Effects of Edaphic Fertilization and Foliar Application of Se and Zn Nanoparticles on Yield and Bioactive Compounds in Malus domestica L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Site, Plant Material, and Treatments
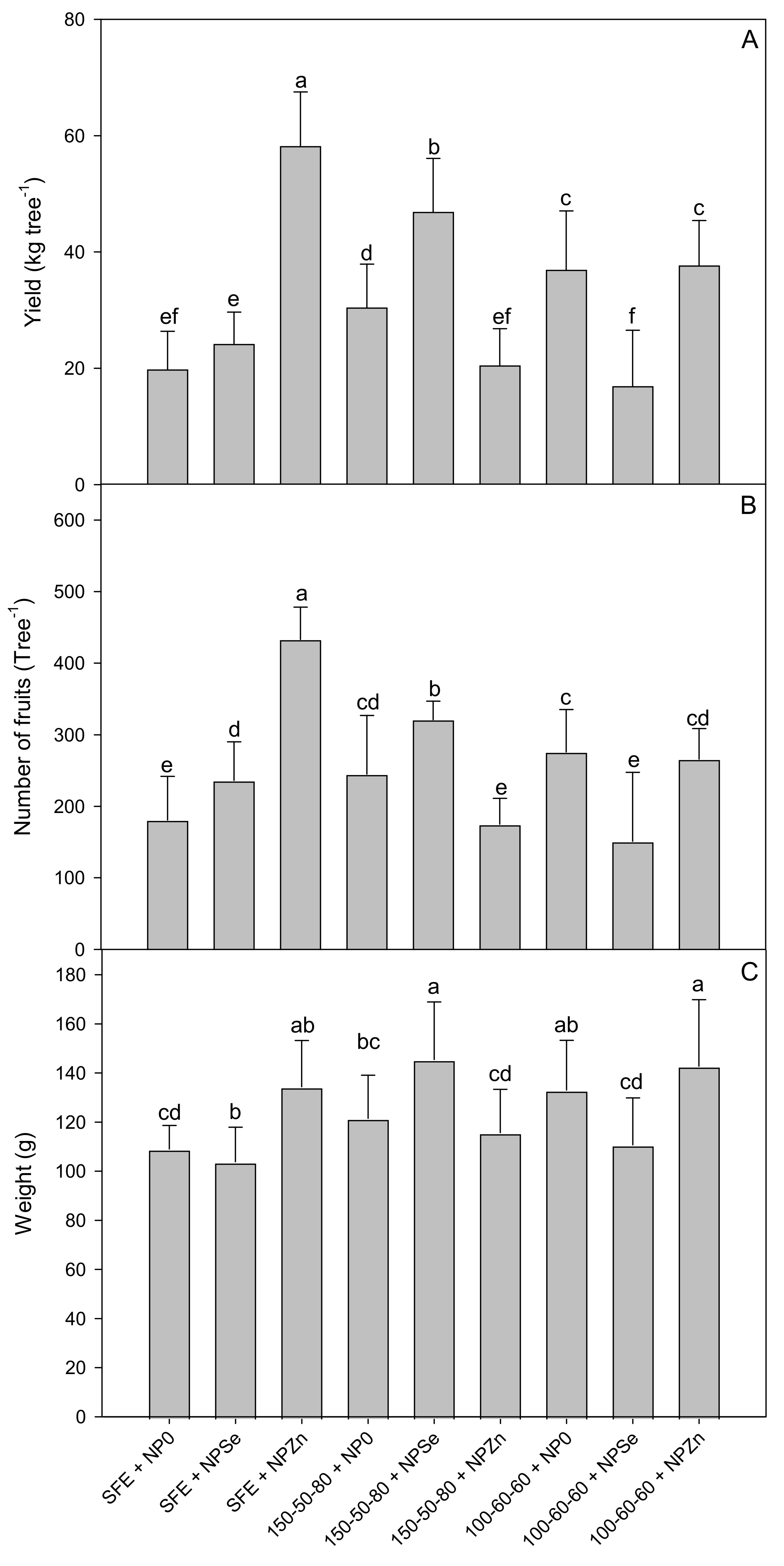
2.3. Fruit Yield, Number, and Weight
2.4. Total, Reducing, and Nonreducing Sugars
2.5. Bioactive Compounds and Antioxidant Activity
2.5.1. Total Phenolic Content, Flavonoids
2.5.2. Vitamin C Content in Apple Fruits
2.5.3. Determination of Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Crop Yield
3.2. Total, Reducing, and Nonreducing Sugars in Apple Fruits
3.3. Contents of Total Phenols, Flavonoids, and Ascorbic Acid
Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 16 January 2022).
