Characterization of Volatile Compounds from Tea Plants (Camellia sinensis (L.) Kuntze) and the Effect of Identified Compounds on Empoasca flavescens Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Plant Materials
2.3. Resistance Selection in Tea Plants
2.4. Analysis of Tea Leaf Volatile Compounds
2.5. Y-Tube Olfactometer Test
2.6. Statistical Analysis
3. Results
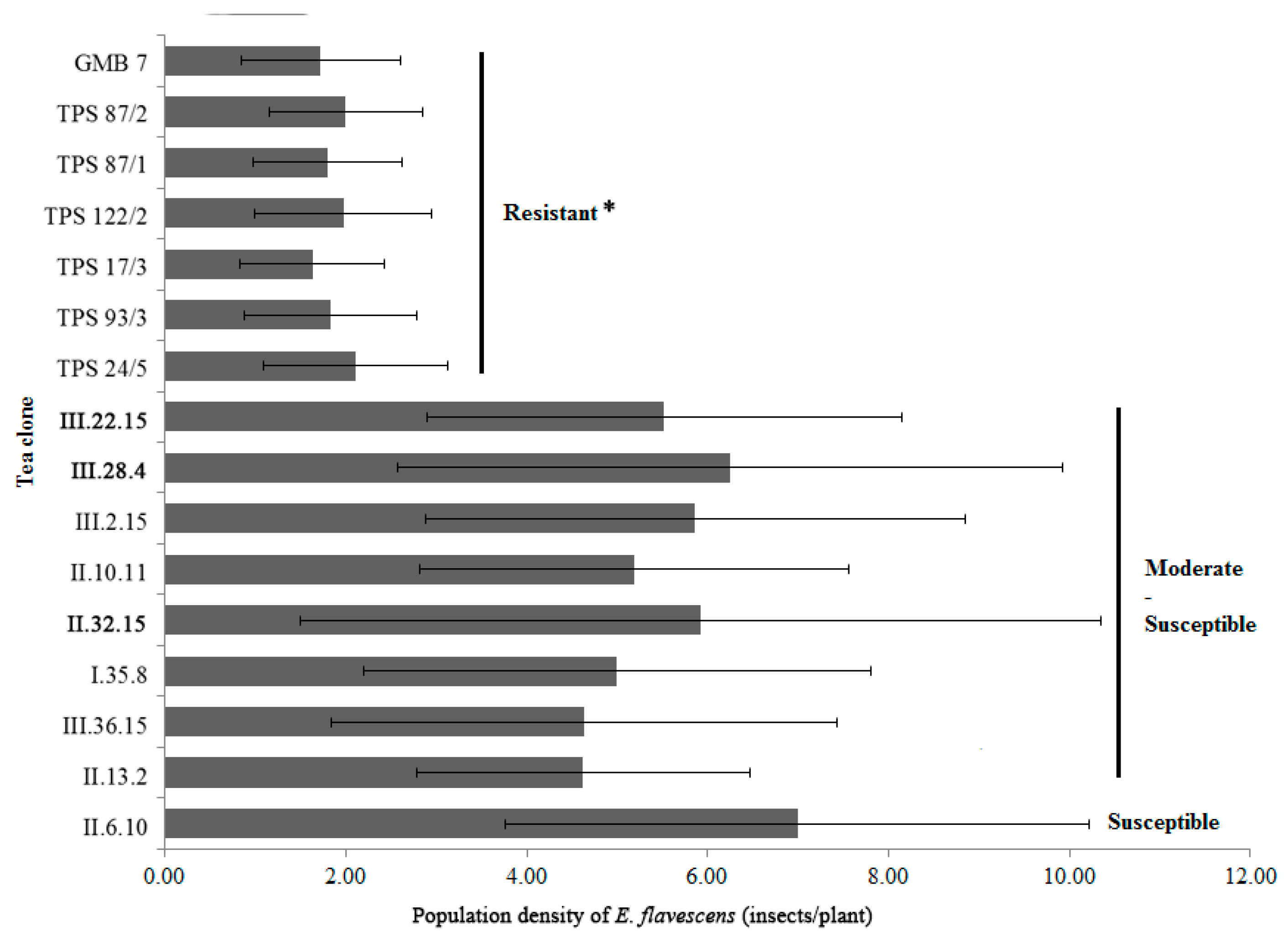
3.1. Resistance Selection of Tea Plant Resistance
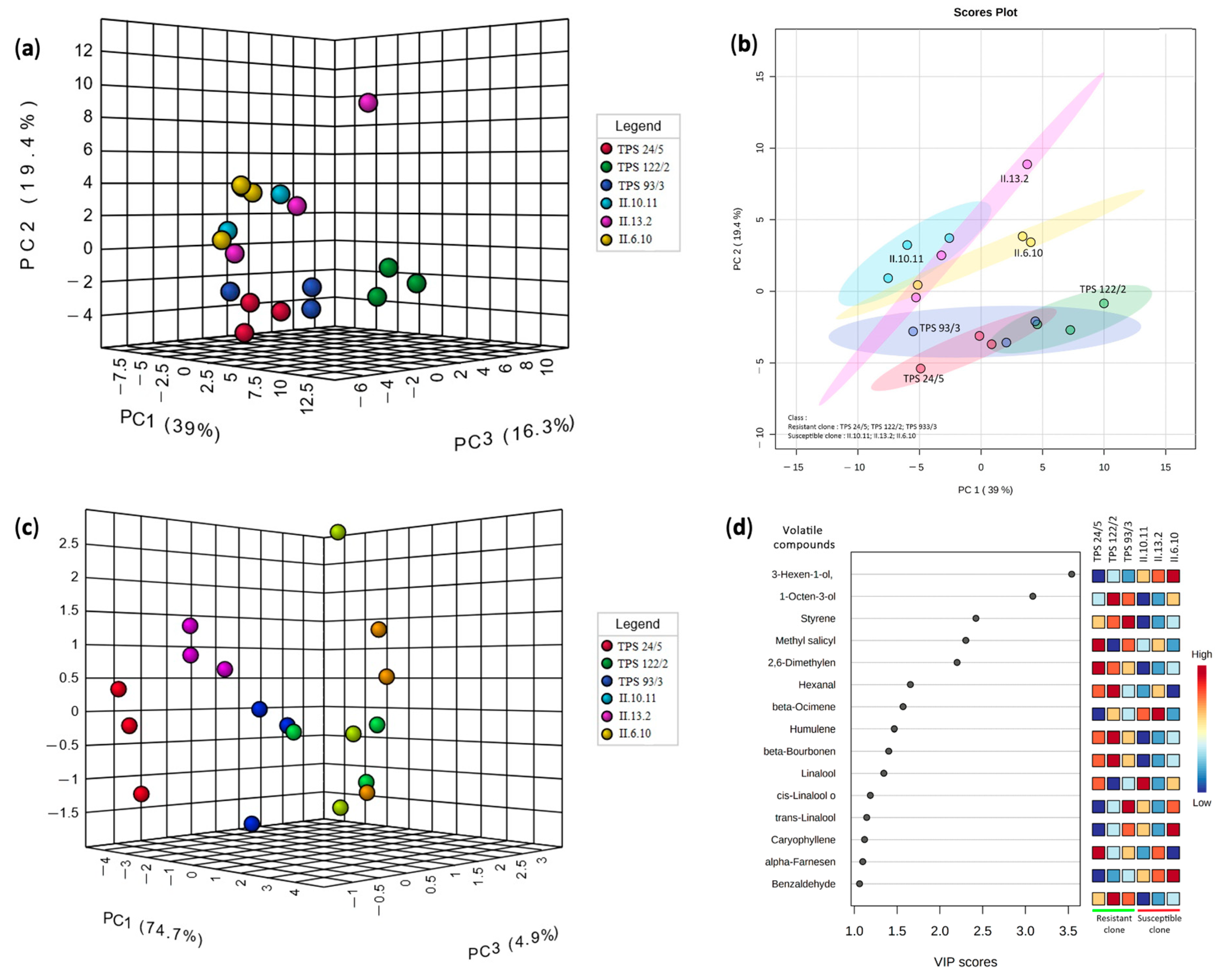
3.2. Profile of Volatile Compounds of Resistant and Susceptible Tea Clones
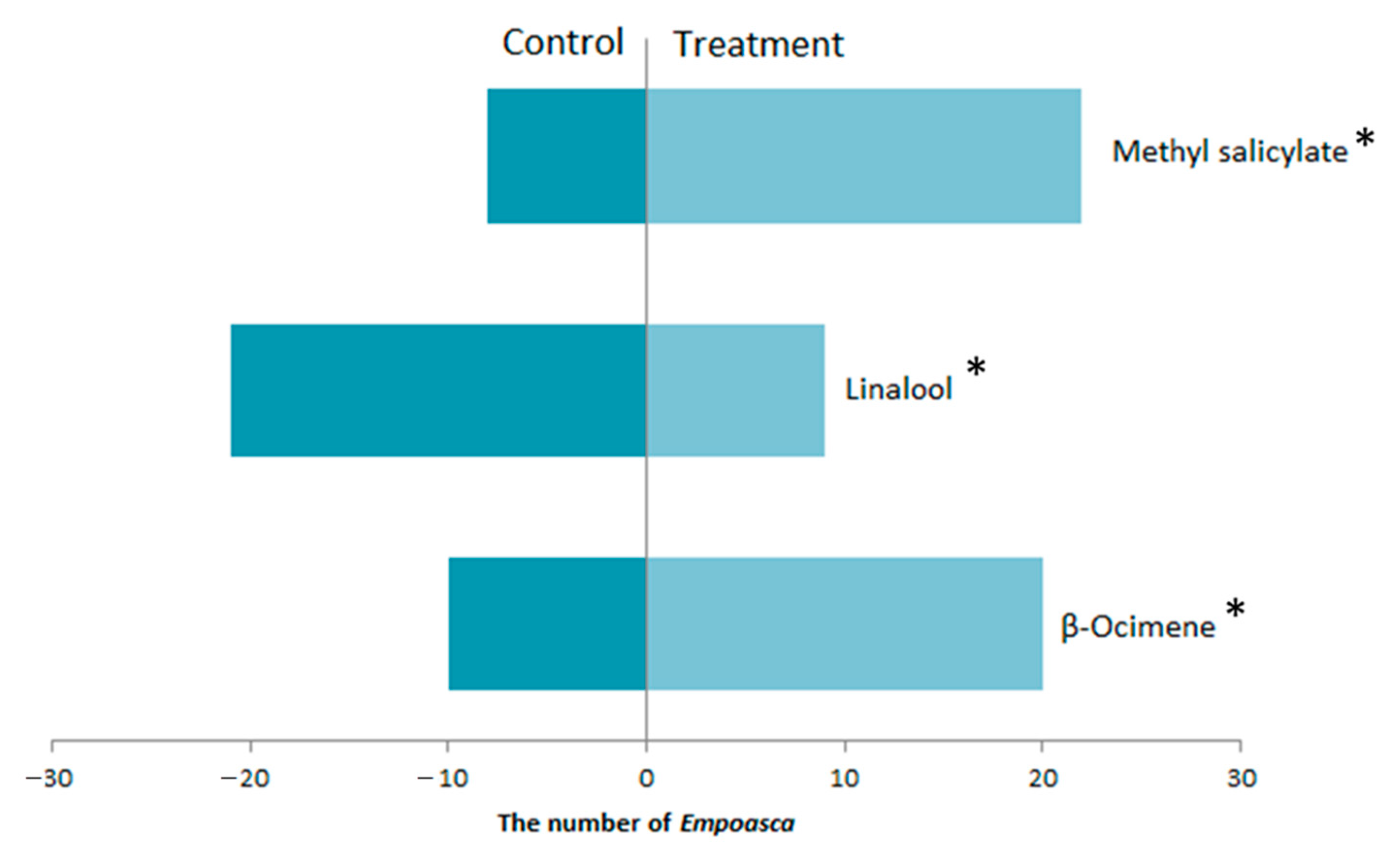
3.3. The Response of E. flavescens Response to the Volatile Compounds Produced by Tea Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mu, D.; Pan, C.; Qi, Z.; Qin, H.; Li, Q.; Liang, K.; Rao, Y.; Sun, T. Multivariate analysis of volatile profiles in tea plant infested by tea green leafhopper Empoasca onukii Matsuda. Plant Growth Regul. 2021, 95, 111–120. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z. Non-host plant essential oil volatiles with potential for a ‘push-pull’ strategy to control the tea green leafhopper, Empoasca vitis. Entomol. Exp. 2015, 156, 77–87. [Google Scholar] [CrossRef]
- Xin, Z.J.; Li, X.W.; Bian, L.; Sun, X.L. Tea green leafhopper, Empoasca vitis, chooses suitable host plants by detecting the emission level of (3Z)-hexenyl acetate. Bull. Entomol. Res. 2017, 107, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, H.; Wang, Q.; Liu, J.; Zhang, L.; Mu, W.; Xu, Y.; Zhang, Z.; Gu, S. Identification and expression analysis of chemosensory genes in the tea green leafhopper, Empoasca onukii Matsuda. J. Appl. Entomol. 2018, 142, 828–846. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Mu, D.; Cui, L.; Ge, J.; Wang, M.-X.; Liu, L.-F.; Yu, X.-P.; Zhang, Q.-H.; Han, B.-Y. Behavioral responses for evaluating the attractiveness of specific tea shoot volatiles to the tea green leafhopper, Empoaca vitis. Insect Sci. 2012, 19, 229–238. [Google Scholar] [CrossRef]
