Salicylic Acid Foliar Spray Enhanced Silybum marianum Growth and Yield, as Well as Its Chemical Constituents and Chalcone Synthase Gene Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of S. marianum L.
2.2. Measurement of S. marianum Growth and Yield
2.3. Measurement of Photosynthetic Pigments in S. marianum Leaves
2.4. Quantification of Nitrogen, Phosphorus, and Potassium Contents in S. marianum Leaves
2.5. Quantification of Silybin (A + B) in S. marianum Fruits by High-Performance Liquid Chromatography (HPLC)
2.5.1. Preparation of Standard Solutions and Standard Curve
2.5.2. Preparation of Methanolic Extracts from Ground S. marianum Dried Fruits
2.5.3. Instrumentation and Analysis Conditions
2.6. Gene Expression Analyses of CHS 1, 2, and 3 by Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6.1. Extraction of Total RNA and Synthesis of Complementary DNA (cDNA)
2.6.2. Analysis of CHS Genes Expression by Quantitative Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
3.1. Effect of Salicylic Acid on S. marianum Vegetative Growth and Yield
3.2. Photosynthetic Pigments and Element Contents in S. marianum Leaves
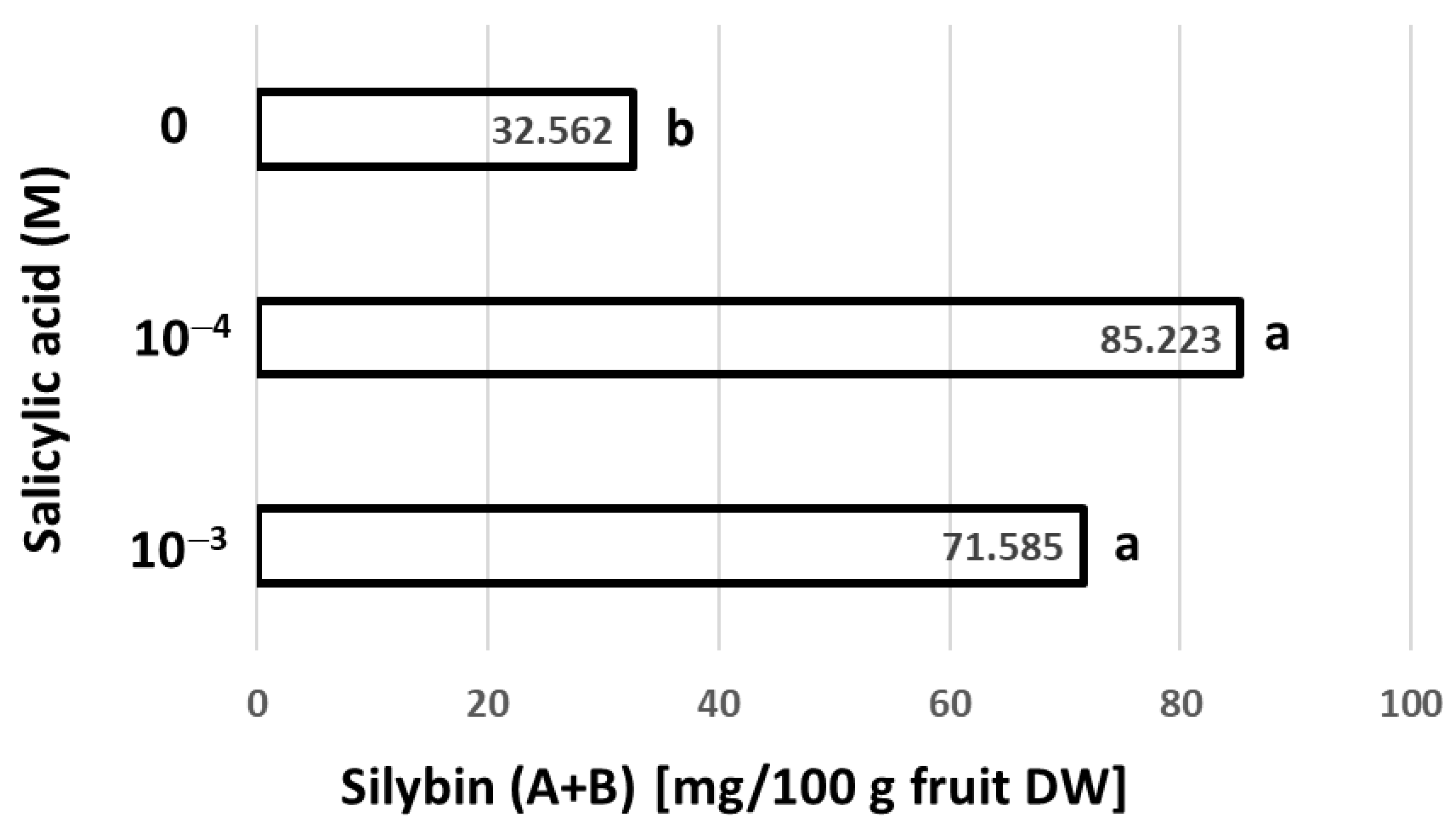
3.3. Effect of Foliar Application of Salicylic Acid on Silybin (A + B) Contents
3.4. The Effect of Foliar Spray of Salicylic Acid on the Expression of CHS 1, 2, and 3 Genes in S. marianum Petals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complementary Altern. Med. AJTCAM/Afr. Netw. Ethnomed. 2013, 10, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P. Milk Thistle (Silybum marianum). In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press, Elsevier Inc.: Cambridge, MA, USA, 2018; pp. 321–325. [Google Scholar] [CrossRef]
- Crocenzi, F.; Roma, M. Silymarin as a New Hepatoprotective Agent in Experimental Cholestasis: New Possibilities for an Ancient Medication. Curr. Med. Chem. 2006, 13, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Q.; Weng, Y.; Wang, S.; Zhao, Y.; Zhang, F.; Zhou, J.; Wu, X. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the Nf-ΚB Pathway. Dig. Dis. Sci. 2018, 63, 3398–3408. [Google Scholar] [CrossRef]
- Khattab, S.; El-Garhy, H.A. Genetic Diversity and in Vitro Assessment of Salt Tolerance Responses and Associated Changes in Gene Expression of Male Poplar (Populus) Trees. J. Hortic. Sci. Biotechnol. 2016, 91, 551–561. [Google Scholar] [CrossRef]
- El Sherif, F.; Khattab, S.; Ibrahim, A.K.; Ahmed, S.A. Improved Silymarin Content in Elicited Multiple Shoot Cultures of Silybum marianum L. Physiol. Mol. Biol. Plants 2013, 19, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Jan, H.; Drouet, S.; Tungmunnithum, D.; Shirazi, J.H.; Hano, C.; Abbasi, B.H. Chitosan Elicitation Impacts Flavonolignan Biosynthesis in Silybum marianum (L.) Gaertn Cell Suspension and Enhances Antioxidant and Anti-Inflammatory Activities of Cell Extracts. Molecules 2021, 26, 791. [Google Scholar] [CrossRef]
