Abstract
The two-spotted spider mite, Tetranychus urticae, is an important pest of horticultural crops worldwide and has developed resistance against multiple kinds of pesticides. To date, information on the resistance to pesticides is inadequate for T. urticae populations in Hainan, south China. In the current study, we determined the resistance to seven pesticides in five field populations of T. urticae that were collected on cucurbit crops in Hainan in 2021. The results showed that T. urticae populations developed high to extremely high resistance to abamectin and bifenthrin and medium to high resistance to pyridaben, profenofos, and cyflumetofen. However, four of the five populations were either susceptible to or had only low resistance to bifenazate and B-azolemiteacrylic. We also determined the frequencies of mutations previously associated with T. urticae resistance to abamectin, pyrethroids, organophosphates, bifenazate, or pyridaben; mutation frequencies as high as 100% were detected for some of the mutations in some of the populations. The results should facilitate the development of rational strategies for the chemical control of T. urticae populations in Hainan, China.
1. Introduction
The two-spotted spider mite, Tetranychus urticae, is a global agricultural and horticultural pest that feeds on the sap of host plant leaves, resulting in chlorosis, drying, and even death. Such damage ultimately reduces crop yield and quality [1]. To control mites, growers commonly spray chemical pesticides. Given the frequent use of pesticides and the characteristics of T. urticae, the resistance of field populations of T. urticae to different kinds of pesticides has become a serious problem worldwide, including in Europe, Australia, Turkey, Canada [2,3,4,5,6], and China. In different regions in China, researchers have reported the resistance levels of some T. urticae field populations to abamectin and bifenthrin and even to some newly developed pesticides [7,8,9].
The resistance of T. urticae to chemical pesticides has mainly been associated with the increased activity of detoxification enzymes (i.e., metabolic resistance) and with mutations that alter the structure of targeted genes [10]. Metabolic resistance usually involves detoxification enzymes such as cytochrome P450 monooxygenases (P450s), carboxy/cholinesterases (CarEs or CCEs), and glutathione S-transferases (GSTs) [1,11]. The target-site mutations G314D and G326E in the glutamate-gated chloride channel genes GluCl1 and GluCl3, respectively, have been related to abamectin resistance in T. urticae [12]. Mutations L1024V, A1215D, and F1538I in the voltage-gated sodium channel gene VGSC were reported to be responsible for pyrethroid (sodium channel modulators) resistance [13], and mutations G119S, A201S, T280A, G328A, and F331W/Y in the acetylcholine gene have been associated with organophosphate resistance [10,14]. A recent study also identified mutations associated with the resistance of T. urticae populations to the newly developed pesticide bifenazate, mitochondrial complex III electron transport inhibitors [15].
Tetranychus urticae is highly polyphagous, feeding on more than 1100 plant species in more than 140 plant families [16], including cucurbits. Cucurbits are commonly cultivated in Hainan Province, south China, in part because of the high temperatures of the region [17]. Although cucurbits are important host plants of T. urticae, the pesticide resistance of populations in Hainan is unclear, which limits the efficient control or resistance management of the mite populations.
In this current study, we evaluated the resistance of five T. urticae field populations collected from Hainan province to seven commonly used and newly-developed pesticides. The mutation frequencies of the targeted resistance genes related to commonly used insecticides were also determined. The results should improve the chemical control of field populations of T. urticae on cucurbit crops in Hainan and should also facilitate resistance management.
2. Materials and Methods
2.1. Tetranychus urticae Populations
A pesticide-susceptible laboratory strain (IPP-SS) was provided by the Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The IPP-SS strain has been kept on leave discs of kidney bean, Phaseolus vulgaris, in a laboratory for more than 10 years without exposure to any pesticides. Five field populations of T. urticae were collected in Hainan, China; each population was a mixture of three subsamples with at least 1000 mites per subsample. Each subsample was collected from the different plants of the same plant species. The plantation size of each sampled cucurbit crop ranged from 667 m2 to 1334 m2. Background information on the five field populations is provided in Table 1. All the cucurbit crops were cultivated in the plastic tunnels, and abamectin, emamectin benzoate, and pyridaben were frequently applied for pest control (personal observation). All of the mites were reared in a growth chamber at 26 ± 1 °C and with 60% ± 5% relative humidity (RH) and a 16 h: 8 h (L: D) photoperiod.

