Abstract
To explore the effect of bagging on the nutritional quality and color of kiwifruit (Actinidia spp.), the fruits of yellow-fleshed kiwifruit cultivars were analyzed after bagging treatment. Bagging treatment promoted the degreening of mesocarp and increased brightness. Bagging significantly reduced the accumulation of dry matter, titratable acids, starch, sucrose, fructose, and glucose during kiwifruit development. Additionally, bagging significantly reduced the accumulation of chlorophyll and carotenoids during development, whereas after debagging, the chlorophyll and carotenoid contents were significantly increased. Gene expression analysis showed that during most of the fruit development periods, the chlorophyll biosynthesis genes AcRCBS, AcGLUTR, and AcCHLG, and degradation genes AcCBR, AcPAO, AcPPH, AcCLH, and AcSGR had significantly lower expression levels in bagged fruit. Bagging also inhibited the expression of carotenoid metabolism genes, especially AcSGR and AcLCYB, which may play a key role in the process of fruit development during bagging by decreasing the accumulation of chlorophyll and carotenoids in kiwifruit. Additionally, bagging significantly reduced the content of AsA. The expression of the AsA biosynthesis genes AcPMI2, AcGPP2, and AcGalDH in bagged fruit was significantly lower than in the control, indicating that these may be the key genes responsible for the difference in the accumulation of AsA after bagging.
1. Introduction
Bagging is an important cultivation method for improving the appearance of fruits, effectively avoiding fruit surface pollution, and reducing contamination with residues of pesticides, which are currently used in diverse fruits, such as apples [1], peaches [2], grapes [3], and kiwifruit (Actinidia spp.) [4]. Bagging can regulate fruit development by altering the fruit microenvironment, such as light quality and temperature, to affect fruit appearance and flavor factors [5].
Fruit color is an important component of fruit quality that mainly depends on chlorophyll, carotenoids, and anthocyanins [6]. In many fruits, such as peppers [7], carrots [8] and oranges [9], the yellow color of the pericarp is caused by carotenoids. During the development of yellow-fleshed kiwifruit, chlorophyll is degraded, causing the green color to fade and the carotenoid color to be revealed [6]. The metabolic pathways of chlorophyll and carotenoid metabolism in kiwifruit have been studied. The metabolism of chlorophyll mainly depends on the catalysis of enzymes such as ribulose-1,5-bisphosphatecarboxylase (RBCS), glutamyl tRNA reductase (GLUTR), chlorophyll synthase (CHLG), chlorophyll b reductase (CBR), pheophorbide a oxygenase (PAO), chlorophyllase (CLH), and pheophytin pheophorbide hydrolase (PPH) [10,11]. The yellowness of kiwifruit results from the degradation of chlorophylls under the control of STAY-GREEN (SGR) [6]. The metabolism of carotenoids mainly depends on the catalysis of enzymes such as phytoene synthase (PSY), phytoene desaturase (PDS), carotenoid isomerase (CRTISO), zetacarotene desaturase (ZDS), lycopene ε-cyclase (LCYE), lycopene β-cyclase (LCYB), cytochrome P450 β-ring carotenoid hydroxylase (CYP97A), cytochrome P450 ε-ring carotenoid hydroxylase (CYP97C), β-carotene hydroxylase (CHYB), zeaxanthin epoxidase (ZEP), violaxanthin de-epoxidase (VDE), carotenoid cleavage dioxygenases (CCD), and 9-cis-epoxycarotenoid dioxygenase (NCED) [12]. Studies have shown that bagging affects the color of fruits. Bagging can significantly reduce the content of chlorophyll and carotenoids in kiwifruits [4]. However, the mechanism by which bagging affects chlorophyll and carotenoid metabolism in kiwifruits remains unclear.
Ascorbic acid (AsA), also known as vitamin C, is essential for normal human growth and development [13]. However, as the human body cannot synthesize AsA, it can only be obtained from the diet, and fresh fruit is one of the most important sources of AsA [13]. Research on the biosynthetic pathways of AsA in plants has made great progress. Four possible pathways for the biosynthesis of AsA in plants have been proposed [14,15], among which the L-galactose pathway is considered to play a dominant role, with the other pathways being complementary to AsA biosynthesis [16]. AsA biosynthesis mainly depends on the catalysis of some enzymes, such as glucose-6-phosphate isomerase (PGI), phosphomannose isomerase (PMI), phosphomannomutase (PMM), GDP-mannose pyrophosphorylase (GMP), GDP-mannose-3,5-epimerase (GME), GDP-L-galactose-1-phosphorylase (GGP), L-galactose-1-phosphatase (GPP), L-galactose dehydrogenase (GalDH), and L-galactono-1,4-lactone dehydrogenase (GalLDH) [17]. AsA biosynthesis requires light, and the process would be blocked by shading, resulting from bagging or exposure to strong light [18].
Kiwifruit is rich in nutrients (e.g., AsA, dietary fiber, carotenoids, flavonoids, phenolics, and minerals), has a regular shape and a pleasant flavor, making it popular among consumers worldwide [19]. During the growth and development of kiwifruit, it is highly susceptible to infection by the pathogenic microorganisms present in the environment, resulting in a decline of fruit quality [20]. Therefore, bagging, which reduces exposure to pathogens, is an important kiwifruit-cultivation measure that helps to preserve the appearance of fruits, reduce pesticide pollution, and improve the commerciality of fruits [21,22]. However, while bagging improves the appearance of the fruit, it also has an adverse effect on its quality [23]. In this study, yellow-fleshed kiwifruit (A. chinensis) was used to determine the effects of bagging and debagging on the external and internal quality of the fruit, as well as transcript levels of key genes involved in chlorophyll and carotenoid metabolism, to lay a foundation for analyzing the effects of light on the molecular mechanism of chlorophyll and carotenoid metabolism in kiwifruit.
2. Materials and Methods
2.1. Plant Materials and Treatments
The experiment was conducted in a commercial orchard (287° N, 115.38° E) in Fengxin County, Jiangxi Province, China. The 6-year-old ‘Jinyan’ kiwifruit (A. chinensis Planch.) grafted onto a rootstock of A. arguta (Siebold et Zucc.) Planch. ex Miq. was selected as the research subjects. Eighteen vines in the full bearing period were selected for bagging treatments. The vines were put into three treatment groups with six trees in each. The following treatments were carried out: (1) no bagging (control); (2) single fruit bagging performed 30 days after anthesis, followed by debagging at 90 days after bagging; (3) single fruit bagging performed 30 days after anthesis until harvesting. About 30 fruitlets from each vine (a total of 540 fruits) were bagged, and 10 fruits from each vine (a total of 180 fruits) were debagged at 90 days after bagging. The bags were 3 mm-thick double-layer opaque paper bags with black inside and yellow outside, and the specification was 16 cm (length) × 12 cm (width) (Guokang, Pingdu, China). Each treatment group was sampled at 0, 30, 60, 90, 105, 120, and 135 days after bagging, and two fruits from four directions (east, west, north, and south) and three biological replicates for a total of 24 fruits were collected and mixed per each treatment plot. All fruits were taken to the laboratory without delay, and the pulp after was frozen with liquid nitrogen for three biological replicates after measuring the color difference and stored at −80 °C.
2.2. Color, Starch, and Dry-Matter Determination
A portable colorimeter CR-400 (Konica Minolta Sensing, Inc., Osaka, Japan) was used to detect the color differences. For epicarp color, the fruits from sampling point were randomly selected and tested at four locations around the equatorial plane of the fruits. For mesocarp color, fruits from sampling point were cut horizontally to determine the chroma of fruits at the inner equator. Eight fruits were determined each time and three biological replicates were performed. The a*, b*, and L* values were recorded, with a* and b* representing the chromaticity components (a*, negative to positive corresponding to a shift from green to red; b*, negative to positive corresponding to a shift from blue to yellow, respectively) and L* representing the brightness of the color (negative to positive corresponding to a shift from dark to light).
Total starch content in 0.1 g of frozen samples based on absorbance at 620 nm was measured using a starch-content kit (No. A148-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Starch content was measured in three biological replicates and expressed as mg g−1 fresh weight (FW).
Dry-matter content was determined using the drying method. After measuring the color difference, 1/4 pulp was taken from each of the 8 fruits and mixed together, and repeated 3 times. The samples were dried in an oven at 105 °C until a constant weight was achieved. Dry-matter content (%) = fruit dry weight (g)/fruit fresh weight (g) × 100, with measurements repeated three times.
2.3. Measurement of Chlorophyll, Total Carotenoid, and AsA Content
Approximately 1.0 g of kiwifruit pulp was used for extraction by grinding to a fine powder with liquid nitrogen and then transferring the powder to a 10 mL centrifuge tube. The powder was mixed with 5.0 mL of ice-cold 80% acetone containing 0.1% butylated hydroxytoluene (BHT). The mixture was incubated in the dark for 60 min until the pulp turned white. The mixture was then centrifuged at 12,000× g for 10 min at 4 °C, and the supernatant was collected for use.