- Janda, M.; Ruelland, E. Magical Mystery Tour: Salicylic Acid Signalling. Environ. Exp. Bot. 2015, 114, 117–128. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Alyemeni, M.N.; Ahmad, A. Salicylic Acid Mediated Changes in Growth, Photosynthesis, Nitrogen Metabolism and Antioxidant Defense System in Cicer arietinum L. Plant Soil Environ. 2012, 58, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Gorni, P.H.; De Oliveira Brozulato, M.; Da Silva Lourenção, R.; Konrad, E.C.G. Increased Biomass and Salicylic Acid Elicitor Activity in Fennel (Foeniculum vulgare Miller). Braz. J. Food Technol. 2017, 20, 1–7. [Google Scholar] [CrossRef]
- Amanullah, M.M.; Sekar, S.; Vincent, S. Plant Growth Substances in Crop Production: A Review. Asian J. Plant Sci. 2010, 9, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Gacnik, S.; Veberič, R.; Hudina, M.; Marinovic, S.; Halbwirth, H.; Mikulič-petkovšek, M. Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest. Plants 2021, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gupta, R.C.; Dey, A.; Malik, T.; Pandey, D.K. Optimization of Salicylic Acid and Chitosan Treatment for Bitter Secoiridoid and Xanthone Glycosides Production in Shoot Cultures of Swertia paniculata Using Response Surface Methodology and Artificial Neural Network. BMC Plant Biol. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gorni, P.H.; Pacheco, A.C. Growth Promotion and Elicitor Activity of Salicylic Acid in Achillea millefolium L. Afr. J. Biotechnol. 2016, 15, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, A.C.; Gorni, P.H. Elicitation with Salicylic Acid as a Tool for Enhance Bioactive Compounds in Plants. In Salicylic Acid—A Versatile Plant Growth Regulator; Hayat, S., Siddiqui, H., Damalas, C.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–15. ISBN 978-3-030-79229-9. [Google Scholar]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Divya, P.; Puthusseri, B.; Neelwarne, B. The Effect of Plant Regulators on the Concentration of Carotenoids and Phenolic Compounds in Foliage of Coriander. LWT—Food Sci. Technol. 2014, 56, 101–110. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The Origin and Evolution of Plant Flavonoid Metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Sanjari, S.; Shobbar, Z.S.; Ebrahimi, M.; Hasanloo, T.; Sadat-Noori, S.-A.; Tirnaz, S. Chalcone Synthase Genes from Milk Thistle (Silybum marianum): Isolation and Expression Analysis. J. Genet 2015, 94, 611–617. [Google Scholar] [CrossRef]