Table 1.
Background information on the field populations of T. urticae collected in Hainan, China.
2.2. Toxicity Bioassay
Toxicity bioassays with abamectin, three traditional pesticides (profenofos, bifenthrin, and pyridaben), and three recently developed pesticides (B-azolemiteacrylic, bifenazate, and cyflumetofen) were carried out using the leaf-dip method as previously described [8]. All of the tested pesticides were commercial formulations and purchased from chemical companies as follows: abamectin 1.8% EC (Veyong Biochemical, Hebei, China), pyridaben 15% EC and profenofos 40% EC (Yongnong Biological Science, Zhejiang, China), bifenthrin 25 g/L EC (Quzhou Chemical Industry, Zhejiang, China), 30% B-azolemiteacrylic SC (Sinochem Pesticide R & D, Shenyang, China), 20% cyflumetofen SC (FMC Plant Protection, Shanghai, China), and bifenazate 43% SC (Macdermid Chemical, Shanghai). Each pesticide was diluted with water to 6 concentrations based on the preliminary experiments, and water alone was used as the control. The fresh leaves of kidney beans were cut into small discs about 2 cm in diameter. The discs were completely immersed in the prepared concentrations of pesticides for 10 s and were then air-dried under a fume hood. The treated discs were placed abaxial side up in a Petri dish (3.5 cm diameter; one disc per dish) with 0.2% agar. A fine brush was used to place about 25 healthy and active female adults of T. urticae on each disc. The dishes were then covered with lids that had holes to prevent the accumulation of water vapor. Each combination of pesticide concentration and population was represented by four replicate dishes, except that the toxicity data for the susceptible IPP-SS strain were obtained from a recent study in our laboratory [9]). The Petri dishes were kept in an incubator (RXZ-380C, ZJ, China) for 24 h before the dead and living mites were counted with the aid of a stereoscope (SZX-7, Olympus, Japan). The mites were considered dead if they did not move when touched by a brush. The bioassay data were considered valid when the mortality rate in the control treatment was less than 20%.
2.3. DNA Extraction and Determination of Mutation Frequencies
The genomic DNA of female adult mites was extracted according to the instructions of the KAPA Express Extract Kit (Roche-Kapa Biosystems, Basel, Switzerland). A single adult female was ground with a plastic grinding rod in a single eight-line tube containing 30 μL of extract buffer (0.5 μL of 1 u/μL Rapid enzyme, 3.0 μL of 1 u/μL Rapid extraction buffer, and 26.5 μL of ddH2O). The extraction was performed with a PCR instrument, which was used to heat the sample to 75 °C for 10 min and then to 95 °C for 5 min. The DNA extracts were then stored at −20 °C.
The mutations in the target genes related to the resistance of abamectin, profenofos, bifenthrin, bifenazate, and pyridaben were determined by sequencing the PCR products generated by specific primers based on previous studies [3,8,9] (Table S1). For each mutation of the target gene in each population, at least 30 female adults were randomly chosen and assessed. The PCR reaction contained 2.0 μL of genomic DNA, 1.25 μL of each primer, 0.5 μL of dNTPs, 0.5 μL of 1 × Q5 reaction buffer, 5 μL of 1 × Q5 High GC enhancer, and 0.25 μL of Q5 High-Fidelity DNA polymerase. PCR amplifications were carried out in an S1000 thermal cycler (Bio-Rad, CA, USA) as follows: initial denaturation for 30 s at 98 °C; followed by 35 cycles of 10 s at 98 °C, 20 s at 53.7–58.0 °C, and 20 s at 72 °C; and a final extension of 2 min at 72 °C. The PCR products were purified and sent to Sangon Biotech Company (Shanghai, China) for bidirectional sequencing using the specific primers same as the PCR amplification.
