In Vitro and In Vivo Performance of Plum (Prunus domestica L.) Pollen from the Anthers Stored at Distinct Temperatures for Different Periods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site and Plum Genotypes Used
2.2. Anthers’ Collection and Storage
2.3. In Vitro Pollen Germination
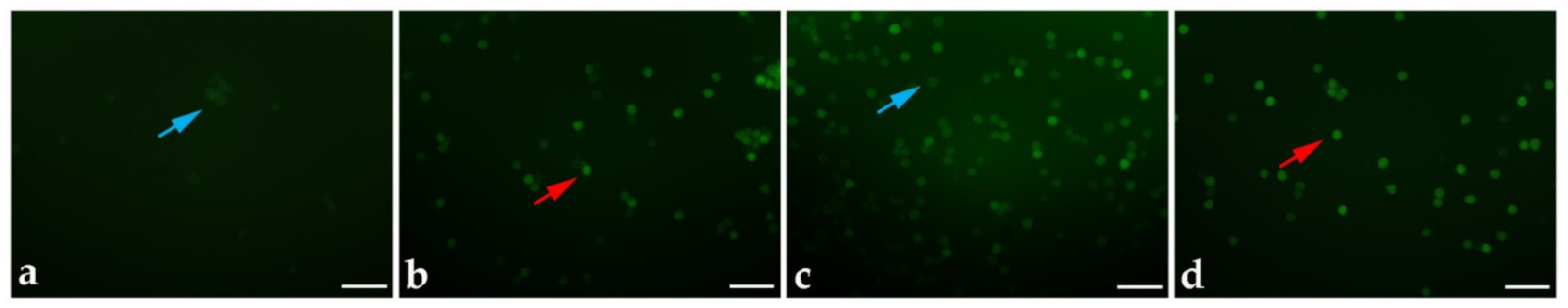
2.4. Pollen Viability Test
2.5. Stigmatic Germinability and Fertilization Ability of Stored Pollen
2.6. Statistical Analysis
3. Results
3.1. Anthers Moisture Content
| Genotype | Moisture Content (%) |
|---|---|
| ‘Čačanska Lepotica’ | 6.78 ± 0.31 |
| ‘Valerija’ | 6.15 ± 0.18 |
| ‘Valjevka’ | 6.72 ± 0.65 |
3.2. Pollen Germinability and Viability
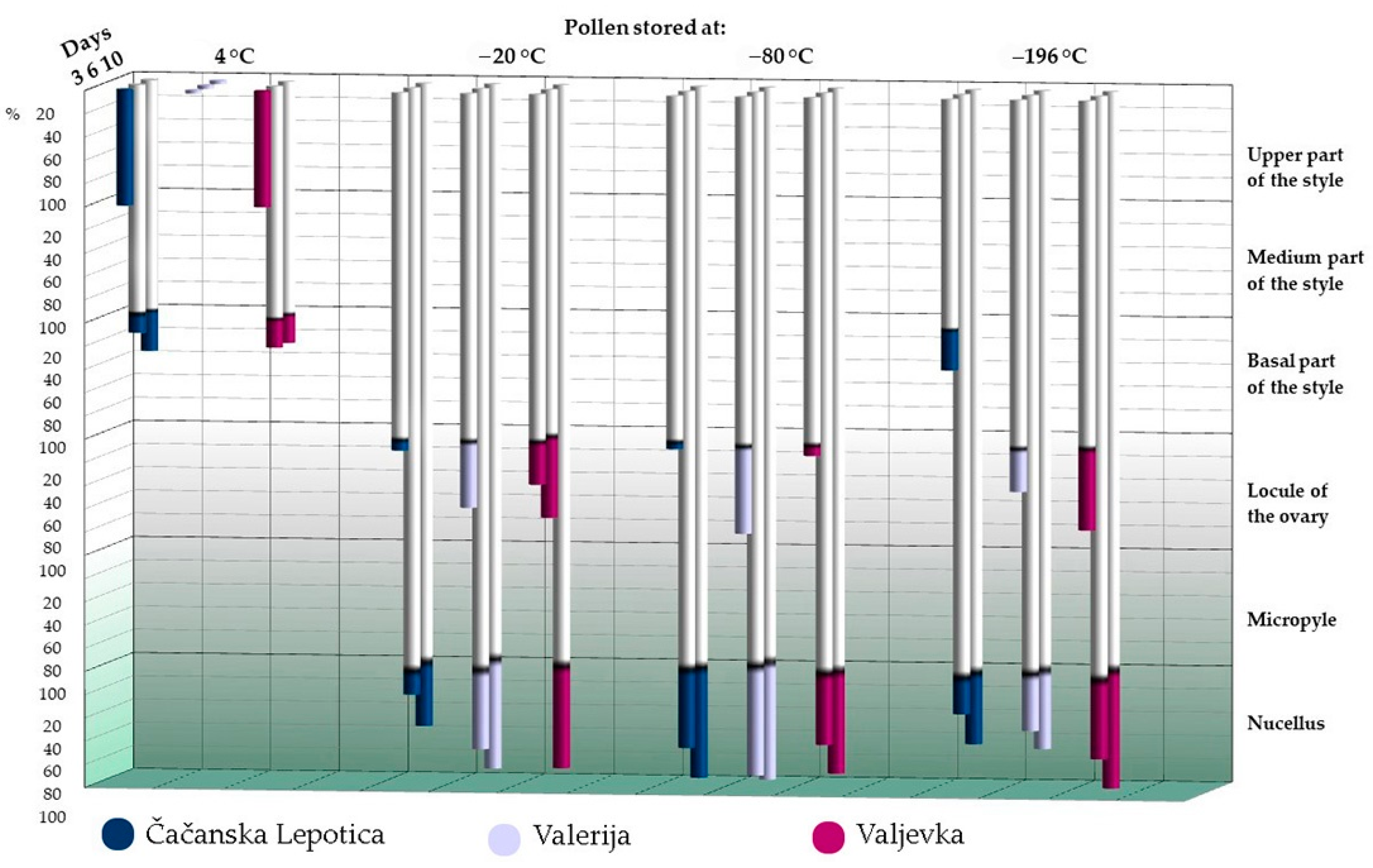
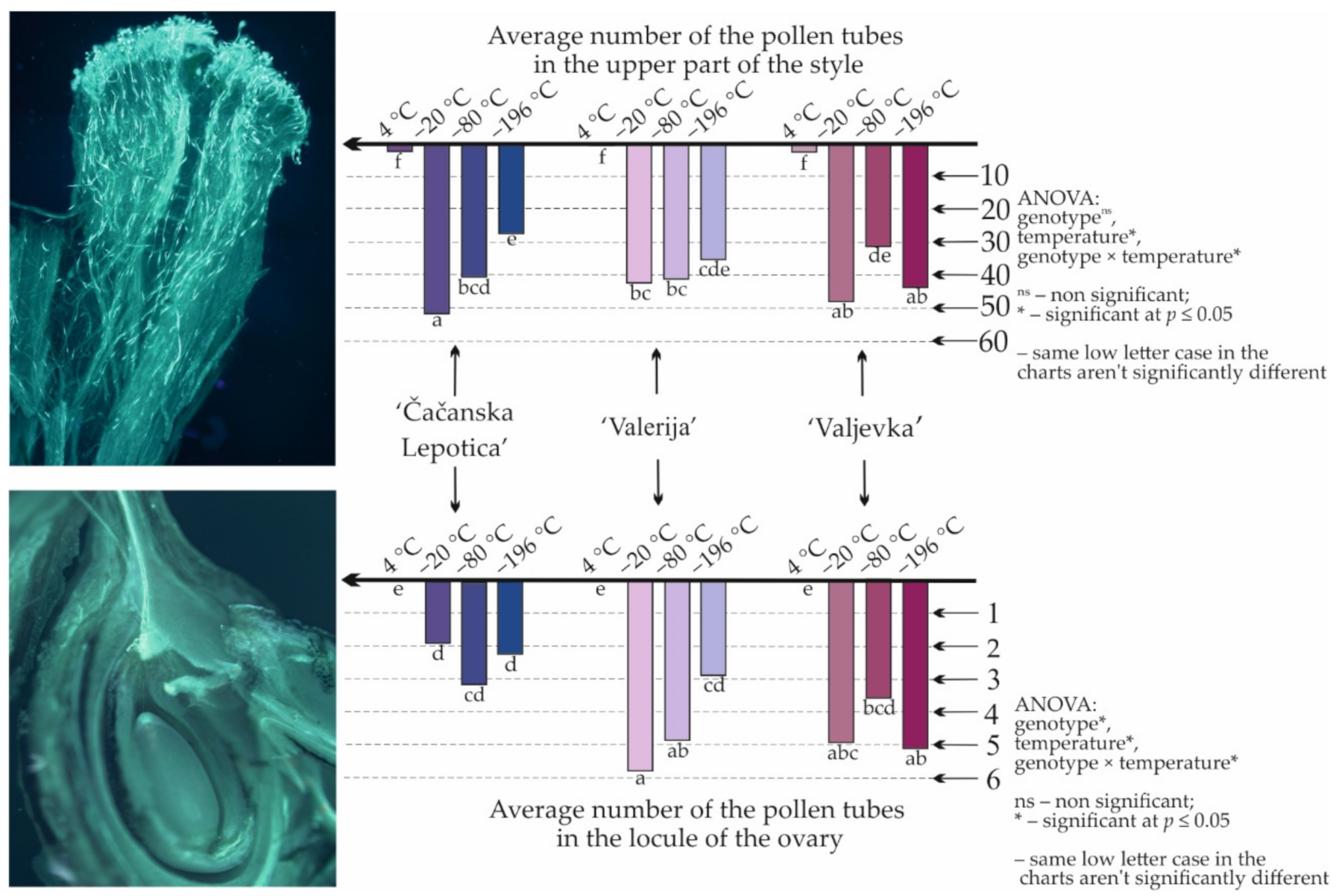
3.3. Stigmatic Germinability of Pollen and Its Further Growth In Vivo
4. Discussion
