stress has transgenerational effects on soybean seed germination and seedling vigor. PLoS ONE 2019, 14, e0214977. [Google Scholar] [CrossRef] [Green Version]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A Rapid Procedure for the Estimation of Available N in the Soils. Curr. Sci. 1956, 25, 259. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Tripti; Kumar, A.; Kumar, V.; Anshumali. Effect of commercial pesticides on plant growth-promoting activities of Burkholderiasp. strain L2 isolated from rhizosphere of Lycopersicon esculentum cultivated in agricultural soil. Toxicol. Environ. Chem. 2015, 97, 1180–1189. [Google Scholar] [CrossRef]
- Catroux, G.; Hartmann, A.; Revellin, C. Trends in rhizobial inoculant production and use. Plant Soil 2001, 230, 21–30. [Google Scholar] [CrossRef]
- Hume, D.J.; Blair, D.H. Effect of numbers of Bradyrhizobium japonicum applied in commercial inoculants on soybean seed yield in Ontario. Can. J. Microbiol. 1992, 38, 588–593. [Google Scholar] [CrossRef]
- Rasool, M.; Akhter, A.; Soja, G.; Haider, M.S. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021, 11, 6902. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S.G. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiato, R.A.; Yuhan, S.; Allen, M.; Zhao, Q. Integrated Processes for Producing Fuels and Biofertilizers from Biomass and Products Produced. U.S. Patent 20140345341 A, 14 June 2014. [Google Scholar]
- Masto, R.E.; Ansari, M.A.; George, J.; Selvi, V.A.; Ram, L.C. Co-application of biochar and lignite fly ash on soil nutri-ents and biological parameters at different crop growth stages of Zea mays. Ecol. Engg. 2013, 58, 314–322. [Google Scholar] [CrossRef]
- Gaind, S.; Gaur, A.C. Evaluation of fly ash as a carrier for diazotrophs and phosphobacteria. Bioresour. Technol. 2004, 95, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Chu, C.; Xu, Z. Effects of different fertilizer formulas on the growth of loquat rootstocks and stem lignification. Sci. Rep. 2020, 10, 1033. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Kushwaha, C.; Tripathi, S.; Singh, K. Soil organic matter and water-stable aggregates under different tillage and residue conditions in a tropical dryland agroecosystem. Appl. Soil Ecol. 2001, 16, 229–241. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, T.Q.; Tan, C.S. Processing tomato phosphorus utilization and post-harvest soil profile phosphorus as affected by phosphorus and potassium additions and drip irrigation. Can. J. Soil Sci. 2011, 91, 417–425. [Google Scholar] [CrossRef]
- Chinnusamy, M.; Kaushik, B.D.; Prasanna, R. Growth, Nutritional, and Yield Parameters of Wetland Rice as Influenced by Microbial Consortia Under Controlled Conditions. J. Plant Nutr. 2006, 29, 857–871. [Google Scholar] [CrossRef]
- Aseri, G.K.; Tarafdar, J.C. Fluorescein Diacetate: A Potential Biological Indicator for Arid Soils. Arid Land Res. Manag. 2006, 20, 87–99. [Google Scholar] [CrossRef]
| Treatment | Composition |
|---|---|
| Control | Soil |
| L2 + FA | Flyash + Soil + Burkholderia sp. LRS02 |
| L2 + BC | Biochar + Soil + Burkholderia sp. LRS02 |
| L2 + FA + BC | Flyash + biochar (1:1) + Soil + Burkholderia sp. LRS02 |
| A30 + FA | Flyash + Soil + Bacillus sp. A30 |
| A30 + BC | +Biochar + Soil+ Bacillus sp. A30 |