2.4. Data Analysis
Polo Plus 2.0 software was used to analyze the bioassay data to obtain the medium lethal concentration (LC50) and the 95% fiducial limits. The resistance fold (RF) of each field population was obtained by dividing its LC50 value by that of strain IPP-SS; as noted, the LC50 value of strain IPP-SS was obtained from a recent study in our laboratory [9]. Based on the RF values, the field populations were considered to be susceptible (1 ≤ RF ≤ 3) or to have low resistance (3 ≤ RF ≤ 10), moderate resistance (10 ≤ RF ≤ 100), high resistance (100 ≤ RF ≤ 1000), or extremely high resistance (RF > 1000) [9]. The homozygous resistant (RR), heterozygous resistant (RS), and homozygous susceptible (SS) were obtained from the sequencing. The frequencies of the resistance alleles were calculated according to the following formula: F (R allele) = ([the number of resistance homozygous mites/total number of detected mites] + [the number of resistance heterozygous mites/the total number of detected mites/2]). The mutation frequency of each population was plotted using the origin software (version 2021).
3. Results
3.1. Resistance of T. urticae to Abamectin and Traditional Pesticides
Based on the LC50 values, the IPP-SS strain was much more susceptible than the five field populations to abamectin and to the traditional pesticides profenofos, bifenthrin, and pyridaben (Table 2). All of the five T. urticae populations obtained from fields in Hainan showed extremely high resistance to abamectin (RF up to 9914.07-fold in population SY-HN3), high resistance to bifenthrin, medium resistance to profenofos, and medium to high resistance to pyridaben (Table 2).

Table 2.
Resistance of T. urticae populations from Hainan to abamectin and three traditional pesticides.
3.2. Resistance of T. urticae to Recently Developed Pesticides
Four of the field populations were susceptible to B-azolemiteacrylic, and one population, LD-HN, exhibited medium resistance. The field populations exhibited low to medium resistance to bifenazate and medium to extremely high resistance to cyflumetofen (Table 3).

Table 3.
Resistance of T. urticae populations from Hainan to recently developed pesticides.
3.3. Mutation Frequencies of Target Sites
The mutation frequencies of the GluCls, VGSC, PSST, Ace, and Cytb genes from the five field populations of T. urticae are indicated in Figure 1. The frequency of the G314D mutation in the GluCl1 gene ranged from 96% to 100% among the five populations. The frequency of the G326E mutation in the GluCl3 gene ranged from 48% to 100% among the populations. In the VGSC gene, the frequency of the F1538I mutation ranged from 47% to 100% among the populations, and the frequency of the A1215D mutation was 100% in all of the five populations. In the Ace gene, the frequencies of G119S and A201S mutations ranged from 20% to 92% and from 1.7% to 80%, respectively, among the populations. In the PSST gene, the frequency of the H92R mutation was 100% in all five populations. In the Cytb gene, the frequency of the G126S mutation was 3% only in the LD-HN population; no other mutations were detected at positions 126, 136, 141, 161, or 262 in the five field populations.
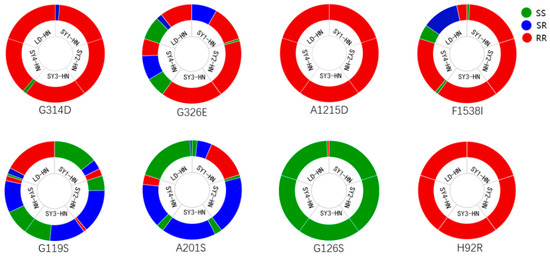
Figure 1.
Mutation frequency of resistance genes in five T. urticae field populations in Hainan. G314D (GluCl1) and G326E (GluCl3) mutations, G119S and A201S mutations (Ace), A1215D and F1538I mutations (VGSC), G126S mutation (Cytb), and H92R mutation (PSST) in T. urticae are presented. SS, RS, and RR indicate homozygous susceptible, heterozygous, and homozygous resistant, respectively.