Chlorophyll content was measured using 80% acetone solution as the blank control for zeroing, and the absorbance of the extracted solution was then determined by colorimetry at wavelengths of 663 nm and 646 nm in a 1 cm light diameter cuvette using a spectrophotometer (Thermo Scientific GENESYS 40, Waltham, MA, USA). Chlorophyll content was calculated as follows: Chlorophyll a = (12.21 A663 − 2.81 A646) × V/(W × 1000); Chlorophyll b = (20.13 A646 − 5.03 A646) × V/(W × 1000); Total chlorophyll = Chlorophyll a + Chlorophyll b. The values of A663 and A646 represent absorbance at 663 nm and 646 nm, respectively, and V and W indicate the total volume of the extract (mL) and the fresh weight of the sample (g). The chlorophyll content was expressed as mg g−1.
Total carotenoid content was determined based on the absorbance of the extract at a wavelength of 450 nm. Total carotenoid content was calculated as follows: Carotenoid = A450 × 1000 × V/(W × 250). The value of A450 represents absorbance at 450 nm, and V and W indicate the total volume of the extract (mL) and the fresh weight of the sample (g), respectively. The carotenoid content was expressed as mg g−1.
The AsA content was measured following the methods described in a previous report, with some modifications [24]. Approximately 1.0 g of kiwifruit pulp was ground to a fine powder with liquid nitrogen and then transferred to a 50 mL centrifuge tube. The powder was mixed with 20.0 mL of a 0.05% (w/v) oxalic-acid solution. The mixture was incubated for 10 min and centrifuged at 10000× g for 15 min. The supernatant was then collected. The supernatant was diluted with 0.05% (w/v) oxalic-acid solution to obtain a 0.5 mg/mL extract for the determination of AsA. The AsA content of kiwifruit was determined by high-performance liquid chromatography (HPLC) (Agilent HPLC 1100, Agilent, Palo Alto, CA, USA) using a C18 column (250 mm × 4.6 mm, 5 μm, Waters). The mobile phase consisted of 0.05% (w/v) oxalic-acid aqueous solution and methanol (98:2), the column temperature was 25 °C, and the flow rate was 0.7 mL/min. L-Ascorbic acid (SV8120, Solarbio, Beijing, China) was used as the standard. The AsA content was identified by comparing the retention times of the peaks with those of the external standard, and quantification was performed using standard calibration curves.
2.4. Gene Expression Analysis
Approximately 0.5 g of kiwifruit pulp was ground to a fine powder with liquid nitrogen. Total RNA was extracted according to the manual of the Quick-RNA isolation kit (Huayueyang, Beijing, China). The concentration of the extracted RNA was determined using a BioDrop spectrophotometer (Bio-Rad, Hercules, CA, USA). Extracted RNA (1 μg) was reverse-transcribed into cDNA using the Hifair® II 1st-strand cDNA synthesis kit (Yeasen, Shanghai, China). Quantitative real-time polymerase chain-reaction (qRT-PCR) analysis was performed using the TB Green Master Mix (RR420A, Takara, Dalian, China) on a CFX96 Touch Real-time PCR instrument (Bio-Rad, Hercules, CA, USA). Each reaction was carried out in triplicate using a reaction volume of 20 μL containing 2.0 μL of cDNA, 0.3 μL of each gene-specific primer (1 μM, Table S1), 10 μL of TB Green Premix Ex Taq, and 7.4 μL of sterile distilled water. The reaction conditions were as follows: 95 °C for 1 min, followed by 40 cycles of 15 s at 95 °C and 25 s at 63 °C. Product accumulation was determined using fluorescence measurement during a 55–95 °C melting-curve analysis. The relative expression of genes was normalized to actin and calculated using the 2−ΔΔCt method [24].
2.5. Statistical Analysis
Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce variance. All results are presented as the mean ± standard error (SE). Data were analyzed and drawn using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). The differences in mean between individual treatments were analyzed by one-way ANOVA, followed by paired-samples t-test using significance thresholds of p < 0.05 or p < 0.01.
3. Results
3.1. Effect of Bagging on the Color of Kiwifruit
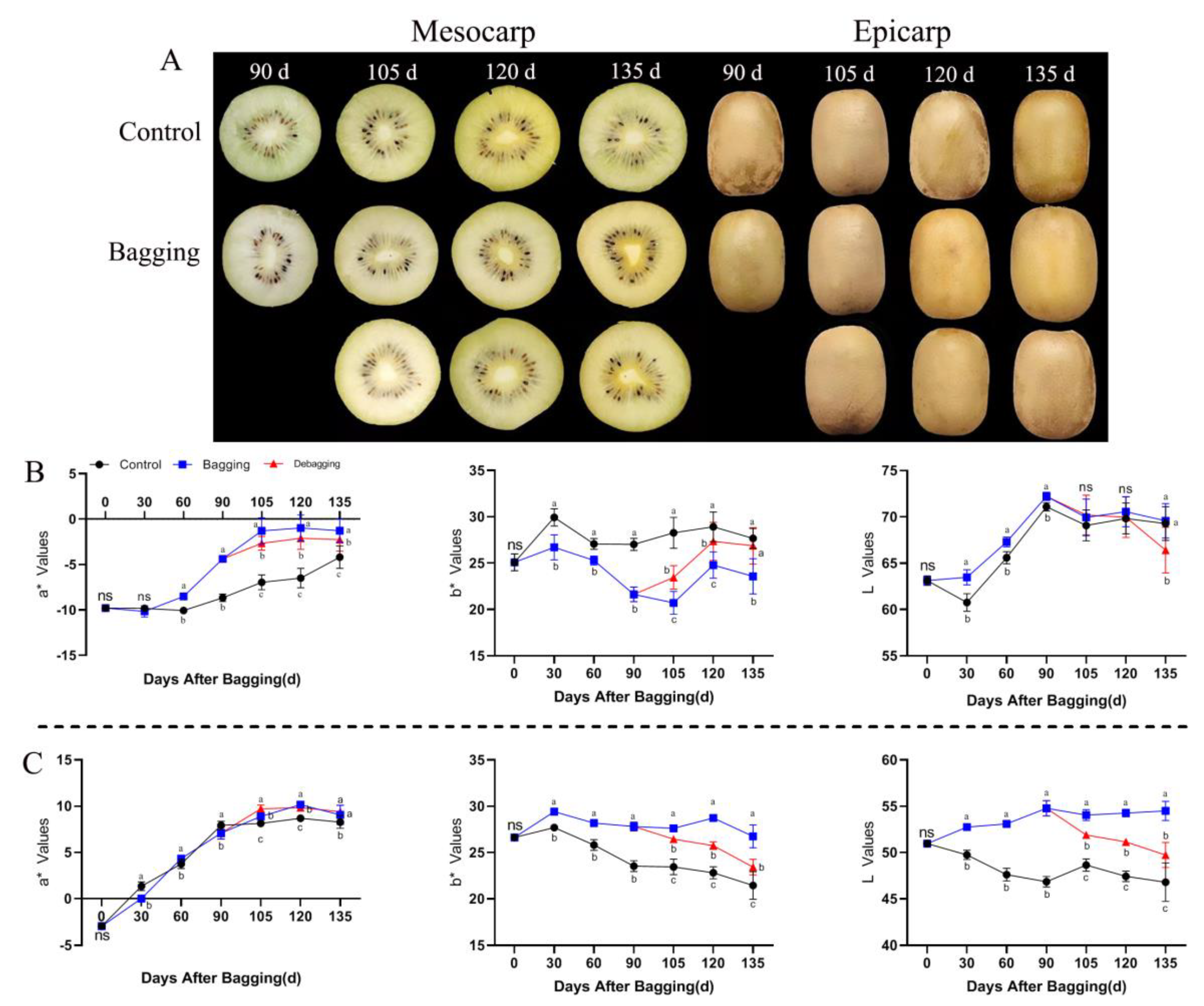
During the growth and development of the yellow-fleshed kiwifruit (‘Jinyan’), the color of the mesocarp and epicarp differed between the bagging and non-bagging treatment groups (Figure 1A). The value of a* showed an upward trend during fruit development, indicating that the greenness of the fruit decreased gradually with fruit growth and development. Bagging affected the a* value during the nondevelopmental period, especially in the mesocarp. The a* value was significantly increased by bagging, and the a* value decreased after debagging, indicating that bagging affects the green appearance of the mesocarp. During the entire fruit development period, bagging decreased the b* value of mesocarp but increased the b* value of the epicarp, indicating that bagging reduced and increased the yellow appearance of the mesocarp and epicarp, respectively. Bagging increased the L value of the mesocarp and epicarp, indicating that the brightness of the fruit improved in response to bagging (Figure 1B,C).