- Schmidt, F.H.; Ferguson, J.H.A. Rainfall Types Based on Wet and Dry Period Ratios for Indonesia with Western New Guinee; Kementerian Perhubungan: Jakarta, Indonesia, 1952. [Google Scholar]
- Sriyadi. Seleksi ketahanan klon teh seri TPS terhadap penyakit cacar. J. Penelit. Teh. Dan Kina 2007, 10, 73–82. [Google Scholar]
- Cai, X.; Luo, Z.; Meng, Z.; Liu, Y.; Chu, B.; Bian, L.; Li, Z.; Xin, Z.; Chen, Z. Primary screening and application of repellent plant volatiles to control tea leafhopper, Empoasca onukii Matsuda. Pest Manag. Sci. 2020, 76, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Wagiman, F.X.; Triman, B. Ketahanan relatif enam belas nomor klon teh PGL terhadap serangan Empoasca sp. J. Perlindungan Tanam. Indones. 2011, 17, 60–65. [Google Scholar]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2021, 73, 449–462. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Huang, S.; Jiang, T.; Wang, C.; Tao, Z.; He, C.; Tang, Q.; Li, P. Volatile DMNT directly protects plants against Plutella xylostella by disrupting the peritrophic matrix barrier in insect midgut. eLife 2021, 10, e63938. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Du, W.; Gao, T.; Wu, Y.; Zhang, N.; Zhao, M.; Jin, J.; Wang, J.; Schwab, W.; Wan, X.; et al. Herbivore-induced DMNT catalyzed by CYP82D47 plays an important role in the induction of JA-dependent herbivore resistance of neighboring tea plants. Plant Cell Environ. 2021, 44, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Dai, Y.; Guo, Y.-N.; Xu, H.-R.; Wang, X.-C. Volatile profile analysis and quality prediction of Longjing tea (Camellia sinensis) by HS-SPME/GC-MS. J. Zhejiang Univ. Sci. B 2012, 13, 972–980. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosynthesis and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.C.S.; Milet-Pinheiro, P.; Bezerra da Silva, P.C.; da Silva, A.G.; da Silva, M.V.; Navarro, D.M.d.A.F.; da Silva, N.H. (E)-caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of Commiphora leptophloeos leaf oil. PLoS ONE 2015, 10, e0144586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Macedo, G.E.; de Brum Vieira, P.; Rodrigues, N.R.; Gomes, K.K.; Martins, I.K.; Franco, J.L.; Posser, T. Fungal compound 1-octen-3-ol induces mitochondrial morphological alterations and respiration dysfunctions in Drosophila melanogaster. Ecotoxicol. Environ. Saf. 2020, 206, 111232. [Google Scholar] [CrossRef]
- Stockton, D.G.; Wallingford, A.K.; Cha, D.H.; Loeb, G.M. Automated aerosol puffers effectively deliver 1-octen-3-ol, an oviposition antagonist useful against spotted-wing drosophila. Pest Manag. Sci. 2021, 77, 389–396. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, L.; He, L.; Zhang, Z.; Zhang, T.; Mu, W.; Lin, J.; Liu, F. Toxicological effects of the fungal volatile compound 1-octen-3-ol against the red flour beetle, Tribolium castaneum (Herbst). Ecotoxicol. Environ. Saf. 2021, 208, 111597. [Google Scholar] [CrossRef]
- Vasconcelos, J.F.; Dias-Pini, N.; Saraiva, W.V.A.; Farias, L.D.L.; Ribeiro, P.R.V.; Melo, J.W.D.S.; Rodrigues, T.H.S.; Macedo, V.H.M. Volatile and phenolic compounds In the resistance of the melon to the vegetable leafminer, Liriomyza sativae Blanchard (Diptera: Agromyzidae). J. Chem. Ecol. 2022, 1–22. [Google Scholar] [CrossRef]
- Salamanca, J.; Souza, B.; Kyryczenko-Roth, V.; Rodriguez-Saona, C. Methyl salicylate increases attraction and function of beneficial arthropods in cranberries. Insects 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derstine, N.T.; Meier, L.; Canlas, I.; Murman, K.; Cannon, S.; Carrillo, D.; Wallace, M.; Cooperband, M.F. Plant volatiles help mediate host plant selection and attraction of the spotted lanternfly (Hemiptera: Fulgoridae): A generalist with a preferred host. Environ. Entomol. 