Sustainable Upcycling of Mushroom Farm Wastewater through Cultivation of Two Water Ferns (Azolla spp.) in Stagnant and Flowing Tank Reactors
Abstract
:1. Introduction
2. Materials and Methods
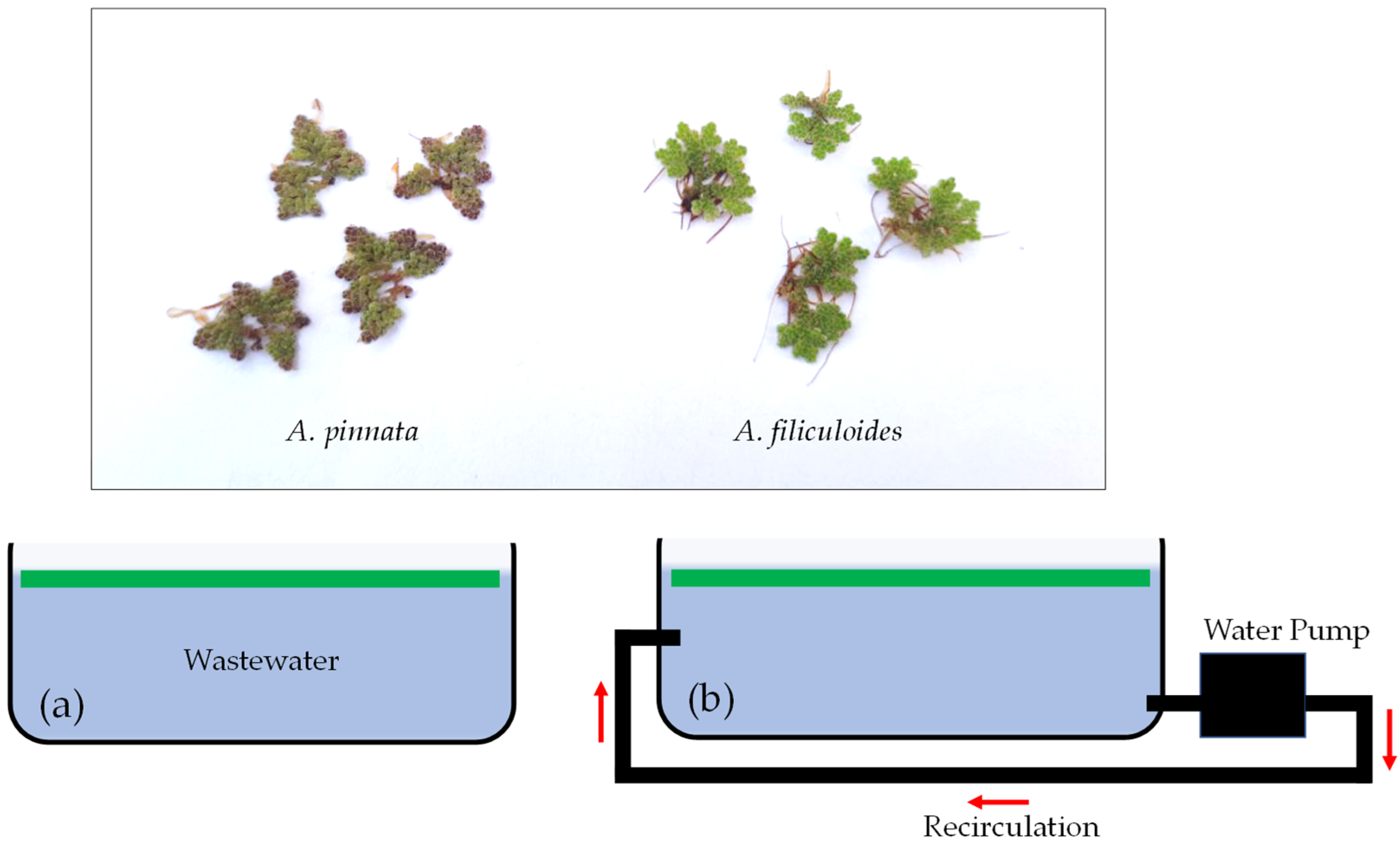
2.1. Collection of Experimental Materials
2.2. Experimental Design and Conditions
2.3. Laboratory Analytical Methods
2.4. Pollutant Removal and Growth Kinetic Modeling
2.5. Statistics and Software
3. Results and Discussion
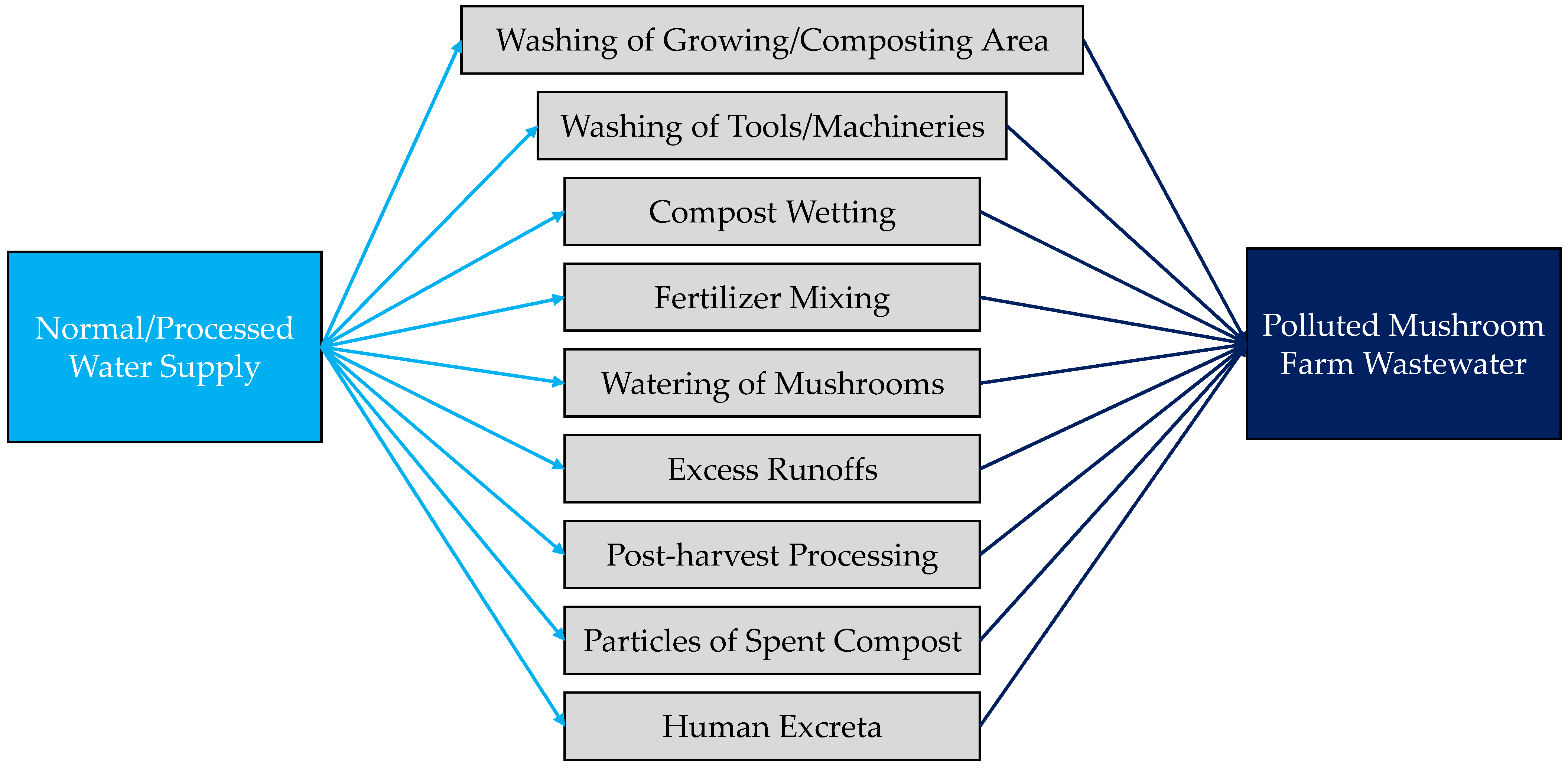
3.1. Properties of Borewell Water and MFW
3.2. Removal of Pollutants from MFW by Azolla spp.
3.3. Effects of MFW and Reactor Type on Growth and Biochemical Parameters of Azolla spp.
3.4. Growth Kinetic Modeling of Azolla spp. Grown in MFW
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monterey Mushroom. 3 Major Reasons Mushrooms Are a Smart, Sustainable Choice; Monterey Mushrooms, LLC 260 Westgate Drive Watsonville, CA 95076; 2020. Available online: https://www.montereymushrooms.com/blog/reasons-mushrooms-are-smart-sustainable-choice (accessed on 1 May 2022).