4. Discussion
4.1. Extremely High Resistance to Abamectin Accompanied with High Frequencies of Point Mutations
In this study, which was conducted in 2021, we evaluated the resistance of T. urticae populations on cucurbit crops in Hainan Province to abamectin, traditional pesticides, and novel pesticides. Growers in Hainan have frequently applied abamectin and pyridaben to control T. urticae and other pests in the field. In recent years, different levels of resistance to abamectin have been reported for T. urticae populations in many regions of the world [5,9,18,19]. In the current study, we found that all five field populations collected from Hainan developed extremely high resistance to abamectin with RF values > 4800, compared to the IPP-SS strain. These results are similar to those reported in 2020 for T. urticae populations on eggplant in Jiyang, a different location in Sanya, Hainan [9], showing that abamectin resistance of T. urticae has possibly existed in wider locations in Hainan. Moreover, the extremely high resistance to abamectin was reported for T. urticae populations in 2018, 2019–2020, and 2021 in Hainan ([8,9], present study), even though the populations are not exactly the same, showing such resistance is relatively stable. In addition, based on the LC50 values from the toxicity bioassay experiments in this study, the susceptibility to abamectin of T. urticae in the five regions in Hainan was low, with LC50 values ranging from 220 mg/L to 456 mg/L. Therefore, abamectin should no longer be applied for the control of T. urticae in Sanya and Ledong in Hainan Province, China currently.
The target site mutations G314D in the GluCl1 gene and G326E in the GluCl3 gene have been reported to be associated with abamectin resistance in T. urticae [12,20,21]. In this study, the frequencies of G314D and G326E mutations in T. urticae collected from Hainan ranged from 94% to 100% and 48% to 100%, respectively. However, no correlation was found between the RF values against abamectin and the frequencies of point mutations (data not shown), suggesting that these populations may have another resistance mechanism or other mechanisms. In addition to target site mutations, for example, detoxification mechanisms have been reported to be involved in the abamectin resistance of T. urticae populations [5,22,23]. A previous study found that the G326E mutation alone or in combination with G314D could not induce high abamectin resistance in a T. urticae population [24], again suggesting the possible existence of multiple abamectin resistant mechanisms in T. urticae field populations. Another point mutation in GluCl3, I321T, was recently detected in a red color morph of T. urticae in Europe [5], but this mutation was not detected in the green color morph (the only morph present) of T. urticae in the current study. So, the complex resistance mechanism to abamectin in different populations needs to be further explored.
4.2. Prudent Application of Traditional Pesticides due to Medium to High Resistance
For many years, bifenthrin, pyridaben, and profenofos have been widely used to control T. urticae, and the resistance to these traditional acaricides in T. urticae populations has been reported in some countries [19,25,26]. In the current study, five T. urticae populations in Hainan showed high resistance to bifenthrin, with RF values ranging from 216 to 784. The frequencies of mutations related to bifenthrin resistance, i.e., A1215D and F1538I [27], were 100% and 47% to 100%, respectively, but as previously noted, the mutation frequencies were not correlated with resistance in T. urticae populations from Hainan. Therefore, we suspect that other resistance mechanisms are involved in the resistance of the tested T. urticae populations to bifenthrin. In support of that possibility, mixed-function oxidase (MFO) was reported to be important for the bifenthrin resistance of T. urticae on cowpea in China [28]. The five T. urticae populations in the current study were moderately resistant to the organophosphate profenofos, even though the frequencies of G119S and A201S mutations, which are important for organophosphate resistance [29], ranged from 20% to 92% and from 2% to 80%, respectively. The frequencies of the G119S mutation previously reported in China and Ethiopia were higher than in the current study, but the G119S mutation had not been detected in Turkey [10,25].
