Bark Extract of Uncaria tomentosa L. for the Control of Strawberry Phytopathogens †
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Reagents
2.2. Phytopathogens Isolates
2.3. Preparation of Bark Extracts, Chitosan Oligomers, and Conjugate Complexes
2.4. Characterization Procedures
2.5. In Vitro Antimicrobial Activity
2.6. Postharvest Protection Studies
2.7. Statistical Analysis
3. Results
3.1. Cat’s Claw Bark Infrared Spectrum
3.2. Cat’s Claw Extract Constituents
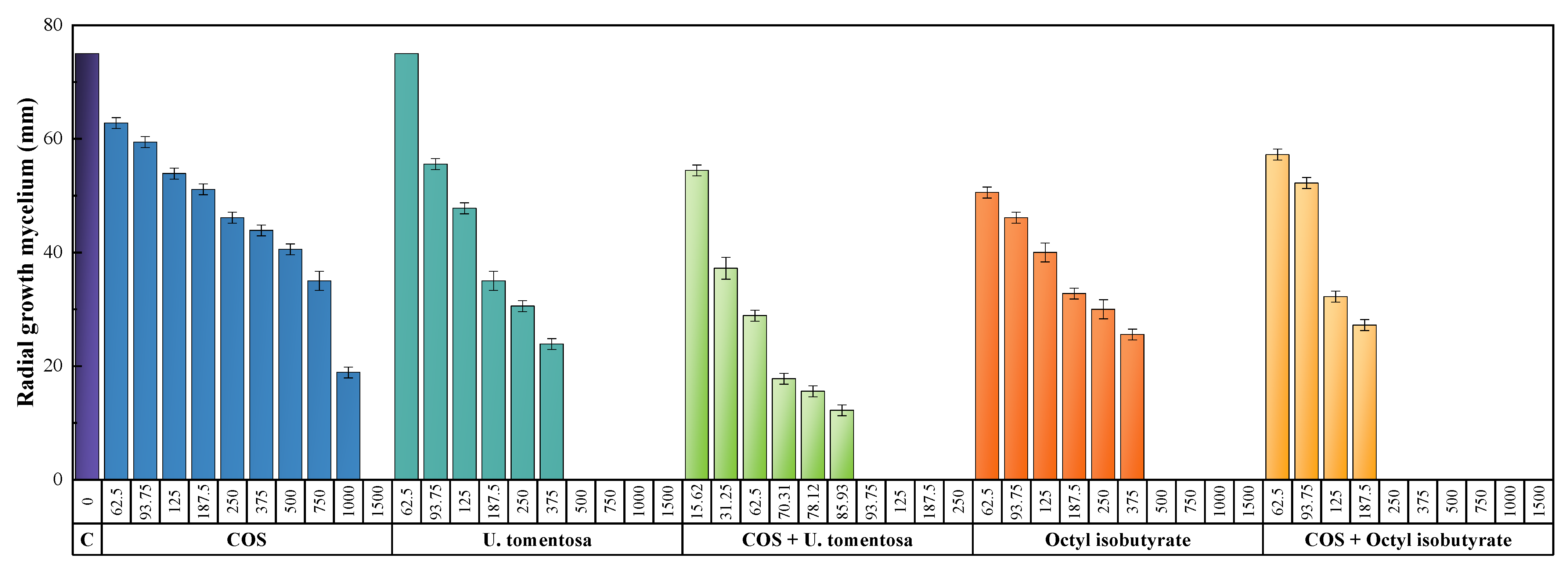
3.3. Antimicrobial Activity
3.4. Postharvest Protection of Strawberry Fruits from Infection by B. cinerea
4. Discussion
4.1. Comparison of In Vitro Activity
4.1.1. Comparison with Other Natural Compounds
4.1.2. Comparison with Conventional Fungicides
4.2. Comparison of Effectiveness of Postharvest Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, E.A.; Mostafa, Y.S.; Alamri, S.; Hashem, M.; Nafady, N.A. Biosafe management of botrytis grey mold of strawberry fruit by novel bioagents. Plants 2021, 10, 2737. [Google Scholar] [CrossRef] [PubMed]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajwa, H.A.; Klose, S.; Nelson, S.D.; Lopez-Aranda, J.; Gullino, M.L.; Lamberti, F.; Minuto, A. Alternatives to methyl bromide in strawberry production in the United States of America and the Mediterranean region. Phytopathol. Mediterr. 2003, 42, 220–244. [Google Scholar] [CrossRef]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Borkovich, K.A.; Zhang, Y.; Wang, C.; Su, P.; Liao, X. Control effect and possible mechanism of the natural compound phenazine-1-carboxamide against Botrytis cinerea. PLoS ONE 2015, 10, e0140380. [Google Scholar] [CrossRef] [Green Version]
- Gomes Honório, I.C.; Bertoni, B.W.; Soares Pereira, A.M. Uncaria tomentosa and Uncaria guianensis an agronomic history to be written. Ciência Rural 2016, 46, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Magdy Beshbishy, A.; Wasef, L.; Elewa, Y.H.A.; Abd El-Hack, M.E.; Taha, A.E.; Al-Sagheer, A.A.; Devkota, H.P.; Tufarelli, V. Uncaria tomentosa (Willd. ex Schult.) DC.: A review on chemical constituents and biological activities. Appl. Sci. 2020, 10, 2668. [Google Scholar] [CrossRef] [Green Version]