- Abou Fayssal, S.; el Sebaaly, Z.; Alsanad, M.A.; Najjar, R.; Bohme, M.; Yordanova, M.H.; Sassine, Y.N. Combined Effect of Olive Pruning Residues and Spent Coffee Grounds on Pleurotus ostreatus Production, Composition, and Nutritional Value. PLoS ONE 2021, 16, e0255794. [Google Scholar] [CrossRef]
- Kumar, P.; Eid, E.M.; Al-Huqail, A.A.; Širić, I.; Adelodun, B.; Abou Fayssal, S.; Valadez-Blanco, R.; Goala, M.; Ajibade, F.O.; Choi, K.S.; et al. Kinetic Studies on Delignification and Heavy Metals Uptake by Shiitake (Lentinula edodes) Mushroom Cultivated on Agro-Industrial Wastes. Horticulturae 2022, 8, 316. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Adelodun, B.; Bedeković, D.; Kos, I.; Širić, I.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M.; Abou Fayssal, S.; et al. Sustainable Use of Sewage Sludge as a Casing Material for Button Mushroom (Agaricus bisporus) Cultivation: Experimental and Prediction Modeling Studies for Uptake of Metal Elements. J. Fungi 2022, 8, 112. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Singh, J.; Kumar, P. Potential of Water Fern (Azolla pinnata R.Br.) in Phytoremediation of Integrated Industrial Effluent of SIIDCUL, Haridwar, India: Removal of Physicochemical and Heavy Metal Pollutants. Int. J. Phytoremediat. 2020, 22, 392–403. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Kumar, P.; Singh, J. Anaerobic Digestion of Azolla pinnata Biomass Grown in Integrated Industrial Effluent for Enhanced Biogas Production and COD Reduction: Optimization and Kinetics Studies. Environ. Technol. Innov. 2020, 17, 100627. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Eid, E.M.; Singh, J.; Adelodun, B.; Kumar, P.; Kumari, S.; Choi, K.S. Modeling of Water Hyacinth Growth and Its Role in Heavy Metals Accumulation from Unoperated Old Ganga Canal at Haridwar, India. Rend. Lincei. Sci. Fis. Nat. 2021, 32, 805–816. [Google Scholar] [CrossRef]
- Riva, V.; Riva, F.; Vergani, L.; Crotti, E.; Borin, S.; Mapelli, F. Microbial Assisted Phytodepuration for Water Reclamation: Environmental Benefits and Threats. Chemosphere 2020, 241, 124843. [Google Scholar] [CrossRef]
- Nwogwu, N.A.; Ajala, O.A.; Ajibade, F.O.; Ajibade, T.F.; Adelodun, B.; Lasisi, K.H.; Ugya, A.Y.; Kumar, P.; Omotade, I.F.; Babalola, T.E.; et al. Phytoremediation Mechanisms of Heavy Metal Removal: A Step Towards a Green and Sustainable Environment. In Innovative Bio-Based Technologies for Environmental Remediation; CRC Press: Boca Raton, FL, USA, 2021; pp. 207–236. [Google Scholar]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of Iron–Lysine on Morpho-Physiological Traits and Combating Chromium Toxicity in Rapeseed (Brassica Napus L.) Plants Irrigated with Different Levels of Tannery Wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar] [CrossRef]