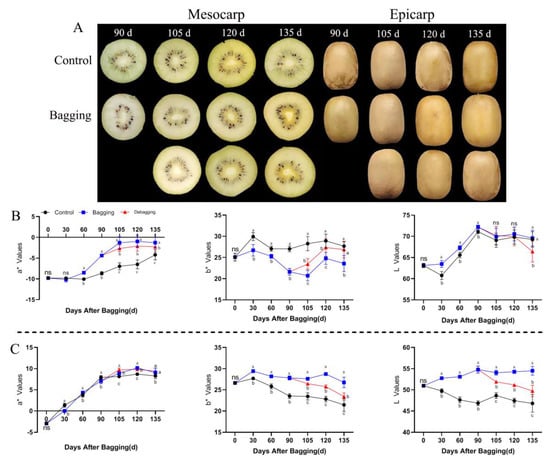
Figure 1.
Effects of bagging on the color change of kiwifruit during development. (A) Appearance of mesocarp and epicarp with three treatments during kiwifruit development. (B,C) Changes in a*, b*, and L values of different treatments in mesocarp (B) and epicarp (C) during kiwifruit development. Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce the variance. All results are presented as mean ± standard error (SE). Bars with the same letter are not significantly different at p > 0.05 among three treatments, according to paired−samples t−test, ‘ns’ means not significant.
3.2. Effect of Bagging on the Internal Quality of Kiwifruit
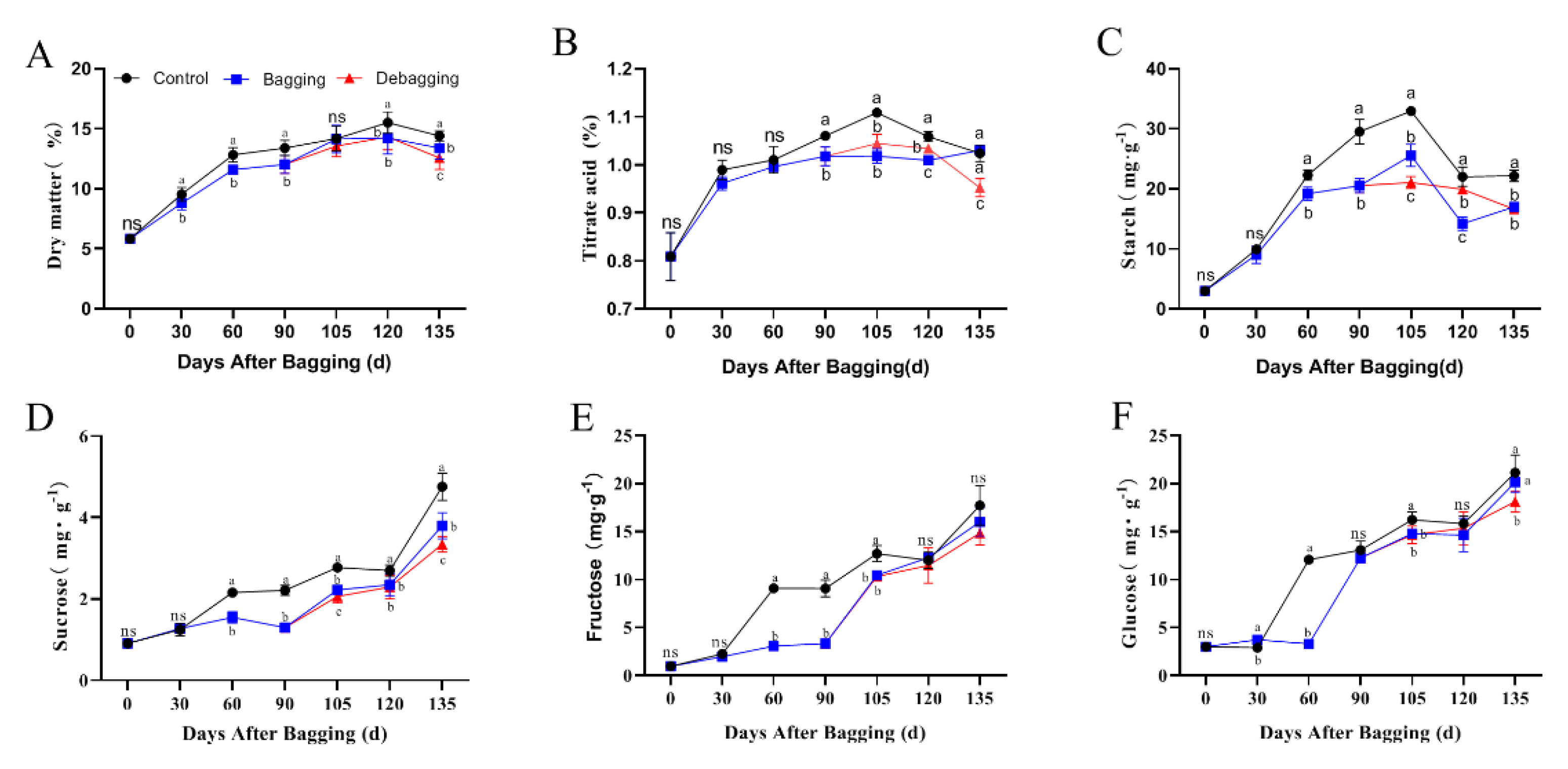
Bagging affected the internal quality of fruits. Bagging significantly reduced the accumulation of dry matter, titratable acids, starch, sucrose, fructose, and glucose during kiwifruit development, and there was no significant increase in these substances after debagging (Figure 2). These results indicated that bagging negatively affects the internal quality of kiwifruit.
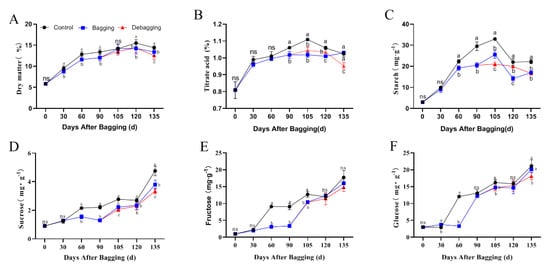
Figure 2.
Effects of bagging on dry matter (A), titratable acids (B), starch (C), sucrose (D), fructose (E), and glucose (F) content during kiwifruit development. Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce the variance. All results are presented as mean ± standard error (SE). Bars with the same letter are not significantly different at p > 0.05 among three treatments, according to paired−samples t−test, ‘ns’ means not significant.
3.3. Effect of Bagging on the Accumulation of Chlorophyll and Carotenoids
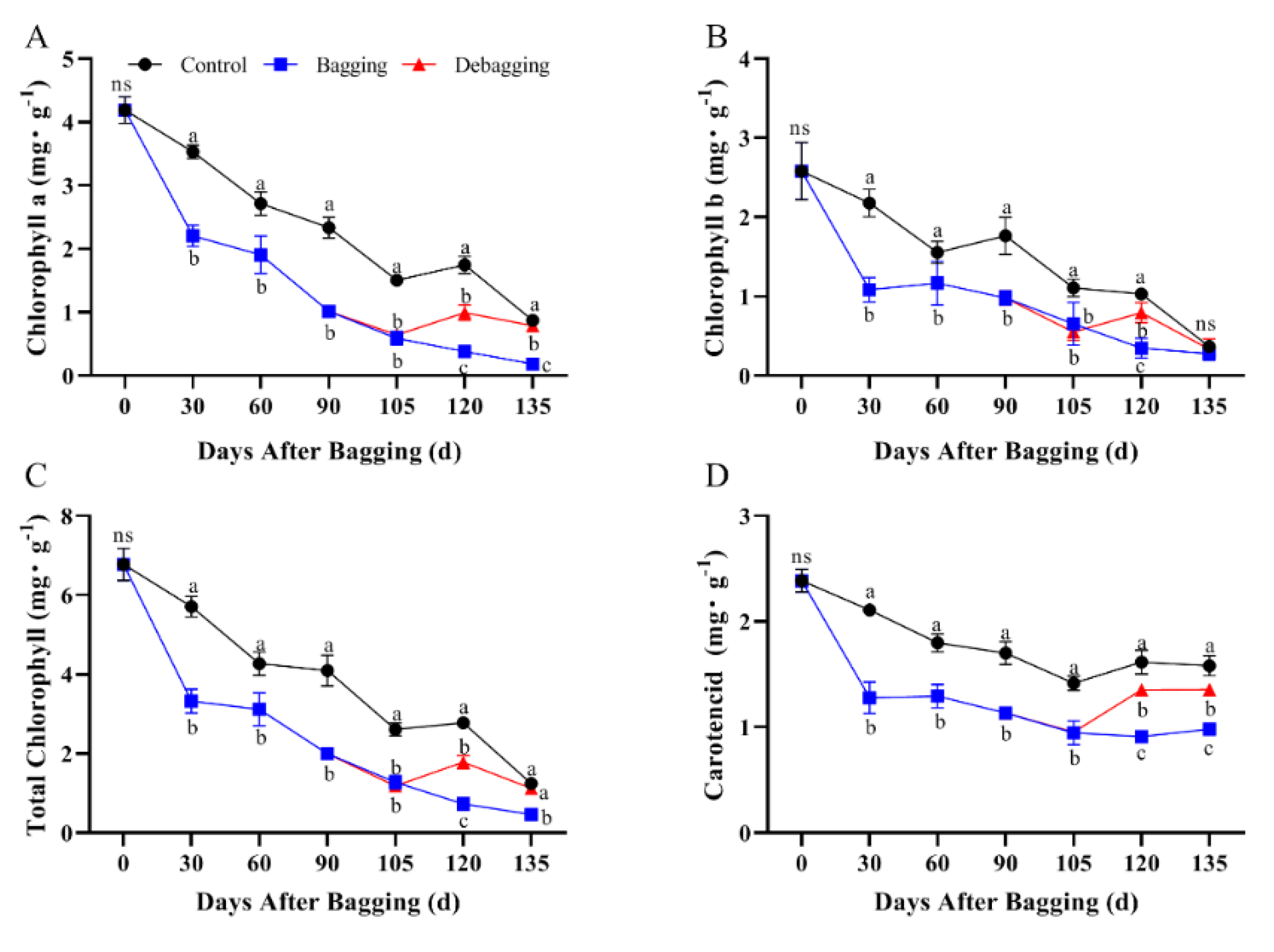
To determine the effect of bagging on color change in kiwifruit, the chlorophyll and carotenoid contents were determined. The contents of chlorophyll a, chlorophyll b, and total chlorophyll showed a decreasing trend during the fruit development period, whereas carotenoid content decreased during early development and remained stable in the late-development stage. Bagging significantly reduced the accumulation of chlorophyll and carotenoids during development, whereas after debagging, the chlorophyll and carotenoid contents increased significantly (Figure 3). These results indicated that bagging has a significant effect on chlorophyll and carotenoid metabolism in kiwifruit.