4.1. Moisture Content of Anthers
4.2. Pollen Viability after Different Storage Periods and Temperatures
4.3. Pollen Germination In Vivo
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayasankar, S.; Dowling, C.; Selvaraj, D.K. Plums and Related Fruits. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Tondrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 401–405. [Google Scholar]
- FAOStat 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 April 2022).
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic characterization of worldwide Prunus domestica (plum) germplasm using sequence-based genotyping. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, W.; Neumüller, M. Plum breeding. In Breeding Plantation Tree Crops Temperate Species; Mohan Jain, S., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 161–231. [Google Scholar] [CrossRef]
- Butac, M. Plum breeding. In Prunus; Küden, A., Ali, A., Eds.; IntechOpen: London, UK, 2020; pp. 1–24. [Google Scholar] [CrossRef]
- Milošević, N.; Glišić, I.; Đorđević, M.; Radičević, S.; Jevremović, D. An overview of plum Breeding at Fruit Research Institute, Čačak. Acta Hortic. 2021, 1322, 7–11. [Google Scholar] [CrossRef]
- Halapija Kazija, D.; Jelačić, T.; Vujević, P.; Milinović, B.; Čiček, D.; Biško, A.; Pejić, I.; Šimon, S.; Žulj Mihaljević, M.; Pecina, M.; et al. Plum germplasm in Croatia and neighboring countries assessed by microsatellites and DUS descriptors. Tree Genet. Genomes 2014, 10, 761–778. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Plum (Prunus spp.) Breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2018; pp. 165–215. Available online: https://lib.ugent.be/catalog/ebk01:4100000005323086 (accessed on 10 May 2022).