- Mustafa, H.M.; Hayder, G. Recent Studies on Applications of Aquatic Weed Plants in Phytoremediation of Wastewater: A Review Article. Ain Shams Eng. J. 2021, 12, 355–365. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Hafez, R.M.; Hegazy, A.K.; Abd-El Fattah, A.M.; Mohamed, N.H.; Mustafa, Y.M.; Gobouri, A.A.; Azab, E. Variations of Structural and Functional Traits of Azolla pinnata r.Br. in Response to Crude Oil Pollution in Arid Regions. Sustainability 2021, 13, 2142. [Google Scholar] [CrossRef]
- Arora, A.; Saxena, S.; Sharma, D.K. Tolerance and Phytoaccumulation of Chromium by Three Azolla Species. World J. Microbiol. Biotechnol. 2006, 22, 97–100. [Google Scholar] [CrossRef]
- Sundararaman, S.; Kumar, P.S.; Deivasigamani, P.; Jagadeesan, A.K.; Devaerakkam, M.; Al-Hashimi, A.; Choi, D. Assessing the Plant Phytoremediation Efficacy for Azolla filiculoides in the Treatment of Textile Effluent and Redemption of Congo Red Dye onto Azolla Biomass. Sustainability 2021, 13, 9588. [Google Scholar] [CrossRef]
- Naghipour, D.; Ashrafi, S.D.; Gholamzadeh, M.; Taghavi, K.; Naimi-Joubani, M. Phytoremediation of Heavy Metals (Ni, Cd, Pb) by Azolla filiculoides from Aqueous Solution: A Dataset. Data Brief 2018, 21, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Stȩpniewska, Z.; Bennicelli, R.P.; Balakhnina, T.I.; Szajnocha, K.; Banach, A.; Wolińska, A. Potential of Azolla caroliniana for the Removal of Pb and Cd from Wastewaters. Int. Agrophysics 2005, 19, 251–255. [Google Scholar]
- Leão, G.A.; de Oliveira, J.A.; Felipe, R.T.A.; Farnese, F.S. Phytoremediation of Arsenic-Contaminated Water: The Role of Antioxidant Metabolism of Azolla caroliniana Willd. (Salviniales). Acta Bot. Bras. 2017, 31, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Kumar, U.; Nayak, A.K. Azolla Germplasms at NRRI: Conservation, Characterization and Utilization; NRRI Research Bulletin No. 19; ICAR—National Rice Research Institute: Cuttack, India, 2019; Volume 68. [Google Scholar]
- Apha, A. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Chromý, V.; Vinklárková, B.; Šprongl, L.; Bittová, M. The Kjeldahl Method as a Primary Reference Procedure for Total Protein in Certified Reference Materials Used in Clinical Chemistry. I. A Review of Kjeldahl Methods Adopted by Laboratory Medicine. Crit. Rev. Anal. Chem. 2015, 45, 106–111. [Google Scholar] [CrossRef]
- De Carvalho, L.M.J.; Gomes, P.B.; de Godoy, R.L.O.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total Carotenoid Content, α-Carotene and β-Carotene, of Landrace pumpkins (Cucurbita Moschata Duch): A Preliminary Study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Kumar, V.; Kumar, P.; Kumar, P. Kinetics and Prediction Modeling of Heavy Metal Phytoremediation from Glass Industry Effluent by Water Hyacinth (Eichhornia crassipes). Int. J. Environ. Sci. Technol. 2022, 19, 5481–5492. [Google Scholar] [CrossRef]
- Petersen, F.; Demann, J.; Restemeyer, D.; Ulbrich, A.; Olfs, H.W.; Westendarp, H.; Appenroth, K.J. Influence of the Nitrate-n to Ammonium-n Ratio on Relative Growth Rate and Crude Protein Content in the Duckweeds Lemna minor and Wolffiella hyalina. Plants 2021, 10, 1741. [Google Scholar] [CrossRef]