The five T. urticae populations in the current study showed a moderate to high level of resistance to pyridaben. Four of the populations had pyridaben LC50 values > 5000 mg/L. The pyridaben resistance status of T. urticae populations in Hainan was much higher in the current study than in a study conducted in 2019–2020 [9], indicating that the resistance of T. urticae populations to pyridaben has rapidly increased in Hainan. This is possibly related to the frequent use of pyridaben in the field. The frequency of the H92R mutation, which has been related to pyridaben resistance [3], was 100% and might explain the high level of resistance. This was not the same case for some other T. urticae populations [30]. The combination of genes that encode detoxification enzymes (including CYP392A3) and the H110R mutation contributes to the resistance increases of the T. urticae field populations to pyridaben [30]. Of course, the involvement of other resistant mechanisms that have yet to be discovered cannot be excluded. Given the increased resistance to pyridaben and the high mutation frequency in resistance genes, pyridaben is unlikely to be effective for controlling T. urticae in Hainan, China.
4.3. Alternative Use of Novel Pesticides for T. urticae Control
Cyflumetofen, bifenazate, and B-azolemiteacrylic are newly developed pesticides, and the latter two, in particular, have always been reported to provide high efficiencies of T. urticae control in the field [31,32]. The current study, however, documented the occurrence of resistance against bifenazate and B-azolemiteacrylic in T. urticae populations in some locations in Hainan. The five populations had low to medium resistance to bifenazate; for B-azolemiteacrylic, some populations exhibited no resistance, but other populations exhibited medium resistance. Given that the LD-HN population had medium resistance to both bifenazate and B-azolemiteacrylic, careful rotation of pesticides is now needed in the area. The frequency of the G126S mutation was 3.45% in the LD-HN population, but this mutation alone does not confer bifenazate resistance in T. urticae, according to a recent report [33]. The resistance to bifenazate should be monitored in Ledong, Hainan continuously, and the underlying resistance mechanism should be determined. Although the LD-HN population had a moderate level of resistance to B-azolemiteacrylic (RF = 12.80), the other four T. urticae populations remained sensitive to B-azolemiteacrylic. Based on the current results and those of a previous study [34], which showed that the use of B-azolemiteacrylic inhibited the developmental rate of T. urticae and did not readily select for resistance, we suggest that B-azolemiteacrylic can still be used to control T. urticae in Hainan.
In Japan in 2007, cyflumetofen was registered as an acaricide for use on fruit trees. To date, different levels of resistance against cyflumetofen have been reported in T. urticae populations in China and Greece [9,28,35]. In the present study, high to extremely high levels of cyflumetofen resistance were detected in T. urticae populations in Hainan; with an RF value of 1050, the LD-HN population had the highest level of cyflumetofen resistance. These results indicate that T. urticae populations in Hainan are rapidly developing resistance to cyflumetofen and that the efficacy of cyflumetofen is predicted to decline in the field. It follows that alternative pesticides should be used in rotation with cyflumetofen.
In this study, the resistance of five T. urticae field populations from Hainan province was determined, which is possibly not very sufficient to reflect the overall resistant status of T. urticae populations feeding on the cucurbit crops cultivated in plastic tunnels in Hainan. In order to demonstrate the resistant status of T. urticae in this area, it is necessary to assess the resistance for more populations in the near future, taking the different host plants, cultivation methods, and other factors into consideration. However, we believe that the results of this study suggest prudent selection and application of pesticides for the control of T. urticae in Hainan, China.
5. Conclusions
In conclusion, the five T. urticae populations from Hainan, China, in the current study exhibited high to extremely high resistance to abamectin and bifenthrin and medium to high resistance to pyridaben, profenofos, and cyflumetofen. In contrast, four of the five populations were either susceptible to or had only low resistance to bifenazate and B-azolemiteacrylic. In addition, the mutation frequencies of genes previously associated with resistance to abamectin, pyrethroids, organophosphates, and pyridaben were high. Finally, we conclude that abamectin and traditional pesticides are no longer suitable for field use in the tested locations in Hainan currently. Bifenazate and B-azolemiteacrylic, in contrast, would provide greater control than abamectin, bifenthrin, pyridaben, profenofos, or cyflumetofen and could slow the development of resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8070590/s1, Table S1: Primers and amplified PCR products used to detect mutations in target sites associated with the pesticide resistance of Tetranychus urticae.