2020, 49, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, D.M.; Borges, M.; Laumann, R.A.; Woodcock, C.M.; Withall, D.M.; Pickett, J.A.; Birkett, M.A.; Blassioli-Moraes, M.C. Identification of volatile compounds involved in host location by Anthonomus grandis (Coleoptera: Curculionidae). Front. Ecol. Evol. 2018, 6, 98. [Google Scholar] [CrossRef] [Green Version]
- Papanastasiou, S.A.; Ioannou, C.S.; Papadopoulos, N.T. Oviposition-deterrent effect of linalool—A compound of citrus essential oils—On female Mediterranean fruit flies, Ceratitis capitata (Diptera: Tephritidae). Pest Manag. Sci. 2020, 76, 3066–3077. [Google Scholar] [CrossRef]


| No | RT (min) | Compound | Area (%) | Group | |
|---|---|---|---|---|---|
| Susceptible | Resistant | ||||
| 1 | 1.4828 | acetaldehyde | 16.61 | 19.35 | Aldehyde/alkane |
| 2 | 2.7629 | cyclobutanol | 4.41 | 5.03 | Alcohol |
| 3 | 3.7990 | hexanal | 0.53 | 1.39 | Aldehyde/alkane |
| 4 | 5.4946 | 3-hexen-1-ol. (E)- | 1.33 | - | Alcohol |
| 5 | 6.6175 | styrene | - | 1.12 | Aromatic |
| 6 | 9.3340 | benzaldehyde | - | 0.18 | Aromatic |
| 7 | 10.2260 | 1-octen-3-ol | 0.32 | 2.72 | Alcohol |
| 8 | 10.6340 | β-myrcene | 2.58 | 2.59 | Monoterpene |
| 9 | 12.0731 | D-limonene | 0.92 | 0.80 | Monoterpene |
| 10 | 12.5249 | trans-β-ocimene | 1.17 | 0.90 | Monoterpene |
| 11 | 12.9299 | β-ocimene | 10.18 | 5.24 | Monoterpene |
| 12 | 13.8942 | trans-linalool oxide | 1.09 | 0.82 | Monoterpene |
| 13 | 14.4410 | terpinolene | 0.05 | 0.05 | Monoterpene |
| 14 | 14.5413 | cis-linalool oxide | 2.23 | 1.90 | Monoterpene |
| 15 | 14.7960 | 4.8-dimethyl-1.3.7-nonatriene | 0.35 | 0.22 | Alcohol |
| 16 | 15.2901 | linalool | 38.86 | 32.29 | Monoterpene |
| 17 | 15.6572 | 2.6-dimethyleneoct-7-en-3-one | - | 0.68 | Ketone |
| 18 | 16.1447 | allo-ocimene | 0.86 | 0.61 | Monoterpene |
| 19 | 16.6140 | neo-allo-ocimene | 0.56 | 0.42 | Monoterpene |
| 20 | 17.1008 | 2.6-nonadienal. (E.Z)- | 0.06 | 0.08 | Aldehyde/alkane |