- Kyurkchiev, N.; Iliev, A. Some Families of Sigmoid Functions: Applications to Growth Theory; Lap Lambert Academic Publishing: Saarbrucken, Germany, 2019. [Google Scholar]
- Rodríguez Pérez, S.; García Oduardo, N.; Bermúdez Savón, R.C.; Fernández Boizán, M.; Augur, C. Decolourisation of Mushroom Farm Wastewater by Pleurotus ostreatus. Biodegradation 2008, 19, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Zarkami, R.; Sabetraftar, K.; van Damme, P. A Review of Some Ecological Factors Affecting the Growth of Azolla spp. CJES Casp. J. Environ. Sci. Casp. J. Env. Sci. 2013, 11, 65–76. [Google Scholar]
- Phearun, L.; Iwai, C.B. Recycling of Piggery Effluent for Azolla microphylla Production and Application in Vermiculture for Enhancing Nutrients Quality and Driving BCG Economy Model. Environ. Asia 2022, 15, 68–86. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Kadali, K.; Gujar, A.; Rochfort, S.; Stevenson, T.; Ball, A.S.; Mouradov, A. Dual Application of Duckweed and Azolla Plants for Wastewater Treatment and Renewable Fuels and Petrochemicals Production. Biotechnol. Biofuels 2014, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Saber, A.; Tafazzoli, M.; Mortazavian, S.; James, D.E. Investigation of Kinetics and Absorption Isotherm Models for Hydroponic Phytoremediation of Waters Contaminated with Sulfate. J. Environ. Manag. 2018, 207, 276–291. [Google Scholar] [CrossRef]
- Goala, M.; Yadav, K.K.; Alam, J.; Adelodun, B.; Choi, K.S.; Cabral-Pinto, M.M.S.; Hamid, A.A.; Alhoshan, M.; Ali, F.A.A.; Shukla, A.K. Phytoremediation of Dairy Wastewater Using Azolla pinnata: Application of Image Processing Technique for Leaflet Growth Simulation. J. Water Process Eng. 2021, 42, 10215. [Google Scholar] [CrossRef]
- Yalçuk, A.; Ugurlu, A. Treatment of Landfill Leachate with Laboratory Scale Vertical Flow Constructed Wetlands: Plant Growth Modeling. Int. J. Phytoremediat. 2020, 22, 157–166. [Google Scholar] [CrossRef] [PubMed]


| Properties | Borewell Water | Mushroom Farm Wastewater | Student’s t-Test | Safe Discharge Limits ^ | |
|---|---|---|---|---|---|
| t-Statistics | p-Value | ||||
| pH | 7.13 ± 0.03 | 8.30 ± 0.10 * | 19.57 | <0.01 | 5.50–9.00 |
| Electrical Conductivity (EC: dS/m) | 0.17 ± 0.01 | 3.55 ± 0.12 * | 48.61 | <0.01 | NA |
| Total Dissolved Solids (TDS: mg/L) | 144.82 ± 2.50 | 1693.40 ± 56.24 * | 47.64 | <0.01 | 1900 |
| Biological Oxygen Demand (BOD: mg/L) | 3.18 ± 0.20 | 1082.10 ± 13.85 * | 134.91 | <0.01 | 100 |
| Chemical Oxygen Demand (COD: mg/L) | 9.07 ± 0.08 | 2176.30 ± 82.66 * | 45.41 | <0.01 | 250 |
| Total Kjeldahl’s Nitrogen (TKN: mg/L) | 1.55 ± 0.01 | 255.90 ± 10.43 * | 42.23 | <0.01 | 100 |
| Azolla spp. | Treatment | pH | EC (dS/m) | TDS (mg/L) | BOD (mg/L) | COD (mg/L) | TKN (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| A. pinnata | Control | Initial | 7.13 ± 0.03 | 0.17 ± 0.01 | 144.82 ± 2.50 | 3.18 ± 0.20 | 9.07 ± 0.08 | 1.55 ± 0.01 |