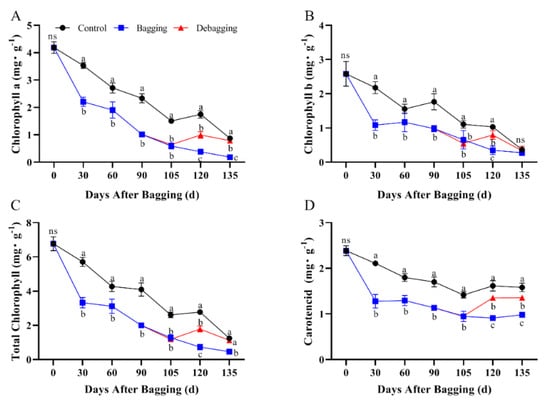
Figure 3.
Effects of bagging on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and carotenoid (D) content during kiwifruit development. Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce the variance. All results are presented as mean ± standard error (SE). Bars with the same letter are not significantly different at p > 0.05 among three treatments, according to paired−samples t−test, ‘ns’ means not significant.
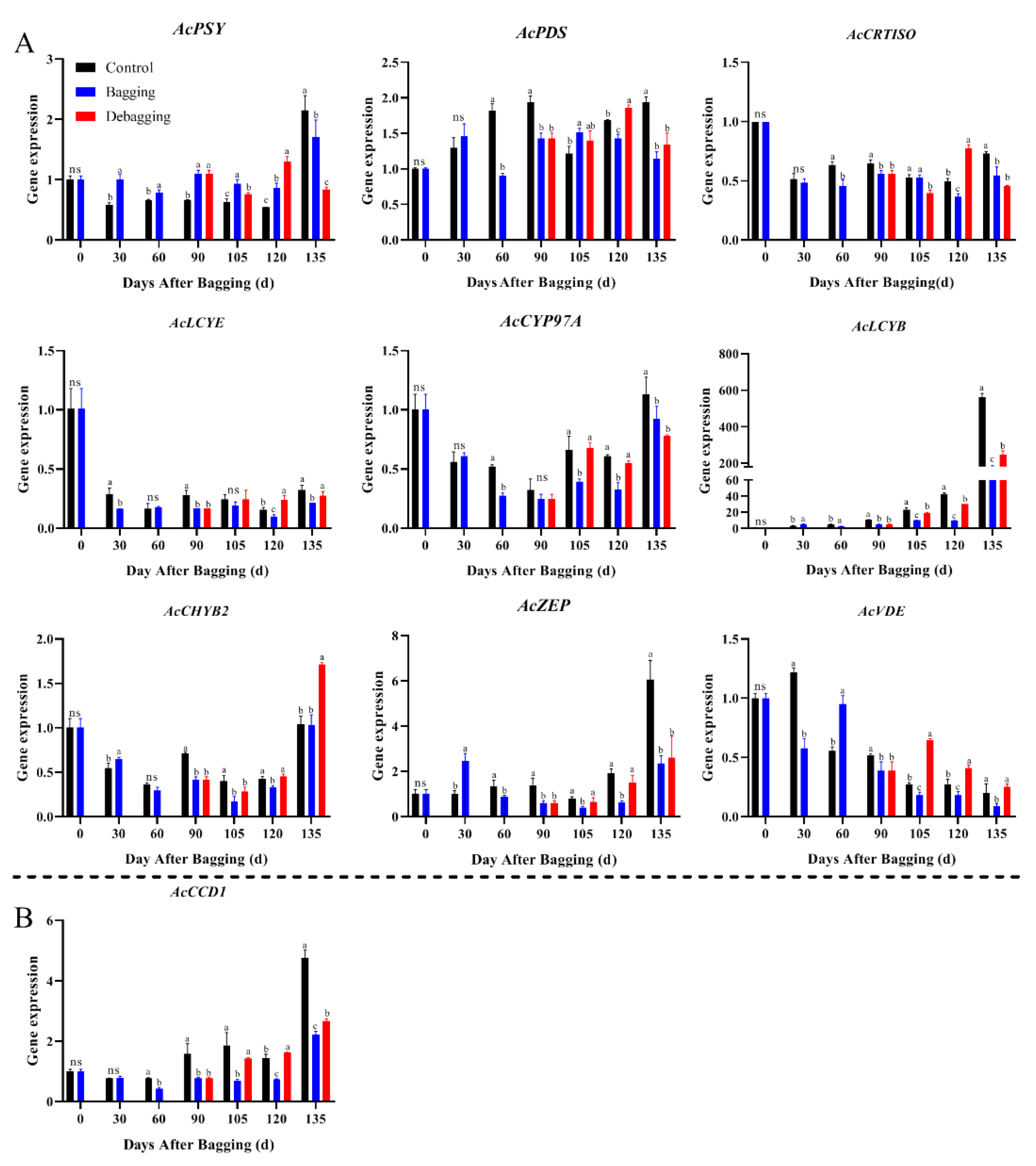
3.4. Effects of Bagging on the Expression of Genes Associated with Chlorophyll and Carotenoids Metabolism-Related Genes in Kiwifruit
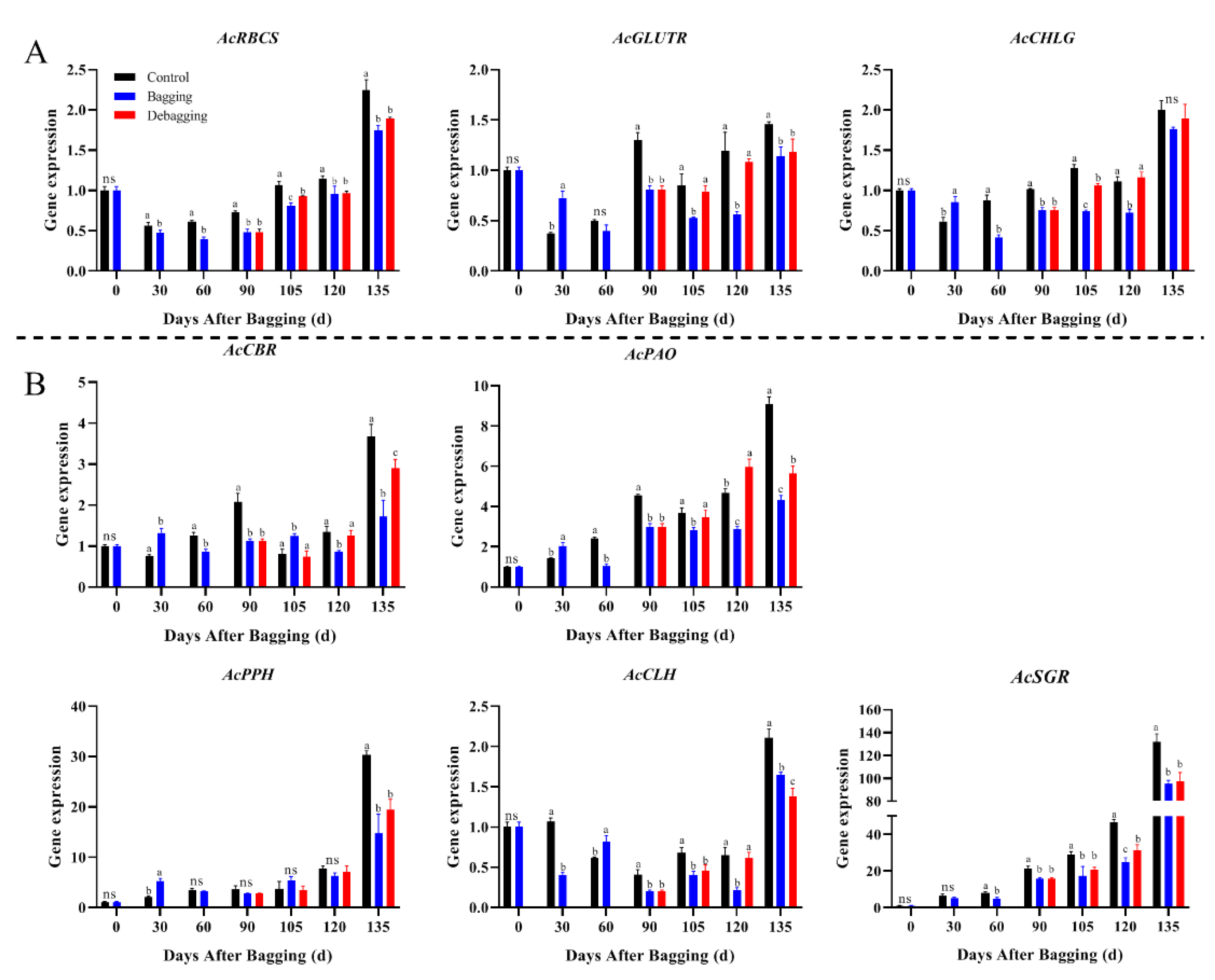
To explore the reasons for the difference in chlorophyll and carotenoid contents after bagging, the expression levels of genes involved in chlorophyll and carotenoid metabolism during fruit development were investigated by qRT-PCR. The expression of chlorophyll metabolism-related genes showed an upward trend during fruit development. During most of the development period, the chlorophyll biosynthesis genes AcRCBS, AcGLUTR, and AcCHLG, and degradation genes AcCBR, AcPAO, AcPPH, AcCLH, and AcSGR had significantly lower expression levels in bagged fruit, while the expression of these genes increased after debagging (Figure 4). The results indicate that the reason for chlorosis during the fruit development period of yellow-fleshed kiwifruit is not due to the reduction of chlorophyll synthesis, but due to the degradation of chlorophyll. Regarding carotenoid metabolism, nine biosynthesis genes and one degradation gene were investigated during fruit development. The expression of AcPSY, AcPDS, AcCRTISO, and AcZEP were stable during the fruit development period, but the expression of AcPSY and AcZEP increased significantly at the fruit ripening stage. The expression of the biosynthesis genes AcLCYE, AcCYP97A, AcCHYB2, and AcVDE were decreased during fruit development, while the expression of AcPSY and AcZEP increased significantly at the fruit ripening stage. Bagging inhibited the expression of carotenoid metabolism genes, whereas debagging promoted the expression to a certain extent (Figure 5). Notably, AcLCYB was highly expressed during late fruit development and was significantly affected by shade bagging (Figure 5A), indicating that it may play an important role in carotenoid accumulation during fruit development.