| 21 | 17.5362 | isoborneol | - | 0.06 | Monoterpene |
| 22 | 17.7293 | ethyl benzoate | 0.10 | 0.08 | Aromatic |
| 23 | 17.9429 | epoxylinalol | 0.22 | 0.20 | Monoterpene |
| 24 | 18.0351 | napthalene | 0.05 | - | Aromatic |
| 25 | 18.7876 | methyl salicylate | 14.88 | 20.00 | Carboxylic acid |
| 26 | 18.8602 | 2-ethoxybenzoic acid | 0.13 | - | Carboxylic acid |
| 27 | 19.2514 | methyl aspirin | 0.02 | 0.03 | Carboxylic acid |
| 28 | 19.8077 | 2-carene | 0.03 | - | Monoterpene |
| 29 | 19.9878 | cis-3-hexenyl-alpha-methylbutyrate | 0.09 | 0.06 | Ester |
| 30 | 20.1350 | cis-3-hexenyl valerate | 0.11 | 0.11 | Ester |
| 31 | 20.3244 | hexyl valerate | 0.02 | 0.02 | Ester |
| 32 | 20.4539 | isogeraniol | 0.09 | - | Monoterpene |
| 33 | 20.8522 | geraniol | 0.15 | 0.41 | Monoterpene |
| 34 | 21.3253 | ethyl salicylate | 0.64 | 0.63 | Carboxylic acid |
| 35 | 22.2461 | indole | 0.12 | 0.18 | Aromatic |
| 36 | 22.8427 | 3-ethoxy-2-pyridinamine | 0.02 | - | Alkaloid |
| 37 | 22.9760 | methyl m-methoxymandelate | 0.05 | 0.04 | Aromatic |
| 38 | 23.2036 | octane. 2.4.6-trimethyl- | 0.02 | - | Fatty acid |
| 39 | 25.1588 | 4-ethoxybenzaldehyde | - | 0.17 | Phenol |
| 40 | 25.1649 | β-bourbonene | - | 0.01 | Sesquiterpene |
| 41 | 25.4249 | β-elemene | - | 0.03 | Sesquiterpene |
| 42 | 25.6863 | cis-jasmone | 0.04 | - | Ketone |
| 43 | 25.8880 | isocaryophyllene | 0.04 | - | Sesquiterpene |
| 44 | 26.0422 | ledene | - | 0.04 | Sesquiterpene |
| 45 | 26.2873 | caryophyllene | 0.49 | 0.79 | Sesquiterpene |
| 46 | 26.3850 | cholestan-2-one oxime | 0.01 | 0.01 | Sterol |
| 47 | 26.6687 | β-cubebene | - | 0.03 | Sesquiterpene |
| 48 | 27.2546 | aromandendrene | 0.06 | 0.03 | Sesquiterpene |
| 49 | 27.3715 | humulene | - | 0.20 | Sesquiterpene |
| 50 | 27.4949 | (E)-β-farnesene | 0.01 | 0.04 | Sesquiterpene |
| 51 | 27.6045 | alloaromadendrene | 0.01 | 0.04 | Sesquiterpene |