| A30 + FA + BC | Soil + Flyash + biochar (1:1) + Bacillus sp. A30 |
| Properties | Flyash | Biochar | Flyash + Biochar * |
|---|---|---|---|
| Physico-chemical characteristics | |||
| MC (%) | 2.3 ± 0.21 | 15.30 ± 0.87 | 10.2 ± 0.47 |
| WHC (%) | 60.9 ± 1.05 | 80.25 ± 2.10 | 64.28 ± 3.24 |
| pH (1:2.5; w/v) | 6.57 ± 0.91 | 7.28 ± 0.32 | 7.03 ± 0.29 |
| EC (dS m−1) | 0.43 ± 0.05 | 0.33 ± 0.05 | 0.39 ± 0.03 |
| DTPA extractable heavy metals (mg kg−1) | |||
| Pb | 3.28 ± 0.05 | 6.82 ± 0.21 | 4.99 ± 1.21 |
| Zn | 15.91 ± 0.09 | 15.02 ± 1.10 | 15.19 ± 1.93 |
| Cu | 7.10 ± 0.05 | 2.15 ± 0.93 | 3.55 ± 0.79 |
| Fe | 9.16 ± 0.06 | 7.08 ± 1.47 | 8.20 ± 1.20 |
| Mn | 5.12 ± 0.19 | 0.29 ± 0.19 | 4.60 ± 0.33 |
| Ni | 0.20 ± 0.03 | <dl | <dl |
| Cd | 0.05 ± 0.01 | <dl | <dl |
| Co | 0.10 ± 0.03 | 1.54 ± 0.23 | 0.31 ± 0.12 |
| Treatments | Seed Germination (%) | Seed Germination Increased over Control (%) | Rate of Seed Germination * |
|---|---|---|---|
| Control | 75.56 ± 5.25 d | - | 7.30 ± 0.80 c |
| L2 + FA | 83.33 ± 2.80 bc | 10.29 | 8.64 ± 0.37 ab |
| L2 + BC | 87.78 ± 3.50 a | 16.18 | 9.08 ± 0.50 a |
| L2 + FA + BC | 88.89 ± 3.24 a | 17.65 | 8.91 ± 0.69 a |
| A30 + FA | 84.44 ± 6.85 b | 11.76 | 7.67 ± 0.30 bc |
| A30 + BC | 84.44 ± 6.85 b | 11.76 | 8.39 ± 0.54 abc |
| A30 + FA + BC | 82.22 ± 4.60 c | 8.82 | 7.94 ± 0.76 abc |
| Treatments | MC (%) | WHC (%) | pH (1:2.5; w/v) | EC (dS m−1) | OC (%) | Avl. P (mg kg−1) | Avi. N (mg kg−1) | DHA (μg TPF g−1 h−1) |
|---|---|---|---|---|---|---|---|---|
| Control | 11.56 ± 1.05 | 49.13 ± 9.94 | 6.60 ± 0.10 | 0.56 ± 0.03 | 0.96 ± 0.03 | 1.59 ± 0.13 | 92.40 ± 3.70 | 3.85 ± 0.33 |
| L2 + FA | 12.98 ± 0.79 | 53.66 ± 19.53 | 6.40 ± 0.10 | 0.63 ± 0.03 | 1.11 ± 0.08 | 2.76 ± 0.19 | 103.13 ± 9.32 | 4.56 ± 0.26 |
| L2 + BC | 11.83 ± 0.97 | 58.07 ± 24.03 | 6.30 ± 0.10 | 0.64 ± 0.02 | 1.04 ± 0.14 | 3.29 ± 0.29 | 118.07 ± 4.04 | 5.27 ± 0.16 |
| L2 + FA + BC | 12.90 ± 0.34 | 59.22 ± 9.76 | 6.25 ± 0.06 | 0.66 ± 0.02 | 1.15 ± 0.11 | 3.19 ± 0.31 | 110.60 ± 4.20 | 5.38 ± 0.47 |
| A30 + FA | 13.87 ± 0.21 | 52.35 ± 26.27 | 6.32 ± 0.03 | 0.65 ± 0.05 | 1.09 ± 0.05 | 2.83 ± 0.16 | 103.60 ± 12.60 | 5.24 ± 0.75 |
| A30 + BC | 12.94 ± 1.29 | 55.36 ± 11.60 | 6.20 ± 0.10 | 0.64 ± 0.01 | 1.19 ± 0.06 | 3.04 ± 0.11 | 110.60 ± 6.10 | 5.20 ± 0.30 |
| A30 + FA + BC | 12.10 ± 1.88 | 54.26 ± 22.11 | 6.42 ± 0.11 | 0.63 ± 0.03 | 1.16 ± 0.11 | 2.65 ± 0.23 | 112.47 ± 5.83 | 4.67 ± 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripti; Kumar, A.; Kumar, V.; Anshumali; Bruno, L.B.; Rajkumar, M. Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant. Horticulturae 2022, 8, 444. https://doi.org/10.3390/horticulturae8050444
Tripti, Kumar A, Kumar V, Anshumali, Bruno LB, Rajkumar M. Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant. Horticulturae. 2022; 8(5):444. https://doi.org/10.3390/horticulturae8050444
Chicago/Turabian StyleTripti, Adarsh Kumar, Vipin Kumar, Anshumali, L. Benedict Bruno, and Mani Rajkumar. 2022. "Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant" Horticulturae 8, no. 5: 444. https://doi.org/10.3390/horticulturae8050444
APA StyleTripti, Kumar, A., Kumar, V., Anshumali, Bruno, L. B., & Rajkumar, M. (2022). Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant. Horticulturae, 8(5), 444. https://doi.org/10.3390/horticulturae8050444