- Neumüller, M. Fundamental and applied aspects of plum (Prunus domestica) breeding. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 139–156. [Google Scholar]
- Aldahadhal, A.; Samarah, N.; Bataineh, A. Effect of storage temperature and duration on pollen viability and in vitro germination of seven pistachio cultivars. J. Appl. Hortic. 2020, 22, 184–188. [Google Scholar] [CrossRef]
- Akihama, T.; Omura, M.; Kozaki, I. Long-term storage of fruit tree pollen and its application in breeding. JARQ 1979, 14, 53–56. [Google Scholar]
- Hanna, W.; Towill, L. Long-term pollen storage. In Plant Breeding Reviews; Janic, J., Ed.; John Wiley and Sons: Chichester, UK, 1995; pp. 197–207. [Google Scholar]
- Dafni, A.; Firmage, D.H. Pollen viability and longevity: Practical, ecological and evolutionary implications. Plant Syst. Evol. 2020, 222, 113–132. [Google Scholar] [CrossRef]
- Özcan, A. Effect of low-temperature storage on sweet cherry (Prunus avium L.) pollen quality. HortScience 2020, 55, 258–260. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970, 45, 115–120. [Google Scholar] [CrossRef]
- Masum, A.; Pounders, C.; Blythe, E.; Wang, X. Longevity of crapemyrtle pollen stored at different temperatures. Sci. Hortic. 2012, 139, 53–57. [Google Scholar] [CrossRef]
- Čalić, D.; Milojević, J.; Belić, M.; Miletić, R.; Zdravkovic Korać, S. Impact of storage temperature on pollen viability and germinability of four Serbian autochthon apple cultivars. Front. Plant Sci. 2021, 12, 709231. [Google Scholar] [CrossRef]
- Franchi, G.G.; Piotto, B.; Nepi, M.; Baskin, C.C.; Baskin, J.M.; Pacini, E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal and survival. J. Exp. Bot. 2011, 62, 5267–5281. [Google Scholar] [CrossRef] [Green Version]
- Frankel, R.; Galun, E. Pollination Mechanisms, Reproduction and Plant Breeding. Monographs on Theoretical and Applied Genetics; Springer: Berlin/Heidelberg, Germany, 1977; pp. 1–284. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Gradziel, T.M.; Ortega, E.; Dicenta, F. Low temperature storage of almond pollen. HortScience 2002, 37, 691–692. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.A.; Dhillon, W.S.; Shafi, R.H.S.; Rather, J.A.; Mir, A.H.; Shafi, W.; Rashid, R.; Bhat, J.A.; Rather, T.R.; Wani, T.A. Influence of storage temperature on viability and in vitro germination capacity of pear (Pyrus spp.) pollen. J. Agric. Sci. 2012, 4, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.K.; Srivastav, M.; Chaudhary, R.; Lal, K.; Patil, P.; Singh, S.K.; Sing, A.K. Low temperature storage of mango (Mangifera inidica L.) pollen. Sci. Hortic. 2013, 161, 193–197. [Google Scholar] [CrossRef]
- Naik, S.; Rana, P.; Rana, V. Pollen storage and use for enhancing fruit production in kiwifruit (Actinidia deliciosa A. Chev.). J. Appl. Hortic. 2013, 15, 128–132. [Google Scholar] [CrossRef]
- Alburquerque, N.; Garcia Montiel, F.; Burgos, L. Influence of storage temperature on the viability of sweet cherry pollen. Span. J. Agric. Res. 2007, 5, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Lukić, M.; Pešaković, M.; Marić, S.; Glišić, I.; Milošević, N.; Radičević, S.; Leposavić, A.; Đorđević, M.; Miletić, R.; Karaklajić Stajić, Ž.; et al. Fruit Cultivars Developed at the Fruit Research Institute, Čačak (1946–2016); Fruit Research Institute: Čačak, Serbia, 2016; pp. 1–180. [Google Scholar]
- Meier, U. Growth stages of mono- and dicotyledonous plants. In Federal Biological Research Centre for Agriculture and Forestry, 2nd ed.; Meier, U., Ed.; BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Berlin/Brunswick, Germany, 2001; pp. 1–158. [Google Scholar]
- Galleta, G.J. Pollen and seed management. In Methods in Fruit Breeding; Moore, J.N., Janick, J., Eds.; Purdue University Press: West Lafayette, IN, USA, 1983; pp. 23–47. [Google Scholar]
- Preil, W. Observing the growth of pollen tubes in pistil and ovarian tissue by means of fluorescence microscopy. Zeiss Inf. 1970, 75, 24–25. [Google Scholar]
- Kho, Y.O.; Baër, J. Fluorescence microscopy in botanical research. Zeiss Inf. 1971, 76, 54–57. [Google Scholar]
- Heslop-Harrison, J. An interpretation of the hydrodynamics of pollen. Am. J. Bot. 1979, 66, 737–743. [Google Scholar] [CrossRef]
- Georgieva, I.D.; Kruleva, M.M. Cytochemical investigation of long-term stored maize pollen. Euphytica 1993, 72, 87–94. [Google Scholar] [CrossRef]