| Final | 6.50 ± 0.03 * | 0.10 ± 0.01 * | 98.35 ± 5.14 * | 2.45 ± 0.50 * | 3.59 ± 0.17 * | 0.70 ± 0.05 * | ||
| S50 | Initial | 8.13 ± 0.02 | 1.80 ± 0.04 | 845.55 ± 14.75 | 526.24 ± 12.38 | 1075.05 ± 14.20 | 127.19 ± 3.71 | |
| Final | 6.80 ± 0.02 * | 0.76 ± 0.05 * | 240.12 ± 6.07 * | 110.24 ± 30.20 * | 260.40 ± 8.36 * | 25.30 ± 2.24 * | ||
| S100 | Initial | 8.30 ± 0.03 | 3.59 ± 0.02 | 1691.10 ± 28.38 | 1052.47 ± 19.65 | 2150.10 ± 17.29 | 254.37 ± 3.20 | |
| Final | 7.03 ± 0.02 * | 1.28 ± 0.05 * | 350.33 ± 8.20 * | 153.32 ± 10.80 * | 388.54 ± 4.36 * | 41.2 ± 4.18 * | ||
| F50 | Initial | 8.12 ± 0.02 | 1.78 ± 0.02 | 846.70 ± 9.45 | 541.05 ± 8.77 | 1088.15 ± 15.65 | 127.95 ± 5.13 | |
| Final | 6.74 ± 0.05 * | 0.42 ± 0.03 * | 210.05 ± 5.60 * | 92.70 ± 3.16 * | 209.13 ± 7.11 * | 22.44 ± 6.52 * | ||
| F100 | Initial | 8.32 ± 0.01 | 3.55 ± 0.04 | 1693.40 ± 24.92 | 1082.10 ± 20.10 | 2176.30 ± 28.05 | 255.90 ± 7.33 | |
| Final | 6.75 ± 0.04 * | 0.70 ± 0.08 * | 218.09 ± 4.83 * | 101.38 ± 5.04 * | 301.55 ± 3.18 * | 34.86 ± 5.41 * | ||
| A. filiculoides | Control | Initial | 7.12 ± 0.02 | 0.18 ± 0.02 | 149.02 ± 7.10 | 3.15 ± 0.16 | 9.12 ± 0.10 | 1.54 ± 0.02 |
| Final | 6.58 ± 0.02 * | 0.12 ± 0.04 * | 103.34 ± 4.05 * | 2.54 ± 0.31 * | 3.78 ± 0.20 * | 0.81 ± 0.04 * | ||
| S50 | Initial | 8.14 ± 0.02 | 1.75 ± 0.03 | 820.10 ± 8.34 | 525.45 ± 6.25 | 1097.63 ± 14.97 | 125.34 ± 3.83 | |
| Final | 6.95 ± 0.04 * | 0.81 ± 0.07 * | 251.40 ± 4.78 * | 114.38 ± 2.61 * | 265.03 ± 9.40 * | 28.12 ± 2.47 * | ||
| S100 | Initial | 8.32 ± 0.02 | 3.49 ± 0.03 | 1640.20 ± 10.55 | 1050.90 ± 12.09 | 2195.26 ± 26.03 | 250.68 ± 3.27 | |
| Final | 7.09 ± 0.02 * | 1.35 ± 0.05 * | 362.90 ± 6.12 * | 167.04 ± 3.53 * | 392.12 ± 8.16 * | 48.36 ± 5.98 * | ||
| F50 | Initial | 8.12 ± 0.01 | 1.80 ± 0.02 | 840.32 ± 11.58 | 545.53 ± 5.90 | 1090.35 ± 15.24 | 127.09 ± 5.30 | |
| Final | 6.69 ± 0.05 * | 0.49 ± 0.04 * | 217.50 ± 6.86 * | 96.82 ± 4.10 * | 210.62 ± 5.03 * | 25.10 ± 6.12 * | ||
| F100 | Initial | 8.35 ± 0.01 | 3.59 ± 0.01 | 1680.63 ± 15.40 | 1091.05 ± 20.24 | 2180.70 ± 27.21 | 254.18 ± 5.08 | |
| Final | 6.80 ± 0.03 * | 0.76 ± 0.04 * | 224.72 ± 8.25 * | 110.18 ± 2.92 * | 315.31 ± 7.72 * | 38.03 ± 4.15 * |
| Azolla spp. | Treatment | Surface Coverage (%) | Fresh Biomass (g) | Relative Growth Rate (g/g/day fwt.) | Chlorophyll (mg/g fwt.) | Carotenoids (mg/g) |
|---|---|---|---|---|---|---|
| A. pinnata | Control | 5.22 ± 0.10 | 20.33 ± 0.10 | 0.02 | 1.20 ± 0.03 | 0.18 ± 0.01 |
| S50 | 49.22 ± 2.06 * | 59.12 ± 1.08 * | 0.05 | 2.00 ± 0.01 * | 0.21 ± 0.02 * | |
| S100 | 62.08 ± 3.50 * | 80.21 ± 2.44 * | 0.06 | 2.16 ± 0.02 * | 0.28 ± 0.02 * | |
| F50 | 76.33 ± 1.72 * | 63.20 ± 1.60 * | 0.05 | 2.13 ± 0.02 * | 0.25 ± 0.01 * | |
| F100 | 84.40 ± 2.03 * | 110.15 ± 2.90 * | 0.07 | 2.40 ± 0.05 * | 0.34 ± 0.03 * | |
| A. filiculoides | Control | 4.50 ± 0.05 | 18.05 ± 0.37 | 0.02 | 1.20 ± 0.02 | 0.16 ± 0.01 |