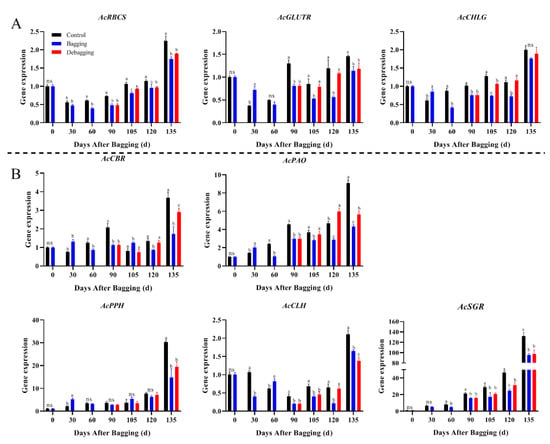
Figure 4.
Effect of bagging on the expression levels of chlorophyll synthesis (A) and cleavage (B) related genes during kiwifruit development. Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce the variance. All results are presented as mean ± standard error (SE). Bars with the same letter are not significantly different at p > 0.05 among three treatments, according to paired−samples t−test, ‘ns’ means not significant.
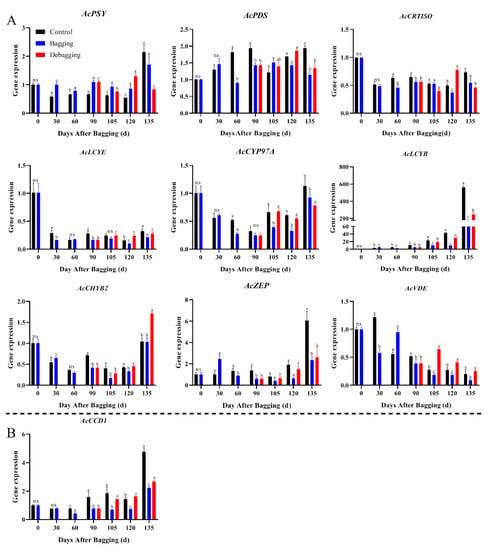
Figure 5.
Effect of bagging on the expression levels of carotenoid synthesis (A) and cleavage (B) related genes during kiwifruit development. Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce the variance. All results are presented as mean ± standard error (SE). Bars with the same letter are not significantly different at p > 0.05 among three treatments, according to paired−samples t−test, ‘ns’ means not significant.
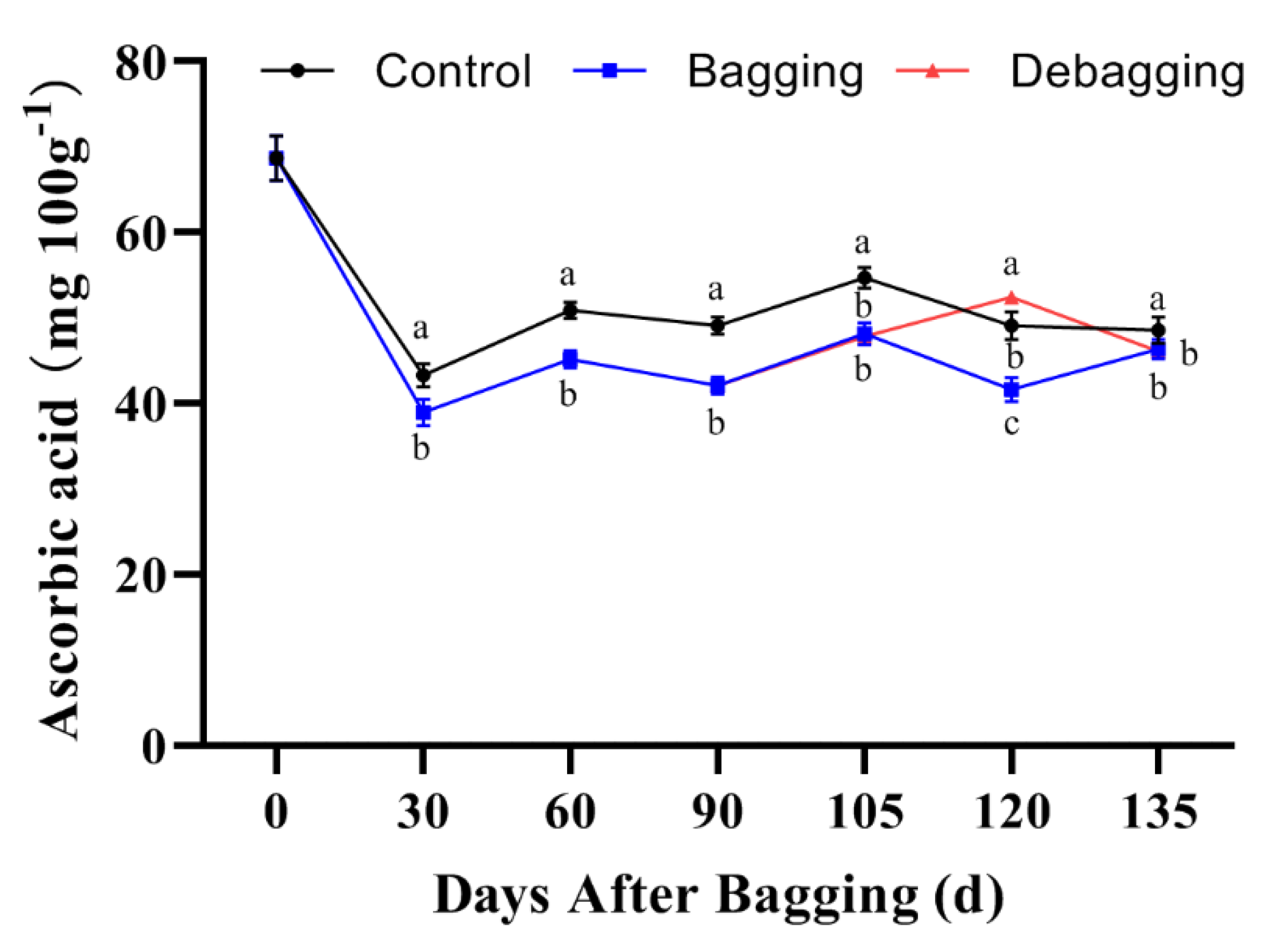
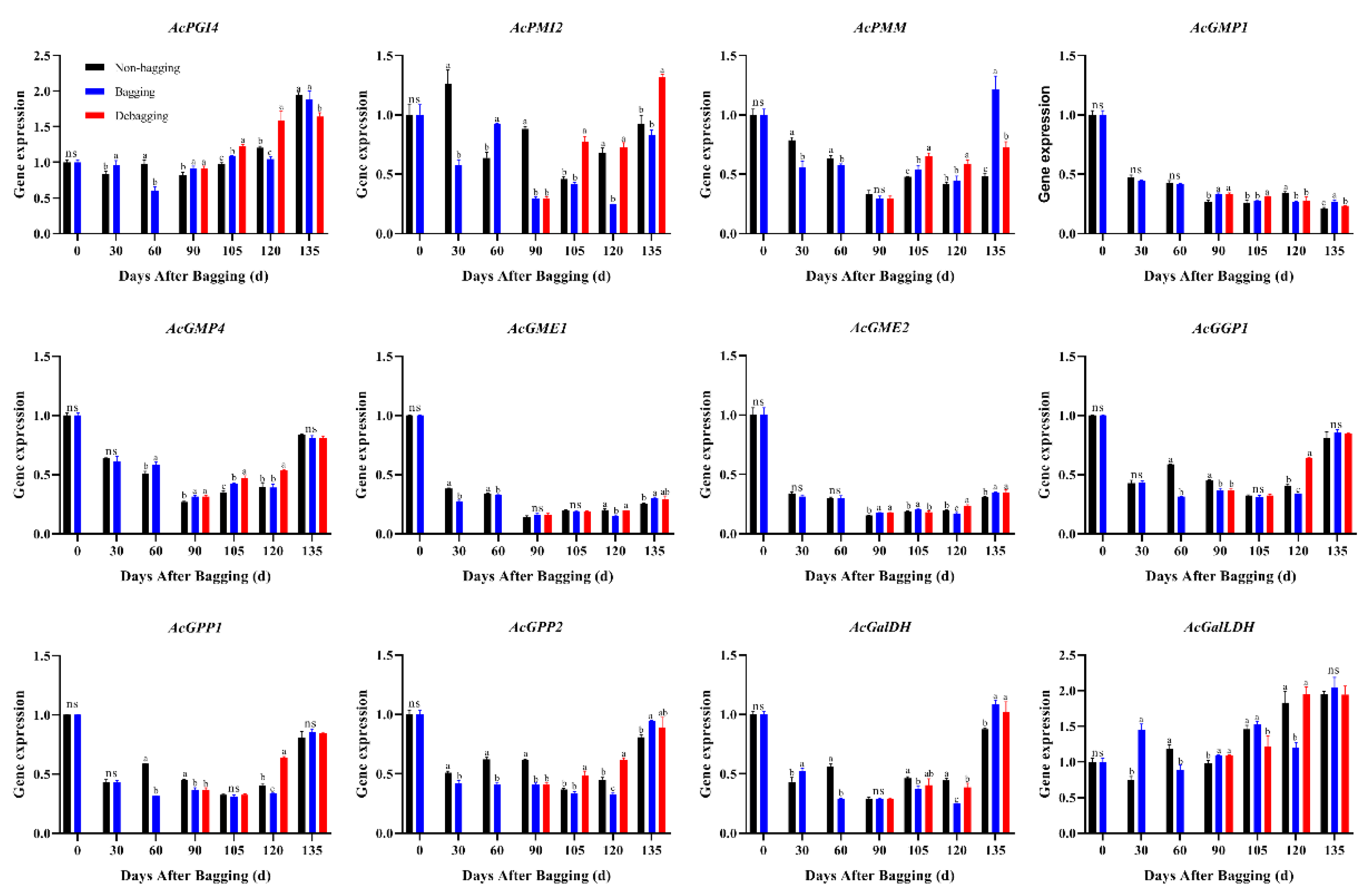
3.5. Effect of Bagging on the AsA Content and the Expression of AsA Biosynthesis Genes
The AsA content of fruits at different developmental stages was assayed using HPLC. The results showed that bagging significantly inhibits the accumulation of AsA; however, the AsA content increased significantly after debagging following bagging for 120 days (Figure 6). L-galactose pathway is considered to play a dominant role of AsA biosynthesis in kiwifruit. To explore the molecular mechanisms through which bagging affects AsA accumulation, we determined the expression of genes related to AsA biosynthesis. The expression of AcPMM, AcGMP1, AcGMP4, AcGME1, AcGME2, AcGGP1, AcGPP1, AcGPP2, and AcGalDH showed high levels at the early stage of fruit development (0 days after bagging). The expression of AcPMI2, AcGPP2, and AcGalDH in bagged fruit was significantly lower than that in the control, indicating that AcPMI2, AcGPP2, and AcGalDH may be the key genes responsible for the difference in AsA accumulation after bagging (Figure 7).