| 52 | 28.1071 | γ-muurolene | 0.01 | - | Sesquiterpene |
| 53 | 28.6874 | longifolene | 0.05 | - | Sesquiterpene |
| 54 | 29.0986 | α-farnesene | 0.23 | 0.07 | Sesquiterpene |
| 55 | 29.2642 | θ-muurolene | 0.01 | - | Sesquiterpene |
| 56 | 29.5492 | calamenene | 0.06 | 0.15 | Sesquiterpene |
| 57 | 30.1420 | α-calacorene | 0.01 | - | Sesquiterpene |
| 58 | 30.6387 | trans-farnesol | 0.01 | - | Sesquiterpene |
| 59 | 30.7600 | E-nerolidol | 0.02 | 0.15 | Sesquiterpene |
| 60 | 30.9116 | 6-octenal. 7-methyl-3-methylene- | 0.01 | - | Aldehyde/alkane |
| 61 | 31.1558 | (Z.E)-farnesol | 0.04 | 0.02 | Sesquiterpene |
| 62 | 31.6900 | 7.9-dimethylhexadecane | 0.01 | 0.01 | Fatty acid |
| 63 | 32.2819 | α-patchoulene | 0.02 | - | Sesquiterpene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauziah, F.; Permana, A.D.; Faizal, A. Characterization of Volatile Compounds from Tea Plants (Camellia sinensis (L.) Kuntze) and the Effect of Identified Compounds on Empoasca flavescens Behavior. Horticulturae 2022, 8, 623. https://doi.org/10.3390/horticulturae8070623
Fauziah F, Permana AD, Faizal A. Characterization of Volatile Compounds from Tea Plants (Camellia sinensis (L.) Kuntze) and the Effect of Identified Compounds on Empoasca flavescens Behavior. Horticulturae. 2022; 8(7):623. https://doi.org/10.3390/horticulturae8070623
Chicago/Turabian StyleFauziah, Fani, Agus Dana Permana, and Ahmad Faizal. 2022. "Characterization of Volatile Compounds from Tea Plants (Camellia sinensis (L.) Kuntze) and the Effect of Identified Compounds on Empoasca flavescens Behavior" Horticulturae 8, no. 7: 623. https://doi.org/10.3390/horticulturae8070623
APA StyleFauziah, F., Permana, A. D., & Faizal, A. (2022). Characterization of Volatile Compounds from Tea Plants (Camellia sinensis (L.) Kuntze) and the Effect of Identified Compounds on Empoasca flavescens Behavior. Horticulturae, 8(7), 623. https://doi.org/10.3390/horticulturae8070623