- Li, Y.M.; Chen, L.B. Vigor change of several grasses pollen stored in different temperature and humidity conditions. Plant Physiol. Commun. 1998, 34, 35–37. [Google Scholar]
- Dinato, N.B.; Imaculada Santos, I.R.; Zanotto Vigna, B.B.; de Paula, A.F.; Fávero, A.P. Pollen cryopreservation for plant breeding and genetic resources conservation. CryoLetters 2020, 41, 115–127. [Google Scholar] [PubMed]
- Milatović, D.; Nikolić, D.; Radović, M. Influence of temperature on pollen germination and pollen tube growth of plum cultivars. In Proceedings of the Sixth International Scientific Agricultural Symposium “Agrosym 2015”, Jahorina, Bosnia and Herzegovina, 15–18 October 2015; pp. 378–382. [Google Scholar]
- Cerović, R.; Fotirić Akšić, M.; Đorđević, M.; Meland, M. The effect of the pollinizers on pollen tube growth and fruit set of European plum (Prunus domestica L.) in a Nordic climate. Sci. Hortic. 2021, 288, 110390. [Google Scholar] [CrossRef]
- Glišić, I.; Milatović, D.; Cerović, R.; Radičević, S.; Đorđević, M.; Milošević, N. Examination of suitability of the cultivar ‘Čačanska Lepotica’ as a pollenizer for promising plum genotypes developed at FRI, Čačak (Serbia). Acta Hortic. 2020, 1289, 213–220. [Google Scholar] [CrossRef]
- Wertheim, S.J. Methods for cross pollination and flowering assessment and their interpretation. Acta Hortic. 1996, 423, 237–241. [Google Scholar] [CrossRef]
- Impre, D.; Reitz, J.; Köpnick, C.; Rolletschek, H.; Börner, A.; Senula, A.; Nagel, M. Assessment of pollen viability for weat. Front. Plant Sci. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Aloni, B.; Peet, M.; Pharr, M.; Karni, L. The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annum) pollen in relation to its germination. Physiol. Plant. 2001, 112, 505–512. [Google Scholar] [CrossRef]
- Cerovic, R.; Micic, N.; Djuric, G.; Nikolic, M. Determination of pollen viability in sweet cherry. Acta Hortic. 1998, 468, 559–566. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Lin, X.-J.; Liang, H.-M.; Wang, F.-F.; Chen, L.-Y. The long journey of pollen tube in the pistil. Int. J. Mol. Sci. 2018, 19, 3529. [Google Scholar] [CrossRef] [Green Version]
- Montal, R.; Cuenca, J.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Influence of temperature on the progamic phase in Citrus. Environ. Exp. Bot. 2019, 166, 103806. [Google Scholar] [CrossRef]
- Qu, H.; Guan, Y.; Wang, Y.; Zhang, S. PLC-mediated signaling pathway in pollen tubes regulates the gametophytic self-incompatibility of Pyrus species. Front. Plant Sci. 2017, 8, 1164. [Google Scholar] [CrossRef] [Green Version]
- Radičević, S.; Cerović, R.; Nikolić, D.; Đorđević, M. The effect of genotype and temperature on pollen tube growth and fertilization in sweet cherry (Prunus avium L.). Euphytica 2016, 209, 121–136. [Google Scholar] [CrossRef]
- Cerović, R.; Ružić, Đ. Pollen tube growth in sour cherry (Prunus domestica L.) at different temperatures. J. Hortic. Sci. Biotechnol. 1992, 67, 333–340. [Google Scholar] [CrossRef]
- Sanzol, J.; Hererro, M. The “effective pollination period” in fruit tree. Sci. Hortic. 2001, 90, 1–17. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Younts, T.L.B.; Muday, G.K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. USA 2018, 115, 11188–11197. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.F.; Jiang, B.; Zhao, J.S.; Li, J.C.; Sun, Q.M. Metabolomics and transcriptomics analysis of pollen germination response to low-temperature in pitaya (Hylocereus polyrhizus). Front. Plant Sci. 2022, 13, 866588. [Google Scholar] [CrossRef]
- Du, G.; Xu, J.; Gao, C.; Lu, J.; Li, Q.; Du, J.; Lv, M.; Sun, X. Effect of low storage temperature on pollen viability of fifteen herbaceous peonies. Biotechnol. Rep. 2019, 21, e00309. [Google Scholar] [CrossRef]
- Đorđević, M.; Cerović, R.; Radičević, S.; Nikolić, D.; Milošević, N.; Glišić, I.; Marić, S.; Lukić, M. Pollen tube growth and embryo sac development in ‘Pozna Plava’ plum cultivar related to fruit set. Erwerbs-Obstbau 2019, 61, 313–322. [Google Scholar] [CrossRef]
- Ogašanović, D. The most suitable pollinators for new plum cultivars. J. Yugosl. Pomol. 1985, 19, 109–115. [Google Scholar]
- Kuzmanović, M.; Cerović, R.; Radičević, S. Study of the progamic phase of pollination in the plum cultivar Čačanska Lepotica. J. Pomol. 2007, 41, 89–93. Available online: https://www.institut-cacak.org/vocarstvo.html (accessed on 10 May 2022).