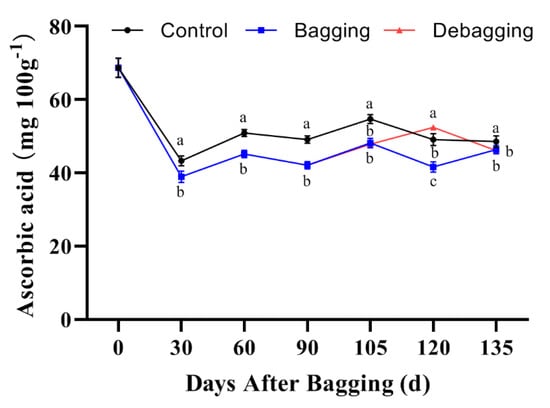
Figure 6.
Effects of bagging on AsA content during kiwifruit development. Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce the variance. All results are presented as mean ± standard error (SE). Bars with the same letter are not significantly different at p > 0.05 among three treatments, according to paired−samples t−test.
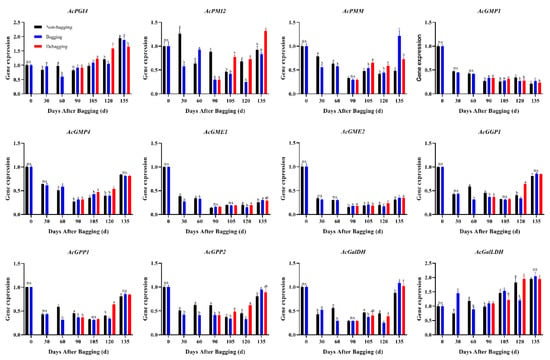
Figure 7.
Effect of bagging on the expression levels of AsA synthesis-related genes during kiwifruit development. Each assay was performed in triplicate to ensure the validity of the experimental data and to reduce the variance. All results are presented as mean ± standard error (SE). Bars with the same letter are not significantly different at p > 0.05 among three treatments, according to paired-samples t-test, ‘ns’ means not significant.
4. Discussion
Fruit bagging is a common practice used in the production of peach [2], apple [1], pear [25], and grapefruit [3], which has multiple effects on internal and external quality of fruit. Bagging can change the growth environment of fruits, and paper bags commonly used in production have a particularly obvious impact on light. Light can affect fruit coloring, and the effect of light on different fruit tissues varies [21]. The amount of light absorbed by different kiwifruit tissues is inconsistent; the light exposure of the inner axial placenta is only 1/30 of that of the surface [26]. In this study, we found that bagging had different effects on the color of the epicarp and mesocarp. Bagging mainly affected the color of the mesocarp and the brightness in the epicarp, indicating that bagging can improve the brightness of the fruit surface and enhance the appearance of the inner fruit. Bagging followed by debagging produces fruit with a bright red color, which is more appealing to consumers [27]. In our study, debagging improves the brightness of the fruit surface, which is consistent with previous research [28].