Author Contributions
Conceptualization, S.W.; methodology, T.T. and M.W. (Mingmei Wu); software, T.T. and M.W. (Mingmei Wu); validation, T.T., M.W. (Mingmei Wu), Y.Z. and D.X.; formal analysis, T.T. and M.W. (Mingmei Wu); investigation, T.T., M.W. (Mingyue Wu) and Y.Z.; resources, M.W. (Mingyue Wu); writing—original draft preparation, W.X. and S.W.; writing—review and editing, Q.S. and S.W.; visualization, T.T. and D.X.; supervision, S.W.; project administration, W.X. and S.W; funding acquisition, W.X. and S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32072458), the China Agriculture Research System of MOF and MARA (CARS-25), the 2020 Research program of Sanya Yazhou Bay Science and Technology City (SKJC-2020-02-012), and the Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables, the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-IVFCAAS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namin, H.H.; Zhurov, V.; Spenler, J.; Grbic, M.; Grbic, V.; Scott, I.M. Resistance to pyridaben in Canadian greenhouse populations of two-spotted spider mites, Tetranychus urticae (Koch). Pestic. Biochem. Physiol. 2020, 170, 104677. [Google Scholar] [CrossRef] [PubMed]
- Bajda, S.; Dermauw, W.; Panteleri, R.; Sugimoto, N.; Douris, V.; Tirry, L.; Osakabe, M.; Vontas, J.; Van Leeuwen, T. A mutation in the PSST homologue of complex I (NADH: Ubiquinone oxidoreductase) from Tetranychus urticae is associated with resistance to METI acaricides. Insect Biochem. Mol. Biol. 2017, 80, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, E.; Cevik, B.; Ay, R. Abamectin resistance and resistance mechanisms in Tetranychus urticae populations from cut flowers greenhouses in Turkey. Int. J. Acarol. 2020, 46, 94–99. [Google Scholar] [CrossRef]
- Xue, W.X.; Snoeck, S.; Njiru, C.; Inak, E.; Dermauw, W.; Van Leeuwen, T. Geographical distribution and molecular insights into abamectin and milbemectin cross-resistance in European field populations of Tetranychus urticae. Pest Manag. Sci. 2020, 76, 2569–2581. [Google Scholar] [CrossRef]
- Herron, G.A.; Langfield, K.L.; Chen, Y.; Wilson, L.J. Development of abamectin resistance in Tetranychus urticae in Australian cotton and the establishment of discriminating doses for T. lambi. Exp. Appl. Acarol. 2021, 83, 325–341. [Google Scholar] [CrossRef]
- Tang, X.F.; Zhang, Y.J.; Wu, Q.J.; Xie, W.; Wang, S.L. Stage -Specific Expression of Resistance to Different Acaricides in Four Field Populations of Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2014, 107, 1900–1907. [Google Scholar] [CrossRef]
- Xu, D.D.; He, Y.Y.; Zhang, Y.J.; Xie, W.; Wu, Q.J.; Wang, S.L. Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic. Biochem. Physiol. 2018, 150, 89–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.D.; Zhang, Y.J.; Wu, Q.J.; Xie, W.; Guo, Z.J.; Wang, S.L. Frequencies and mechanisms of pesticide resistance in Tetranychus urticae field populations in China. Insect Sci. 2022, 29, 827–839. [Google Scholar] [CrossRef]
- Inak, E. Geographical distribution and origin of acetylcholinesterase mutations conferring acaricide resistance in Tetranychus urticae populations from Turkey. Exp. Appl. Acarol. 2022, 86, 49–59. [Google Scholar] [CrossRef]
- Xu, Z.F.; Zhu, W.Y.; Liu, Y.C.; Liu, X.; Chen, Q.S.; Peng, M.; Wang, X.Z.; Shen, G.M.; He, L. Analysis of insecticide resistance-related genes of the carmine spider mite Tetranychus cinnabarinus based on a de novo assembled transcriptome. PLoS ONE 2014, 9, e94779. [Google Scholar] [CrossRef] [Green Version]