| Genotype | Temperature | Fresh Pollen/Day 0 of the Storage | 3 Months of Storage | 6 Months of Storage | 9 Months of Storage | 12 Months of Storage | |
|---|---|---|---|---|---|---|---|
| ‘Čačanska Lepotica’ | 4 °C | 63.79 ± 0.87 a/A | 57.73 ± 1.06 a/BCD | 55.38 ± 0.70 a/DEF | 48.56 ± 0.64 b/F | 3.17 ± 1.97 d/G | genotype *, temperature *, genotype × temperature * |
| −20 °C | 59.60 ± 0.98 a/ABCD | 57.61 ± 1.19 a/CD | 56.81 ± 0.45 a/CDE | 52.41 ± 0.91 a/EFG | |||
| −80 °C | 59.83 ± 0.71 a/ABC | 56.61 ± 0.98 a/CDE | 55.73 ± 0.68 a/CDEF | 55.27 ± 0.54 a/DEF | |||
| −196 °C | 62.04 ± 0.93 a/AB | 58.94 ± 0.57 a/BCD | 57.68 ± 1.38 a/CD | 51.77 ± 0.89 a/FG | |||
| ‘Valerija’ | 4 °C | 44.73 ± 0.94 b/A | 33.93 ± 0.38 b/E | 25.05 ± 0.78 d/F | 18.40 ± 0.19 f/G | 0.00 ± 0.00 f/H | genotype *, temperature *, genotype × temperature * |
| −20 °C | 41.32 ± 1.08 b/BCD | 38.33 ± 0.19 bc/CD | 37.71 ± 0.03 cd/CDE | 37.41 ± 1.43 bc/DE | |||
| −80 °C | 40.30 ± 1.08 b/BCD | 38.24 ± 0.68 bc/CD | 37.74 ± 0.07 cd/CDE | 36.77 ± 0.27 bc/DE | |||
| −196 °C | 43.87 ± 1.14 b/AB | 41.03 ± 2.57 b/ABC | 40.87 ± 0.56 c/ABCD | 40.77 ± 0.36 b/ABCD | |||
| ‘Valjevka’ | 4 °C | 42.35 ± 0.92 c/A | 30.65 ± 1.18 c/C | 21.43 ± 1.08 e/D | 13.66 ± 0.36 g/D | 1.68 ± 1.28 e/E | genotype *, temperature *, genotype × temperature * |
| −20 °C | 40.30 ± 1.03 c/BC | 34.63 ± 1.55 c/BC | 33.49 ± 0.54 e/BC | 35.27 ± 0.83 c/BC | |||
| −80 °C | 37.80 ± 0.76 c/AB | 36.80 ± 1.57 bc/B | 34.57 ± 1.37 de/BC | 35.58 ± 0.52 c/B | |||
| −196 °C | 41.92 ± 0.94 c/A | 41.75 ± 0.76 b/A | 36.45 ± 1.02 cd/AB | 33.81 ± 1.21 c/BC | |||
| genotype *, temperature ns, genotype × temperature ns | genotype *, temperature *, genotype × temperature ns | genotype *, temperature *, genotype × temperature * | genotype *, temperature *, genotype × temperature * | genotype *, temperature *, genotype × temperature * | ANOVA | ||
| Genotype | Temperature | Fresh Pollen/Day 0 of the Storage | 3 Months of Storage | 6 Months of Storage | 9 Months of Storage | 12 Months of Storage | |
|---|---|---|---|---|---|---|---|
| ‘Čačanska Lepotica’ | 4 °C | 59.25 ± 3.98 b/A | 56.25 ± 1.51 a/AB | 51.09 ± 2.87 c/BC | 48.89 ± 1.03 bc/CD | 39.31 ± 1.59 c/D | genotype *, temperature *, genotype × temperature ns |
| −20 °C | 58.02 ± 2.36 a/AB | 57.90 ± 0.27 a/BC | 54.25 ± 2.40 ab/CD | 53.36 ± 1.80 a/D | |||
| −80 °C | 57.82 ± 0.26 a/AB | 56.82 ± 0.93 a/BC | 52.24 ± 0.18 ab/CD | 51.97 ± 1.80 a/D | |||
| −196 °C | 57.72 ± 3.82 a/AB | 56.24 ± 0.43 ab/BC | 55.56 ± 0.71 a/CD | 54.30 ± 2.58 a/D | |||