Although bagging improves fruit’s appearance quality, it also has some negative effects on fruit quality [27]. Studies on kiwifruit showed that bagging reduced the soluble solids of ‘Jinkui’ (A. deliciosa) and ‘Jinyan’ (A. chinensis) [29], but had no effect on the soluble solids, dry matter, soluble sugars, and titratable acids of A. eriantha [4]. In this study, bagging reduced the accumulation of dry matter, titratable acid, and starch during fruit development, which is consistent with previous findings [29]. Fructose, glucose, and sucrose are the main components of soluble sugars in fruits [30]. The proportion of soluble sugar components constantly changes with the development and maturity of the fruit. The content of fructose and glucose in mature kiwifruit is the highest, followed by sucrose [31]. Studies on pears have shown that bagging fruits reduces sugar content and the activities of enzymes related to sucrose metabolism [32]. The reason why bagging reduces the edible quality of fruits may be that after bagging, fruits are in shading condition for a long time, and photosynthesis of fruit skins is weakened or almost absent, which reduces the assimilates of fruits. Meanwhile, respiration in bags needs to consume certain assimilates [28]. Soluble sugar content plays an important role in fruit development and ripening. Sucrose treatment can promote the accumulation of anthocyanin in strawberry fruit, thereby accelerating fruit ripening [33]. Fruit ripening was significantly delayed in low-sugar-content orange mutant [34]. Exogenous sucrose treatment accelerates color change of postharvest tomato fruit [35]. In this study, the contents of sucrose, fructose, and glucose gradually increased as the fruits matured, but the sugar content of bagged fruits decreased compared to that in the control. This implies that the changes of soluble sugar content and color are correlated during the development and ripening of kiwifruit.
Most fleshy fruits are green during the early stage of development. With the maturation of the fruits, the contents of various components in the fruits change significantly. Chlorophyll and carotenoids are the main pigments involved in the development of yellow-fleshed fruits [6]. During fruit development and ripening, chlorophyll is degraded, and carotenoids are synthesized and accumulated [11]. Both green-fleshed kiwifruit and yellow-fleshed kiwifruit are green in color when immature, and their carotenoid content is stable [36]. As the fruit matures, chlorophyll in the mesocarp of yellow-fleshed kiwifruit is degraded into colorless metabolites, and the mesocarp color changes to yellow as carotenoids become dominant. However, in green-fleshed kiwifruit, the degree of chlorophyll degradation is far less than that in yellow-fleshed kiwifruit, and therefore green-fleshed kiwifruit contains higher chlorophyll at maturity and the mesocarp remains green in color [36,37]. In conclusion, the main factor contributing to the yellow color of kiwifruit mesocarp is the degradation of chlorophyll rather than carotenoid synthesis. In this study, chlorophyll content decreased during fruit development and ripening, and bagging treatment accelerated the rate of chlorophyll degradation. Carotenoid content was relatively stable in the later stages of development, and bagging reduced the accumulation of carotenoids. Notably, chlorophyll and carotenoid contents increased after debagging, indicating that light plays an important role in chlorophyll and carotenoid metabolism.
Recent studies on chlorophyll and carotenoid metabolism have also been reported. The metabolism of chlorophyll and carotenoids in plants is a dynamic process involving synthesis and degradation [11]. Chlorophyll degradation was shown to result in chlorosis of the mesocarp, in response to the combined effect of downregulated chlorophyll-synthesis genes and upregulated chlorophyll-degradation genes [6]. In this study, with the development of kiwifruit, the expression of chlorophyll-synthesis genes was slightly upregulated, whereas its degradation genes were significantly upregulated. Although bagging treatment inhibited the expression of chlorophyll-synthesis and -degradation genes, the upregulation trend of chlorophyll-degradation genes remained unchanged, which may be the reason for chlorosis during the development of ‘jinyan’ kiwifruit. Studies have shown that SGR proteins play a key role in the degradation of plant chlorophyll [38]. Chlorophyll degradation in kiwifruit is regulated by SGR proteins [10]. The chlorophyll content and expression of chlorophyll-metabolism genes in green and yellow kiwifruit during development showed that SGR was positively correlated with chlorophyll degradation [6]. In this study, it was also found that the upregulation of AcSGR expression during fruit development was much higher than that of other chlorophyll-degradation genes, which once again proved that chlorophyll degradation in kiwifruit is regulated by the SGR gene. Similar to chlorophyll metabolism, carotenoid synthesis and degradation are also regulated by the associated genes. In this study, bagging reduced the expression of most genes related to carotenoid metabolism, which is consistent with previous research results of studies on peaches [39]. Previous studies on kiwifruit have shown that β-carotene is the main carotenoid in kiwifruit [40], and its content is positively correlated with the expression of the LCYB gene [40], indicating that LCYB may play an important role in the process by which light affects the accumulation of carotenoids in kiwifruit.
The effect of light on AsA content has been confirmed in many plants. A bagging study on kiwifruit showed that bagging reduces the AsA content of A. deliciosa, A. chinensis, and A. eriantha [4]. The results of the present study also support this conclusion. The L-galactose pathway is the dominant biosynthesis pathway of AsA in plants, and all structural genes in this pathway have been identified [16]. Analysis of the promoter sequences of these structural genes revealed that light-responsive elements were distributed on most gene promoters and their activity could be induced by light [18]. Studies on kiwifruit showed that only GalLDH, GalUR, and DHAR expression were affected by bagging, while a study on ‘Jinyan’ (A. chinensis) found that only the expression of AcPMI2, AcGPP2, and AcGalDH could be significantly suppressed by darkness, indicating that the synthesis of AsA in kiwifruit is regulated by multiple structural genes.
5. Conclusions
The results of this study show that bagging reduces the fruit quality and decreases the accumulation of chlorophyll and carotenoids in kiwifruit. The content of chlorophyll, carotenoids, and AsA simultaneously not change and increase after debagging. Analysis of gene expression showed that AcSGR and AcLCYB were the key factors behind the decrease in chlorophyll and carotenoid content after bagging. This study is a preliminary attempt to investigate the effect of bagging on chlorophyll, carotenoids, and AsA in A. chinensis, and further studies are required to explore the specific mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8060478/s1, Table S1: Primers for gene expression.