- Dermauw, W.; Ilias, A.; Riga, M.; Tsagkarakou, A.; Grbić, M.; Tirry, L.; Van Leeuwen, T.; Vontas, J. The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: Implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochem. Mol. Biol. 2012, 42, 455–465. [Google Scholar] [CrossRef]
- Kwon, D.H.; Clark, J.M.; Lee, S.H. Cloning of a sodium channel gene and identification of mutations putatively associated with fenpropathrin resistance in Tetranychus urticae. Pestic. Biochem. Physiol. 2010, 97, 93–100. [Google Scholar] [CrossRef]
- Binyang, A.J.; Elanga -Ndille, E.; Tene-Fossog, B.; Ndo, C.; Nouage, L.; Assatse, T.; Fotso -Toguem, Y.; Tabue, R.; Zeukeng, F.; Nguiffo, D.N.; et al. Distribution of acetylcholinesterase (Ace-1(R)) target-site G119S mutation and resistance to carbamates and organophosphates in Anopheles gambiae sensu lato populations from Cameroon. Parasit. Vectors 2022, 15, 53. [Google Scholar] [CrossRef]
- Fotoukkiaii, S.M.; Tan, Z.; Xue, W.X.; Wybouw, N.; Van Leeuwen, T. Identification and characterization of new mutations in mitochondrial cytochrome b that confer resistance to bifenazate and acequinocyl in the spider mite Tetranychus urticae. Pest Manag. Sci. 2020, 76, 1154–1163. [Google Scholar] [CrossRef]
- Grbić, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouzé, P.; Grbić, V.; Osborne, E.J.; Dermauw, W.; Ngoc, P.C.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.F.; Ji, X.C.; Pan, F.; Liang, Y.P.; Xiao, T.B.; Xie, S.H. Investigation and analysis of the current status of pesticide application in winter melon and vegetable pest control in Hainan Province, southern China. Acta Entomol. Sinica 2016, 59, 1282–1290. [Google Scholar]
- Ferreira, C.B.S.; Andrade, F.H.N.; Rodrigues, A.R.S.; Siqueira, H.A.A.; Gondim, M.G.C. Resistance in field populations of Tetranychus urticae to acaricides and characterization of the inheritance of abamectin resistance. Crop Prot. 2015, 67, 77–83. [Google Scholar] [CrossRef]
- Emre, İ.; Sultan, Ç.; Ahmet, G.F. Monitoring of acaricide resistance and target site mutations in Tetranychus urticae Koch (Acari: Tetranychidae) populations collected from bean fields in Central Anatolia. Int. J. Acarol. 2022, 48, 279–285. [Google Scholar]
- Kwon, D.H.; Yoon, K.S.; Clark, J.M.; Lee, S.H. A point mutation in a glutamate-gated chloride channel confers abamectin resistance in the two-spotted spider mite, Tetranychus urticae Koch. Insect Mol. Biol. 2010, 19, 583–591. [Google Scholar] [CrossRef]
- Mermans, C.; Dermauw, W.; Geibel, S.; Van Leeuwen, T. A G326E substitution in the glutamate-gated chloride channel 3 (GluCl3) of the two-spotted spider mite Tetranychus urticae abolishes the agonistic activity of macrocyclic lactones. Pest Manag. Sci. 2017, 73, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.; Tsakireli, D.; Ilias, A.; Morou, E.; Myridakis, A.; Stephanou, E.G.; Nauen, R.; Dermauw, W.; Van Leeuwen, T.; Paine, M.; et al. Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochem. Mol. Biol. 2014, 46, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.D.; Zhang, Y.; Zhang, Y.J.; Wu, Q.J.; Guo, Z.J.; Xie, W.; Zhou, X.M.; Wang, S.L. Transcriptome profiling and functional analysis suggest that the constitutive overexpression of four cytochrome P450s confers resistance to abamectin in Tetranychus urticae from China. Pest Manag. Sci. 2021, 77, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Simma, E.A.; Hailu, B.; Jonckheere, W.; Rogiers, C.; Duchateau, L.; Dermauw, W.; Van Leeuwen, T. Acaricide resistance status and identification of resistance mutations in populations of the two-spotted spider mite Tetranychus urticae from Ethiopia. Exp. Appl. Acarol. 2020, 82, 475–491. [Google Scholar] [CrossRef]