- Yap, Y.K.; El-sherif, F.; Habib, E.S.; Khattab, S. Moringa Oleifera Leaf Extract Enhanced Growth, Yield and Silybin Content While Mitigating Salt-induced Adverse Effects on the Growth of Silybum marianum. Agronomy 2021, 11, 2500. [Google Scholar] [CrossRef]
- El-Garhy, H.A.S.; Khattab, S.; Moustafa, M.M.A.; Abou Ali, R.; Abdel Azeiz, A.Z.; Elhalwagi, A.; El Sherif, F. Silybin Content and Overexpression of Chalcone Synthase Genes in Silybum marianum L. Plants under Abiotic Elicitation. Plant Physiol. Biochem. 2016, 108, 191–202. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Karimi, E. Involvement of Salicylic Acid on Antioxidant and Anticancer Properties, Anthocyanin Production and Chalcone Synthase Activity in Ginger (Zingiber officinale Roscoe) Varieties. Int. J. Mol. Sci. 2012, 13, 14828–14844. [Google Scholar] [CrossRef] [PubMed]
- Campos, Â.D.; Ferreira, A.G.; Hampe, M.M.V.; Antunes, I.F.; Brancão, N.; Silveira, E.P.; Da Silva, J.B.; Osório, V.A. Induction of Chalcone Synthase and Phenylalanine Ammonia-Lyase by Salicylic Acid and Colletotrichum lindemuthianum in Common Bean. Braz. J. Plant Physiol. 2003, 15, 129–134. [Google Scholar] [CrossRef]
- Hesami, S.; Nabizadeh, E.; Rahimi, A.; Rokhzadi, A. Effects of Salicylic Acid Levels and Irrigation Intervals on Growth and Yield of Coriander (Coriandrum sativum) in Field Conditions. Environ. Exp. Biol. 2012, 10, 113–116. [Google Scholar]
- Preciado-Rangel, P.; Reyes-Pérez, J.J.; Ramírez-Rodríguez, S.C.; Salas-Pérez, L.; Fortis-Hernández, M.; Murillo-Amador, B.; Troyo-Diéguez, E. Foliar Aspersion of Salicylic Acid Improves Phenolic and Flavonoid Compounds, and Also the Fruit Yield in Cucumber (Cucumis sativus L). Plants 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Es-sbihi, F.Z.; Hazzoumi, Z.; Amrani Joutei, K. Effect of Salicylic Acid Foliar Application on Growth, Glandular Hairs and Essential Oil Yield in Salvia officinalis L. Grown under Zinc Stress. Chem. Biol. Technol. Agric. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Method of Analysis of the Association of Official Chemist. In Official Methods of Analysis; Howitz William, Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1984. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis—A Laboratory Bombay; Hans Publishers: Delhi, India, 1947. [Google Scholar]
- Jackson, M.L. Soil Chemica Analysis; Prentice Hall: New Delhi, India, 1967. [Google Scholar]
- Murphy, J.; Riley, J.P.A. Modified Single-Solution Method for the Determination of Phosphorus in Natural Water. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mazumdar, B.; Majumder, K. Methods of Physiochemical Analysis of Fruits; Daya Publishing House: Delhi, India, 2003. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- StatSoft STATISTICA for Windows, Version 6; StatSoft Inc.: Tulsa, OK, USA, 2001.