Author Contributions
Conceptualization, Y.X. and Y.L. (Yafang Liu); methodology, Y.L. (Yafang Liu); software, Y.L. (Yafang Liu); validation, W.L., C.Y. and Y.L. (Yujia Lin); formal analysis, Y.W.; investigation, Y.X.; resources, Y.X.; data curation, C.C.; writing—original draft preparation, Z.G.; writing—review and editing, J.C.; visualization, C.W.; supervision, J.C. and Z.G.; project administration, Z.G.; funding acquisition, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32060704), Natural Science Foundation of Jiangxi Province of China (20202BAB215005), and Jiangxi 2011 Collaborative Innovation Center of Postharvest Key Technology and Quality Safety of Fruits and Vegetables, China (JXGS-05).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.G.; Di, B.; Li, Y.L.; Chen, S.H.; Zhang, J.Q.; Liu, Y.F. Effect of bagging on antioxidative capability of peel tissue in apples. Acta Agric. Boreali-Sin. 2005, 20, 54–56. [Google Scholar] [CrossRef]
- Liu, T.; Song, S.; Yuan, Y.; Wu, D.; Chen, M.; Sun, Q.; Zhang, B.; Xu, C.; Chen, K. Improved peach peel color development by fruit bagging. Enhanced expression of anthocyanin biosynthetic and regulatory genes using white non-woven polypropylene as replacement for yellow paper. Sci. Hortic. 2015, 184, 142–148. [Google Scholar] [CrossRef]
- Zhang, E.; Chai, F.; Zhang, H.; Li, S.; Liang, Z.; Fan, P. Effects of sunlight exclusion on the profiles of monoterpene biosynthesis and accumulation in grape exocarp and mesocarp. Food Chem. 2017, 237, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.L.; He, Y.Q.; Li, X.S.; Zhong, M.; Huang, C.H.; Yi, S.Y.; Liu, Q.; Xu, X.B. Effects of bagging on fruit flavor quality and related gene expression of AsA synthesis in Actinidia eriantha. Sci. Hortic. 2019, 256, 108511. [Google Scholar] [CrossRef]
- He, L.; Xu, X.Q.; Wang, Y.; Vanderweide, J.; Sun, R.Z.; Cheng, G.; Chen, W.; Li, S.D.; Li, S.P.; Duan, C.Q.; et al. Differential influence of timing and duration of bunch bagging on volatile organic compounds in Cabernet Sauvignon berries (Vitis vinifera L.). Aust. J. Grape Wine Res. 2022, 28, 75–85. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhou, B.; Qi, Y.W.; Chen, X.; Liu, C.H.; Liu, Z.D.; Ren, X.L. Expression differences of pigment structural genes and transcription factors explain flesh coloration in three contrasting kiwifruit cultivars. Front. Plant Sci. 2017, 8, 1507. [Google Scholar] [CrossRef]
- Guo, Y.M.; Bai, J.J.; Duan, X.D.; Wang, J. Accumulation characteristics of carotenoids and adaptive fruit color variation in ornamental pepper. Sci. Hortic. 2021, 275, 109699. [Google Scholar] [CrossRef]
- Li, T.; Deng, Y.J.; Liu, J.X.; Duan, A.Q.; Liu, H.; Xiong, A.S. DcCCD4 catalyzes the degradation of alpha-carotene and beta-carotene to affect carotenoid accumulation and taproot color in carrot. Plant J. 2021, 108, 1116–1130. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, G.; Pan, S.; Wang, L. Influence of ethylene and ethephon treatments on the peel color and carotenoids of Gannan Newhall navel orange during postharvest storage. J. Food Biochem. 2018, 42, e12534. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Montefiori, M.; Jameson, P.E.; Allan, A.C. The control of chlorophyll levels in maturing kiwifruit. Planta 2012, 236, 1615–1628. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Pan, D.L.; Jia, Z.H.; Wang, T.; Guo, Z.R. Chlorophyll, carotenoid and vitamin C metabolism regulation in Actinidia chinensis ‘Hongyang’ outer pericarp during fruit development. PLoS ONE 2018, 13, e0194835. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, A.S.; Zhou, X.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid pigment accumulation in horticultural plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Nicholas, S. Botanical Briefing: The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Wolucka, B.A.; Van, M.M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, G.L.; Chen, L.; He, Y.Q.; Li, X.S.; Lv, Z.X.; Yi, S.Y.; Zhong, M.; Huang, C.H.; Jia, D.F.; Qu, X.Y.; et al. Three metabolic pathways are responsible for the accumulation and maintenance of high AsA content in kiwifruit (Actinidia eriantha). BMC Genom. 2021, 22, 13. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, P.; Wang, R.C.; Du, X.F.; Xie, Y.; Du, K.; Deng, H.; Li, M.Z.; Zhang, Y.; Grierson, D.; et al. Ethylene response factor AcERF91 affects ascorbate metabolism via regulation of GDP-galactose phosphorylase encoding gene (AcGGP3) in kiwifruit. Plant Sci. 2021, 313, 111063. [Google Scholar] [CrossRef]
- Li, J.; Li, M.J.; Liang, D.; Cui, M.; Ma, F.W. Expression patterns and promoter characteristics of the gene encoding Actinidia deliciosa L-galactose-1-phosphate phosphatase involved in the response to light and abiotic stresses. Mol. Biol. Rep. 2013, 40, 1473–1485. [Google Scholar] [CrossRef]
- Zhuang, Z.Q.; Chen, M.; Niu, J.H.; Qu, N.; Ji, B.; Duan, X.; Liu, Z.D.; Liu, X.B.; Wang, Y.T.; Zhao, B.T. The manufacturing process of kiwifruit fruit powder with high dietary fiber and its laxative effect. Molecules 2019, 24, 3813. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Xiong, G.H.; He, Z.; Yan, M.F.; Zou, M.F.; Jiang, J.X. Transcriptome analysis of Actinidia chinensis in response to Botryosphaeria dothidea infection. PLoS ONE 2020, 15, e0227303. [Google Scholar] [CrossRef]
- Li, Y.K.; Qi, X.J.; Lin, M.; Zhi, L.I.; Fang, J. Effect of bagging on fruit pigmentation in two types of red-fleshed kiwifruit. J. Fruit Sci. 2016, 33, 1492–1501. [Google Scholar] [CrossRef]
- Shi, C.H.; Luo, J.; Zhang, C.X.; Zheng-Wen, Y.E.; Wang, X.Q. Effects of different fruit bags on fruit color and quality of kiwifruit variety ‘Hongyang’. Acta Agric. Shanghai 2013, 29, 32–35. [Google Scholar]
- Li, Y.K.; Qi, X.J.; Cui, W.; Lin, M.M.; Qiao, C.K.; Zhong, Y.P.; Fang, J.B.; Hu, C.G. Restraint of bagging on fruit skin coloration in on-tree kiwifruit (Actinidia arguta). J. Plant Growth Regul. 2021, 40, 603–616. [Google Scholar] [CrossRef]
- Wang, L.B.; Ma, M.; Zhang, S.L.; Wu, Z.F.; Li, j.; Luo, W.Q.; Guo, L.; Lin, W.; Zhang, S.L. Characterization of genes involved in pear ascorbic acid metabolism and their response to bagging treatment during ‘Yali’ fruit development. Sci. Hortic. 2021, 285, 110178. [Google Scholar] [CrossRef]
- Bai, S.L.; Sun, Y.W.; Qian, M.J.; Yang, F.X.; Ni, J.B.; Tao, R.Y.; Li, L.; Shu, Q.; Zhang, D.; Teng, Y.W. Transcriptome analysis of bagging-treated red Chinese sand pear peels reveals light-responsive pathway functions in anthocyanin accumulation. Sci. Rep. 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Possingham, J.V.; Coote, M.; Hawker, J.S. The plastids and pigments of fresh and dried chinese gooseberries (Actinidia chinensis). Ann. Bot. 1980, 45, 529–533. [Google Scholar] [CrossRef]
- Feng, F.J.; Li, M.J.; Ma, F.W.; Cheng, L.L. The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Sci. Hortic. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pal, R.K.; Asrey, R.; Sagar, V.R.; Rana, M.R. Pre-harvest fruit bagging influences fruit color and quality of apple cv. Delicious. Agric. Sci. 2013, 4, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Liu, X.L.; Zhong, C.H. Effects of different types of fruit bags on the quality of kiwifruit ‘Jinkui’. China Fruits 2017, 3, 45–49. [Google Scholar] [CrossRef]
- Wang, R.C.; Shu, P.; Zhang, C.; Zhang, J.L.; Chen, Y.; Zhang, Y.X.; Du, K.; Xie, Y.; Li, M.Z.; Ma, T.; et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef]
- Hu, X.; Kuang, S.; Zhang, A.D.; Zhang, W.S.; Chen, M.J.; Yin, X.R.; Chen, K.S. Characterization of starch degradation related genes in postharvest kiwifruit. Int. J. Mol. Sci. 2016, 17, 2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.F.; He, Z.S.; Tao, S.T.; Zhang, S.L.; Zhang, H.P. Effects of bagging on soluble sugars contents during fruit development of ’Korla fragrant pear’. J. Fruit Sci. 2014, 06, 1072–1078. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Wu, J.; Chen, S.; Chen, H.; Chai, L.; Yi, H. Comparative transcriptome analyses between a spontaneous late-ripening sweet orange mutant and its wild type suggest the functions of ABA, sucrose and ja during citrus fruit ripening. PLoS ONE 2014, 9, e116056. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Mou, W.; Wang, Y.; Li, L.; Mao, L.; Ying, T.; Luo, Z. Exogenous sucrose treatment accelerates postharvest tomato fruit ripening through the influence on its metabolism and enhancing ethylene biosynthesis and signaling. Acta Physiol. Plant. 2016, 38, 225. [Google Scholar] [CrossRef]
- Mcghie, T.K.; Ainge, G.D. Color in fruit of the genus Actinidia: Carotenoid and chlorophyll compositions. J. Agric. Food Chem. 2002, 50, 117–121. [Google Scholar] [CrossRef]
- Ampomah-Dwamena, C.; Mcghie, T.; Wibisono, R.; Montefiori, M.; Hellen, R.P.; Allan, A.C. The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accumulation in fruit. J. Exp. Bot. 2009, 60, 3765–3779. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.D.; Zhang, J.H.; Li, J.H.; Yang, C.X.; Wang, T.T.; Ouyang, B.; Li, H.X.; Giovannoni, J.; Ye, Z.B. A STAY-GREEN protein SlSGR1 regulates lycopene and beta-carotene accumulation by interacting directly with SlPSY1 during ripening processes in tomato. New Phytol. 2013, 198, 442–452. [Google Scholar] [CrossRef]
- Zhu, M.T.; Fang, W.C.; Chen, C.W.; Wang, L.R.; Cao, K. Effects of shading by bagging on carotenoid accumulation in peach fruit flesh. J. Plant Growth Regul. 2021, 40, 1912–1921. [Google Scholar] [CrossRef]
- Ampomah-Dwamena, C.; Thrimawithana, A.H.; Dejnoprat, S.; Lewis, D.; Espley, R.V.; Allan, A.C. A kiwifruit (Actinidia deliciosa) R2R3-MYB transcription factor modulates chlorophyll and carotenoid accumulation. New Phytol. 2019, 221, 309–325. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).