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.F.; Couto, M.; Judacewski, P.; Mafra, L.I.; Nogueira, A. Distribution of phenolic compounds and antioxidant capacity in apples tissues during ripening. J. Food Sci. Technol. 2017, 54, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Verma, M.K.; Sharma, S.K. Nutritional characterization of apple as a function of genotype. J. Food Sci. Technol. 2018, 55, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Iordănescu, O.A.; Băla, M.; Iuga, A.C.; Pane, D.G.; Dascălu, I.; Bujancă, G.S.; David, I.; Hădărugă, N.G.; Hădărugă, D.I. Antioxidant Activity and Discrimination of Organic Apples (Malus domestica Borkh.) Cultivated in the Western Region of Romania: A DPPH. Kinetics–PCA Approach. Plants 2021, 10, 1957. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2021, 10, 2. [Google Scholar] [CrossRef]
- Brunetto, G.; De Melo, G.W.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- Sayah, Z.N.; Jameel, D.A. Effect of Nano NPK Balanced Fertilizer (20-20-20) on Some Vegetative and Fruiting Growth of Cucurbita pepo L. Eurasia J. Biosci. 2020, 14, 6627–6633. [Google Scholar] [CrossRef]
- Agustí, M. Fruticultura, 2nd ed.; Mundi-Press Books: Madrid, Spain, 2010; pp. 97–106. [Google Scholar]
- Soto-Parra, J.M.; Piña-Ramírez, F.J.; Sánchez-Chávez, E.; Pérez-Leal, R.; Basurto-Sotelo, M. Fertigation with Macronutrients in Apple “Golden Delicious”: Impact on Yield and Quality of Fruit. Electrónica Nova Sci. 2016, 18, 162–180. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Akanbi-Gada, M.A.; Ogunkunle, C.O.; Vishwakarma, V.; Viswanathan, K.; Fatoba, P.O. Environmental Technology & Innovation Phytotoxicity of Nano-Zinc Oxide to Tomato Plant (Solanum lycopersicum L.): Zn Uptake, Stress Enzymes Response and Influence on Non-Enzymatic Antioxidants in Fruits. Environ. Technol. Innov. 2019, 14, 100325. [Google Scholar] [CrossRef]
- Attia, T.M.S.; Elsheery, N.I. Nanomaterials: Scope, Applications, and Challenges in Agriculture and Soil Reclamation. In Sustainable Agriculture Reviews 41; Springer: Cham, Switzerland, 2020; ISBN 9783030339968. [Google Scholar]
- Elsheery, N.I.; Sunoj, V.S.J.; Wen, Y.; Zhu, J.J.; Muralidharan, G.; Cao, K.F. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol. Biochem. 2020, 149, 50–60. [Google Scholar] [CrossRef]
- Helaly, M.N.; El-Metwally, M.A.; El-Hoseiny, H.; Omar, S.A.; El-Sheery, N.I. Effect of nanoparticles on biological contamination of in vitro cultures and organogenic regeneration of banana. Aust. J. Crop Sci. 2014, 8, 612–624. [Google Scholar]
- Pinedo-Guerrero, Z.H.; Hernández-Fuentes, A.D.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Cu Nanoparticles in Hydrogels of Chitosan-PVA Affects the Characteristics of Post-Harvest and Bioactive Compounds of Jalapeño Pepper. Molecules 2017, 22, 926. [Google Scholar] [CrossRef] [Green Version]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Romenus, K.D.A.; De la Fuente, M.C.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef] [Green Version]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Valdés-Reyna, J.; Pinedo-Espinoza, J.M.; López-Palestina, C.U.; Hernández-Fuentes, A.D. Foliar Application of Cu Nanoparticles Modified the Content of Bioactive Compounds in Moringa oleifera Lam. Agronomy 2018, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of Foliar Application of Zinc Sulfate and Zinc Nanoparticles in Coffee (Coffea arabica L.). Plant. Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.-S.P. Alleviation of the Effect of Salinity on Growth and Yield of Strawberry by Foliar Spray of Selenium-Nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Teixeira da Silva, J.A. The Use of Nanotechnology to Increase Quality and Yield of Fruit Crops. J. Sci. Food Agric. 2020, 100, 25–31. [Google Scholar] [CrossRef]