- Mohammad, F.; Wajid, M.A.; Bhat, M.A. Effect of Salicylic Acid Sprays on the Performance of Fenugreek Grown with Graded Levels of Salinity. Haya: Saudi J. Life Sci. 2019, 4, 346–354. [Google Scholar] [CrossRef]
- Ahmad, I.; Basra, S.M.A.; Wahid, A. Exogenous Application of Ascorbic Acid, Salicylic Acid and Hydrogen Peroxide Improves the Productivity of Hybrid Maize at Low Temperature Stress. Int. J. Agric. Biol. 2014, 16, 825–830. [Google Scholar]
- Ali, Z.H.; Ali, F.H. Effect of Salicylic Acid on Chlorophyll A, B and Carotene Content of Petunia Sp. Indian J. Ecol. 2019, 46, 188–191. [Google Scholar]
- Ismail, A.; Shahidan, N.; Mat, N.; Othman, R. Effect of Salicylic Acid on Carotenoids and Chlorophyll Content in Mas Cotek (Ficus deltoidea Jack Var. Trengganuensis) Leaves and Its Retinol Activity Equivalents (RAE). J. Pharm. Nutr. Sci. 2020, 10, 25–33. [Google Scholar] [CrossRef]
- Parashar, A.; Yusuf, M.; Fariduddin, Q.; Ahmad, A. Salicylic Acid Enhances Antioxidant System in Brassica juncea Grown under Different Levels of Manganese. Int. J. Biol. Macromol. 2014, 70, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Cao, H.; Cui, X.; Zheng, W.; Wang, S.; Yu, J.; Chen, Z. Molecules Silymarin’ s Inhibition and Treatment Effects for Alzheimer’ s Disease. Molecules 2019, 24, 1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossainzadeh, S.; Ranji, N.; Naderi Sohi, A.; Najafi, F. Silibinin encapsulation in polymersome: A promising anticancer nanoparticle for inducing apoptosis and decreasing the expression level of miR-125b/miR-182 in human breast cancer cells. J. Cell. Physiol. 2019, 234, 22285–22298. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.S.; Moawad, A.S.; AbouZid, S.F.; Owis, A.I. Salicylic Acid Increases Flavonolignans Accumulation in the Fruits of Hydroponically Cultured Silybum marianum. Saudi Pharm. J. 2020, 28, 593–598. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.P.; Zhang, L.; Yan, P.; Ahammed, G.J.; Han, W.Y. Methyl Salicylate Enhances Flavonoid Biosynthesis in Tea Leaves by Stimulating the Phenylpropanoid Pathway. Molecules 2019, 24, 362. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, R.; Ma, G.; Zhang, L.; Hirai, M.; Yahata, M.; Yamawaki, K.; Shimada, T.; Fujii, H.; Endo, T.; Kato, M. Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin in Vitro. Appl. Sci. 2020, 10, 8916. [Google Scholar] [CrossRef]
- Pacheco, A.C.; Cabral, S.; Sabrina, É. Salicylic Acid-Induced Changes to Growth, Flowering and Flavonoids Production in Marigold Plants. J. Med. Plants Res. 2013, 7, 3158–3163. [Google Scholar] [CrossRef]
- Bhasker, P.; Gupta, P.; Sharma, H. Role of Salicylic Acid on Growth, Yield, Quality and Disease Pest Reaction of Onion (Allium cepal.) CV. Agrifound Light Red. SAARC J. Agric. 2020, 18, 39–49. [Google Scholar] [CrossRef]