| S50 | 42.60 ± 1.96 * | 54.64 ± 1.10 * | 0.05 | 1.80 ± 0.05 * | 0.20 ± 0.02 * | |
| S100 | 57.10 ± 2.35 * | 75.06 ± 2.02 * | 0.06 | 2.10 ± 0.07 * | 0.25 ± 0.01 * | |
| F50 | 71.09 ± 0.87 * | 60.38 ± 1.45 * | 0.05 | 2.12 ± 0.04 * | 0.24 ± 0.02 * | |
| F100 | 78.82 ± 2.46 * | 96.10 ± 2.14 * | 0.07 | 2.28 ± 0.03 * | 0.30 ± 0.03 * |
| Azolla spp. | Treatment | Model Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Logistic Model | Modified Gompertz Model | ||||||||||
| R2 | y | P | k | xc | R2 | y | P | k | xc | ||
| A. pinnata | Control | 0.99 | 20.35 | 22.64 | 0.16 | 1.51 | 0.99 | 20.40 | 24.18 | 0.11 | 1.03 |
| S50 | 0.99 | 60.01 | 65.23 | 0.29 | 6.79 | 0.98 | 60.82 | 77.55 | 0.15 | 5.57 | |
| S100 | 0.99 | 82.20 | 88.21 | 0.34 | 7.34 | 0.98 | 83.42 | 104.91 | 0.17 | 6.41 | |
| F50 | 0.99 | 64.66 | 74.52 | 0.27 | 8.14 | 0.98 | 65.38 | 99.15 | 0.12 | 7.99 | |
| F100 | 0.99 | 112.02 | 116.63 | 0.40 | 7.07 | 0.98 | 113.74 | 129.68 | 0.22 | 5.93 | |
| A. filiculoides | Control | 0.99 | 18.17 | 19.64 | 0.16 | 0.03 | 0.99 | 18.21 | 20.48 | 0.12 | 2.54 |
| S50 | 0.99 | 55.47 | 60.93 | 0.28 | 6.94 | 0.98 | 56.24 | 74.32 | 0.14 | 6.08 | |
| S100 | 0.99 | 76.69 | 82.67 | 0.33 | 7.39 | 0.98 | 77.88 | 99.89 | 0.16 | 6.56 | |
| F50 | 0.98 | 62.03 | 77.58 | 0.24 | 9.43 | 0.97 | 62.64 | 127.42 | 0.09 | 11.39 | |
| F100 | 0.99 | 97.54 | 101.73 | 0.33 | 6.99 | 0.98 | 99.14 | 113.98 | 0.21 | 5.85 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.E.; Osman, H.E.M.; Al-Bakre, D.A.; Adelodun, B.; Abou Fayssal, S.; Andabaka, Ž.; Goala, M.; et al. Sustainable Upcycling of Mushroom Farm Wastewater through Cultivation of Two Water Ferns (Azolla spp.) in Stagnant and Flowing Tank Reactors. Horticulturae 2022, 8, 506. https://doi.org/10.3390/horticulturae8060506
Kumar P, Eid EM, Taher MA, El-Morsy MHE, Osman HEM, Al-Bakre DA, Adelodun B, Abou Fayssal S, Andabaka Ž, Goala M, et al. Sustainable Upcycling of Mushroom Farm Wastewater through Cultivation of Two Water Ferns (Azolla spp.) in Stagnant and Flowing Tank Reactors. Horticulturae. 2022; 8(6):506. https://doi.org/10.3390/horticulturae8060506
Chicago/Turabian StyleKumar, Pankaj, Ebrahem M. Eid, Mostafa A. Taher, Mohamed H. E. El-Morsy, Hanan E. M. Osman, Dhafer A. Al-Bakre, Bashir Adelodun, Sami Abou Fayssal, Željko Andabaka, Madhumita Goala, and et al. 2022. "Sustainable Upcycling of Mushroom Farm Wastewater through Cultivation of Two Water Ferns (Azolla spp.) in Stagnant and Flowing Tank Reactors" Horticulturae 8, no. 6: 506. https://doi.org/10.3390/horticulturae8060506
APA StyleKumar, P., Eid, E. M., Taher, M. A., El-Morsy, M. H. E., Osman, H. E. M., Al-Bakre, D. A., Adelodun, B., Abou Fayssal, S., Andabaka, Ž., Goala, M., Singh, J., Kumari, S., Arya, A. K., Choi, K. S., Kumar, V., & Širić, I. (2022). Sustainable Upcycling of Mushroom Farm Wastewater through Cultivation of Two Water Ferns (Azolla spp.) in Stagnant and Flowing Tank Reactors. Horticulturae, 8(6), 506. https://doi.org/10.3390/horticulturae8060506