- Genaidy, E.A.E.; Abd-Alhamid, N.; Hassan, H.S.A.; Hassan, A.M.; Hagagg, L.F. Effect of Foliar Application of Boron Trioxide and Zinc Oxide Nanoparticles on Leaves Chemical Composition, Yield and Fruit quality of Olea europaea L. cv. Picual. Bull. Natl. Res. Cent. 2020, 44, 106. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of Foliar Applications of Zinc and Boron Nano-Fertilizers on Pomegranate (Punica granatum cv. Ardestani) Fruit Yield and Quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of Nano-Calcium and Calcium Chloride Spray on Postharvest Quality and Cell Wall Enzymes Activity in Apple Cv. Red Delicious. Sci. Hortic. 2018, 240, 57–64. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Teixeira da Silva, J.A. Foliar Application of Selenium and Nano-Selenium Affects Pomegranate (Punica Granatum cv. Malase Saveh) fruit yield and quality. S. Afr. J. Bot. 2019, 124, 350–358. [Google Scholar] [CrossRef]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef]
- Treviño-López, E.A.; Sandoval-Rangel, A.; Benavides Mendoza, A.; Ortega Ortiz, H.; Cadenas Pliego, G.; Cabrera de la Fuente, M. Nanopartículas de selenio absorbidas en hidrogeles de quitosán-polivinil alcohol en la producción de pepino injertado. Rev. Mex. Cienc. Agríc. 2021, 26, 159–169. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; González-García, Y.; Cadenas-Pliego, G.; Olivares-Sáenz, E.; Trejo-Téllez, L.I.; Benavides-Mendoza, A. Seed priming with ZnO nanoparticles promotes early growth and bioactive compounds of Moringa oleifera. Not. Bot. Horti. Agrobo. 2021, 49, 12546. [Google Scholar] [CrossRef]
- Ávila, N.R.; Rivas, P.B.; Hernández, M.R. Contenido de azúcares totales, reductores y no reductores en Agave cocui Trelease. Multiciencias 2012, 12, 129–135. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Lahlou, F.A.; Hmimid, F.; Loutfi, M.; Bourhim, N. Antioxidant activity and determination of total phenolic compounds content of Euphorbia Regis-Jubae (webb and berth) from methanol and aqueous extracts. Int. J. Pure App. Biosci. 2014, 2, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Dürüst, N.; Sümengen, D.; Dürüst, Y. Ascorbic Acid and Element Contents of Foods of Trabzon (Turkey). J. Agric. Food Chem. 1997, 45, 2085–2087. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ghazi, D.A. Effect of Iron and Selenium on Growth, Yield and Quality of Eggplant under Different Mineral Fertilization Levels. J. Soil Sci. Agric. Eng. 2018, 9, 525–532. [Google Scholar] [CrossRef]
- Rivera-Gutiérrez, R.G.; Presiado-Rangel, P.; Fortis-Hernández, M.; Betancourt-Galindo, R.; Yescas-coronado, P.; Orozco-Vidal, J.A. Zinc oxide nanoparticles and their effect on melon yield and quality. Rev. Mex. Cienc. Agríc. 2021, 12, 791–803. [Google Scholar] [CrossRef]
- Saini, S.; Kumar, P.; Sharma, N.C.; Sharma, N.; Balachandar, D. Nano-enabled Zn fertilization against conventional Zn analogues in strawberry (Fragaria × Ananassa Duch.). Sci. Hortic. 2021, 282, 110016. [Google Scholar] [CrossRef]
- El-Hak El-Said, R.; El-Aty El- Shazly, S.A.; El-Gazzar, A.A.E.-F.; El-Azeez Shaaban, E.A.; Saleh, M.M.S. Efficiency of Nano-Zinc Foliar Spray on Growth, Yield and Fruit Quality of Flame Seedless Grape. J. Appl. Sci. 2019, 19, 612–617. [Google Scholar] [CrossRef]