- Gondor, O.K.; Janda, T.; Soós, V.; Pál, M.; Majláth, I.; Adak, M.K.; Balázs, E.; Szalai, G. Salicylic Acid Induction of Flavonoid Biosynthesis Pathways in Wheat Varies by Treatment. Front. Plant Sci. 2016, 7, 1447. [Google Scholar] [CrossRef] [Green Version]
| Salinity Level (ppm) | Cations (meq L−1) | Anions (meq L−1) | Sodium Absorption Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 864 | Ca2+ | Mg2+ | Na+ | K+ | CO32− | HCO3− | SO42− | Cl− | 3.43 |
| 5.72 | 2.02 | 7.27 | 0.38 | 0.28 | 2.68 | 4.03 | 8.4 | ||
| Salicylic Acid (M) | Plant Height (cm) | Number of Leaves (n) | Branch Number (n) | Root DW (g) | Aerial Part DW (g) |
|---|---|---|---|---|---|
| 0 | 50.75 b * | 50.50 b | 3.25 b | 6.56 b | 38.83 b |
| 10−4 | 92.00 a | 96.25 a | 5.75 a | 17.49 a | 132.53 a |
| 10−3 | 72.25 a | 52.50 b | 3.50 b | 9.03 b | 59.49 b |
| Salicylic Acid (M) | Capitula Number (n) | Fruits DW (g) |
|---|---|---|
| 0 | 5.00 b * | 16.65 b |
| 10−4 | 17.00 a | 56.95 a |
| 10−3 | 7.25 b | 20.53 b |
| Salicylic Acid (M) | Chl-a (mg/100g FW) | Chl-b (mg/100g FW) | Carotenoids (mg/100g FW) |
|---|---|---|---|
| 0 | 28.28 b * | 6.43 b | 38.88 b |
| 10−4 | 49.98 a | 12.45 a | 48.07 a |
| 10−3 | 29.18 b | 6.86 b | 36.82 b |
| Salicylic Acid (M) | N%/DW | P%/DW | K%/DW |
|---|---|---|---|
| 0 | 1.035 b * | 0.305 b | 1.52 b |
| 10−4 | 1.145 a | 0.698 a | 2.84 a |
| 10−3 | 1.115 a | 0.415 b | 1.73 b |
| Salicylic Acid (M) | CHS 1 (Fold) | CHS 2 (Fold) | CHS 3 (Fold) |
|---|---|---|---|
| 0 | 1.000 c * ± 0.439 | 1.000 c ± 0.168 | 1.000 c ± 0.147 |
| 10−4 | 2.984 a ± 0.990 | 1.682 a ± 0.343 | 2.479 a ± 0.063 |
| 10−3 | 1.912 b ± 0.104 | 1.449 b ± 0.249 | 1.425 b ± 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattab, S.; Yap, Y.-K.; El Sherif, F. Salicylic Acid Foliar Spray Enhanced Silybum marianum Growth and Yield, as Well as Its Chemical Constituents and Chalcone Synthase Gene Activity. Horticulturae 2022, 8, 556. https://doi.org/10.3390/horticulturae8060556
Khattab S, Yap Y-K, El Sherif F. Salicylic Acid Foliar Spray Enhanced Silybum marianum Growth and Yield, as Well as Its Chemical Constituents and Chalcone Synthase Gene Activity. Horticulturae. 2022; 8(6):556. https://doi.org/10.3390/horticulturae8060556
Chicago/Turabian StyleKhattab, Salah, Yun-Kiam Yap, and Fadia El Sherif. 2022. "Salicylic Acid Foliar Spray Enhanced Silybum marianum Growth and Yield, as Well as Its Chemical Constituents and Chalcone Synthase Gene Activity" Horticulturae 8, no. 6: 556. https://doi.org/10.3390/horticulturae8060556
APA StyleKhattab, S., Yap, Y.-K., & El Sherif, F. (2022). Salicylic Acid Foliar Spray Enhanced Silybum marianum Growth and Yield, as Well as Its Chemical Constituents and Chalcone Synthase Gene Activity. Horticulturae, 8(6), 556. https://doi.org/10.3390/horticulturae8060556