- Ilias, A.; Vassiliou, V.A.; Vontas, J.; Tsagkarakou, A. Molecular diagnostics for detecting pyrethroid and abamectin resistance mutations in Tetranychus urticae. Pestic. Biochem. Physiol. 2017, 135, 9–14. [Google Scholar] [CrossRef]
- Riga, M.; Bajda, S.; Themistokleous, C.; Papadaki, S.; Palzewicz, M.; Dermauw, W.; Vontas, J.; Van Leeuwen, T. The relative contribution of target-site mutations in complex acaricide resistant phenotypes as assessed by marker assisted backcrossing in Tetranychus urticae. Sci. Rep. 2017, 7, 9202. [Google Scholar] [CrossRef] [Green Version]
- Ilias, A.; Vontas, J.; Tsagkarakou, A. Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae. Insect Biochem. Mol. Biol. 2014, 48, 17–28. [Google Scholar] [CrossRef]
- Liu, Z.X.; Zhou, L.J.; Yao, Q.; Liu, Y.Q.; Bi, X.Y.; Huang, J.G. Laboratory selection, resistance risk assessment, multi-resistance, and management of Tetranychus urticae Koch to bifenthrin, bifenazate and cyflumetofen on cowpea. Pest Manag. Sci. 2020, 76, 1912–1919. [Google Scholar] [CrossRef]
- Khajehali, J.; Van Leeuwen, T.; Grispou, M.; Morou, E.; Alout, H.; Weill, M.; Tirry, L.; Vontas, J.; Tsagkarakou, A. Acetylcholinesterase point mutations in European strains of Tetranychus urticae (Acari: Tetranychidae) resistant to organophosphates. Pest Manag. Sci. 2010, 66, 220–228. [Google Scholar] [CrossRef]
- Itoh, Y.; Shimotsuma, Y.; Jouraku, A.; Dermauw, W.; Van Leeuwen, T.; Osakabe, M. Combination of target site mutation and associated CYPs confers high-level resistance to pyridaben in Tetranychus urticae. Pestic. Biochem. Physiol. 2022, 181, 105000. [Google Scholar] [CrossRef]
- Qiao, X.F.; Xu, D.D.; Zhang, Y.J.; Xu, B.Y.; Wang, S.L. Effects of bifenazate·etoxazole on the oviposition and development of dominant mite species on vegetables and its control efficacy. J. Plant Prot. 2019, 46, 1310–1315. [Google Scholar]
- Li, J.J.; Hu, T.Y.; Wang, G.B.; Nie, P.C.; Xiao, H.C.; Shang, S.Q. Toxicity and control efficacy of 9 acaricides on the Tetranychus urticae in strawberry. Acta Agric. Boreali -Occident. Sin. 2020, 29, 921–927. [Google Scholar]
- Xue, W.X.; Wybouw, N.; Van Leeuwen, T. The G126S substitution in mitochondrially encoded cytochrome b does not confer bifenazate resistance in the spider mite Tetranychus urticae. Exp. Appl. Acarol. 2021, 85, 161–172. [Google Scholar] [CrossRef]
- Shang, S.Q.; Chang, Y.; Li, W.Z.; Wang, C.Q.; Nie, P.C. Effects of B-azolemiteacrylic on life-history traits and demographic parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2022, 86, 61–71. [Google Scholar] [CrossRef]
- Papapostolou, K.M.; Riga, M.; Charamis, J.; Skoufa, E.; Souchlas, V.; Ilias, A.; Dermauw, W.; Ioannidis, P.; Van Leeuwen, T.; Vontas, J. Identification and characterization of striking multiple-insecticide resistance in a Tetranychus urticae field population from Greece. Pest Manag. Sci. 2021, 77, 666–676. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).