| ‘Valerija’ | 4 °C | 62.15 ± 2.78 a/A | 54.56 ± 0.56 a/A | 42.72 ± 0.51 de/E | 33.50 ± 3.70 e/ F | 0.00 ± 0.00 e/G | genotype *, temperature *, genotype × temperature * |
| −20 °C | 57.19 ± 0.50 a/ABC | 52.62 ± 0.71 bc/CD | 51.67 ± 0.95 ab/ CD | 47.67 ± 6.29 ab/DE | |||
| −80 °C | 59.32 ± 1.25 a/AB | 56.28 ± 1.32 ab/ABC | 53.76 ± 1.08 ab/ BCD | 51.29 ± 2.62 a/CD | |||
| −196 °C | 60.01 ± 0.77 a/AB | 57.20 ± 0.93 a/ABC | 55.70 ± 2.62 a/ BD | 54.59 ± 3.65 a/BC | |||
| ‘Valjevka’ | 4 °C | 54.58 ± 0.49 b/A | 45.74 ± 3.65 b/A | 36.66 ± 0.77 f/E | 19.20 ± 2.13 f/ F | 17.60 ± 1.09 d/F | genotype *, temperature *, genotype × temperature * |
| −20 °C | 52.52 ± 1.09 b/A | 41.81 ± 2.43 de/CDE | 38.17 ± 1.27 de/ DE | 36.30 ± 1.92 c/E | |||
| −80 °C | 54.17 ± 2.68 b/A | 41.33 ± 1.70 e/CDE | 40.54 ± 2.46 d/ CDE | 38.93 ± 2.00 c/DE | |||
| −196 °C | 51.59 ± 2.18 b/AB | 45.75 ± 0.47 d/BC | 43.39 ± 0.98 cd/CD | 41.43 ± 0.85 bc/CDE | |||
| genotype *, temperature ns, genotype × temperature ns | genotype *, temperature ns, genotype × temperature ns | genotype *, temperature *, genotype × temperature * | genotype *, temperature *, genotype × temperature * | genotype *, temperature *, genotype × temperature * | ANOVA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đorđević, M.; Vujović, T.; Cerović, R.; Glišić, I.; Milošević, N.; Marić, S.; Radičević, S.; Fotirić Akšić, M.; Meland, M. In Vitro and In Vivo Performance of Plum (Prunus domestica L.) Pollen from the Anthers Stored at Distinct Temperatures for Different Periods. Horticulturae 2022, 8, 616. https://doi.org/10.3390/horticulturae8070616
Đorđević M, Vujović T, Cerović R, Glišić I, Milošević N, Marić S, Radičević S, Fotirić Akšić M, Meland M. In Vitro and In Vivo Performance of Plum (Prunus domestica L.) Pollen from the Anthers Stored at Distinct Temperatures for Different Periods. Horticulturae. 2022; 8(7):616. https://doi.org/10.3390/horticulturae8070616
Chicago/Turabian StyleĐorđević, Milena, Tatjana Vujović, Radosav Cerović, Ivana Glišić, Nebojša Milošević, Slađana Marić, Sanja Radičević, Milica Fotirić Akšić, and Mekjell Meland. 2022. "In Vitro and In Vivo Performance of Plum (Prunus domestica L.) Pollen from the Anthers Stored at Distinct Temperatures for Different Periods" Horticulturae 8, no. 7: 616. https://doi.org/10.3390/horticulturae8070616
APA StyleĐorđević, M., Vujović, T., Cerović, R., Glišić, I., Milošević, N., Marić, S., Radičević, S., Fotirić Akšić, M., & Meland, M. (2022). In Vitro and In Vivo Performance of Plum (Prunus domestica L.) Pollen from the Anthers Stored at Distinct Temperatures for Different Periods. Horticulturae, 8(7), 616. https://doi.org/10.3390/horticulturae8070616