- Garciá-López, J.I.; Zavala-Garcia, F.; Olivares-Saénz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Ninõ-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; De La Fuente, M.C.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of Selenium and Copper Nanoparticles on Yield, Antioxidant System, and Fruit Quality of Tomato Plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef] [Green Version]
- Abou El-Nasr, M.; El-Hennawy, H.M.; Samaan, M.S.F.; Salaheldin, T.A.; Abou El-Yaziel, A.; El-Kereamy, A. Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless. Plants 2021, 10, 1285. [Google Scholar] [CrossRef]
- Singh, Y.; Bhatnagar, P.; Kumar Meena, N.; Chandra Gurjar, S. The effect of foliar spray of Zn, Cu and B on physico-chemical parameters of sweet orange (Citrus sinensis L.) cv. Mosambi. J. Pharmacogn. Phytochem. 2018, 7, 1606–1610. [Google Scholar]
- Ikram, M.; Raja, N.I.; Mashwani, Z.U.R.; Omar, A.A.; Mohamed, A.H.; Satti, S.H.; Zohra, E. Phytogenic Selenium Nanoparticles Elicited the Physiological, Biochemical, and Antioxidant Defense System Amelioration of Huanglongbing-Infected ‘Kinnow’ Mandarin Plants. Nanomaterials 2022, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, C.; Yan, Y.; Fan, X.; Li, M.; Wang, Y. Foliar Application of Sugar Alcohol Zinc Increases Sugar Content in Apple Fruit and Promotes Activity of Metabolic Enzymes. Hortscience 2014, 49, 1067–1070. [Google Scholar] [CrossRef] [Green Version]
- Ponce-García, O.C.; Soto-Parra, J.M.; Noperi-Mosqueda, L.C.; Alvarez-Holguín, A.; Ochoa-Rivero, J.M.; Holguín-Gutiérrez, F. Production and quality of apple golden delicious by fertilization with macronutrients. Cienc. E Innovación 2021, 3, 573–581. [Google Scholar]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. ZnO nanoparticles: Recent advances in ecotoxicity and risk assessment. Drug Chem. Toxicol. 2018, 43, 322–333. [Google Scholar] [CrossRef]
- Hezaveh, T.A.; Rahmani, F.; Alipour, H.; Pourakbar, L. Effects of Foliar Application of ZnO Nanoparticles on Secondary Metabolite and Micro-Elements of Camelina (Camelina sativa L.) Under Salinity Stress. J. Stress Physiol. Biochem. 2020, 16, 54–69. [Google Scholar]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef]
- Chang-Zheng, S.; Mei-Ying, L.; Jiang-Fei, M.; Ming, C.; Zhu-Mei, X.; Zhen-Wen, Z. Promoting Effect of Foliage Sprayed Zinc Sulfate on Accumulation of Sugar and Phenolics in Berries of Vitis Vinifera cv. Merlot Growing on Zinc Deficient Soil. Molecules 2015, 20, 2536–2554. [Google Scholar] [CrossRef] [Green Version]
- Soliman, S.S.; Alebidi, A.I.; Al-Obeed, R.S.; Al-Saif, A.M. Effect of potassium fertilizer on fruit quality and mineral composition of Fig (Ficus carica L. cv. brown Turky). Pak. J. Bot. 2018, 50, 1753–1758. [Google Scholar]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the in Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of Phytochemical Composition and Antioxidant Capacity of 22 Old Apple Cultivars Grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
| Treatments | Total Sugars (mg GE g−1 DW) | Reducing Sugars (mg GE g−1 DW) | Nonreducing Sugars (mg GE g−1 DW) |
|---|---|---|---|
| SFE + NP0 | 0.38 ± 0.03 d,e | 0.292 ± 0.02 c,d | 0.10 ± 0.01 d |
| SFE + NPSe | 0.63 ± 0.01 b | 0.329 ± 0.03 b | 0.30 ± 0.03 a |
| SFE + NPZn | 0.73 ± 0.01 a | 0.393 ± 0.04 a | 0.33 ± 0.01 a |
| 150-50-80 + NP0 | 0.34 ± 0.01 f | 0.304 ± 0.03 b,c | 0.04 ± 0.02 e |
| 150-50-80 + NPSe | 0.38 ± 0.04 e,f | 0.302 ± 0.02 b,c | 0.08 ± 0.03 d,e |
| 150-50-80 + NPZn | 0.42 ± 0.04 c,d | 0.268 ± 0.02 d,e | 0.15 ± 0.02 c |
| 100-60-60 + NP0 | 0.45 ± 0.03 c | 0.293 ± 0.01 c,d | 0.16 ± 0.03 c |
| 100-60-60 + NPSe | 0.46 ± 0.05 c | 0.251 ± 0.01 e | 0.21 ± 0.04 b |
| 100-60-60 + NPZn | 0.38 ± 0.03 d,e,f | 0.291 ± 0.03 c,d | 0.09 ± 0.02 d |
| Treatments | Phenols (mg GAE g−1 DW) | Flavonoids (mg QE g−1 DW) | Ascorbic Acid (mg AA g−1 DW) | DPPH (µM TE g−1 DW) | ABTS (µM TE g−1 DW) |
|---|---|---|---|---|---|
| SFE + NP0 | 4.79 ± 0.26 c,d | 8.02 ± 1.71 d,e | 1.03 ± 0.09 d | 14.95 ± 1.72 d,e | 21.77 ± 2.01 d |
| SFE + NPSe | 5.86 ± 0.45 b,c | 11.24 ± 1.52 c,d,e | 1.43 ± 0.07 b,c | 18.50 ± 0.46 b | 26.83 ± 0.12 b |
| SFE + NPZn | 7.64 ± 0.73 a | 15.82 ± 1.69 a | 1.51 ± 0.05 a,b | 23.23 ± 0.47 a | 34.05 ± 0.99 a |
| 150-50-80 + NP0 | 4.35 ± 0.75 d | 8.72 ± 1.60 c,d,e | 1.25 ± 0.10 c | 13.86 ± 0.93 e | 20.56 ± 1.75 d |
| 150-50-80 + NPSe | 6.62 ± 0.46 b | 16.86 ± 1.84 a | 1.54 ± 0.17 a,b | 17.65 ± 0.49 b | 25.62 ± 0.14 b |
| 150-50-80 + NPZn | 5.50 ± 0.58 b,c,d | 12.26 ± 1.73 b,c | 1.62 ± 0.18 a,b | 16.42 ± 0.40 b,c | 25.72 ± 0.79 b |
| 100-60-60 + NP0 | 4.34 ± 0.78 d | 7.75 ± 1.72 e | 1.36 ± 0.07 c | 14.68 ± 1.68 d,e | 22.60 ± 2.13 d |
| 100-60-60 + NPSe | 6.06 ± 0.72 b,c | 11.46 ± 1.59 c,d | 1.49 ± 0.11 a,b | 17.40 ± 0.77 b,c | 25.36 ± 0.86 b,c |
| 100-60-60 + NPZn | 4.96 ± 0.75 c,d | 10.32 ± 2.23 c,d,e | 1.78 ± 0.24 a | 15.27 ± 0.26 c,d | 22.83 ± 0.31c |
| Variables | TP | FL | DPPH | ABTS | AAS |
|---|---|---|---|---|---|
| FT | 1.00 | 0.903 (p = 8.65 × 10−31) | 0.906 (p = 3.70 × 10−31) | 0.881 (p = 2.10 × 10−27) | 0.261 (p = 0.0184) |
| FL | 1.00 | 0.756 (p = 3.23 × 10−16) | 0.749 (p = 8.67 × 10−17) | 0.373 (p = 0.00060) | |
| DPPH | 1.00 | 0.973 (p = 4.55 × 10−52) | 0.185 (p = 0.0991) | ||
| ABTS | 1.00 | 0.261 (p = 0.0187) | |||
| AAS | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montaño-Herrera, A.; Santiago-Saenz, Y.O.; López-Palestina, C.U.; Cadenas-Pliego, G.; Pinedo-Guerrero, Z.H.; Hernández-Fuentes, A.D.; Pinedo-Espinoza, J.M. Effects of Edaphic Fertilization and Foliar Application of Se and Zn Nanoparticles on Yield and Bioactive Compounds in Malus domestica L. Horticulturae 2022, 8, 542. https://doi.org/10.3390/horticulturae8060542
Montaño-Herrera A, Santiago-Saenz YO, López-Palestina CU, Cadenas-Pliego G, Pinedo-Guerrero ZH, Hernández-Fuentes AD, Pinedo-Espinoza JM. Effects of Edaphic Fertilization and Foliar Application of Se and Zn Nanoparticles on Yield and Bioactive Compounds in Malus domestica L. Horticulturae. 2022; 8(6):542. https://doi.org/10.3390/horticulturae8060542
Chicago/Turabian StyleMontaño-Herrera, Anay, Yair Olovaldo Santiago-Saenz, César Uriel López-Palestina, Gregorio Cadenas-Pliego, Zeus H. Pinedo-Guerrero, Alma Delia Hernández-Fuentes, and José Manuel Pinedo-Espinoza. 2022. "Effects of Edaphic Fertilization and Foliar Application of Se and Zn Nanoparticles on Yield and Bioactive Compounds in Malus domestica L." Horticulturae 8, no. 6: 542. https://doi.org/10.3390/horticulturae8060542
APA StyleMontaño-Herrera, A., Santiago-Saenz, Y. O., López-Palestina, C. U., Cadenas-Pliego, G., Pinedo-Guerrero, Z. H., Hernández-Fuentes, A. D., & Pinedo-Espinoza, J. M. (2022). Effects of Edaphic Fertilization and Foliar Application of Se and Zn Nanoparticles on Yield and Bioactive Compounds in Malus domestica L. Horticulturae, 8(6), 542. https://doi.org/10.3390/horticulturae8060542