- Bertol, G.; Franco, L.; de Oliveira, B.H. HPLC analysis of oxindole alkaloids in Uncaria tomentosa: Sample preparation and analysis optimisation by factorial design. Phytochem. Anal 2012, 23, 143–151. [Google Scholar] [CrossRef]
- Montoro, P.; Carbone, V.; de Dioz Zuniga Quiroz, J.; De Simone, F.; Pizza, C. Identification and quantification of components in extracts of Uncaria tomentosa by HPLC-ES/MS. Phytochem. Anal 2004, 15, 55–64. [Google Scholar] [CrossRef]
- Peñaloza, E.M.C.; Kaiser, S.; Resende, P.E.d.; Pittol, V.; Carvalho, Â.R.; Ortega, G.G. Chemical composition variability in the Uncaria tomentosa (cat’s claw) wild population. Quim. Nova 2015, 38, 378–386. [Google Scholar] [CrossRef]
- Navarro-Hoyos, M.; Alvarado-Corella, D.; Moreira-Gonzalez, I.; Arnaez-Serrano, E.; Monagas-Juan, M. Polyphenolic composition and antioxidant activity of aqueous and ethanolic extracts from Uncaria tomentosa bark and leaves. Antioxidants 2018, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Arnaez, E.; Moreira, I.; Hurtado, A.; Monge, D.; Monagas, M. Polyphenolic composition and antioxidant activity of Uncaria tomentosa commercial bark products. Antioxidants 2019, 8, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.; Monagas, M.; Bartolomé, B. Proanthocyanidin characterization and bioactivity of extracts from different parts of Uncaria tomentosa L. (cat’s claw). Antioxidants 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, G.; Bourbonnais-Spear, N.; Garner, F. Antibacterial constituents from Uncaria tomentosa. Phytopharmacology 2011, 1, 16–19. [Google Scholar]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Agudelo-Romero, P.; Erban, A.; Rego, C.; Carbonell-Bejerano, P.; Nascimento, T.; Sousa, L.; Martínez-Zapater, J.M.; Kopka, J.; Fortes, A.M. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. J. Exp. Bot. 2015, 66, 1769–1785. [Google Scholar] [CrossRef] [Green Version]
- Charles, S.J.; Russell, G.K. Chemical products from bark digested in ammonia. U.S. Patent No. 2,823,223, 11 February 1958. [Google Scholar]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Ruano-Rosa, D.; Sánchez-Hernández, E.; Baquero-Foz, R.; Martín-Ramos, P.; Martín-Gil, J.; Torres-Sánchez, S.; Casanova-Gascón, J. Chitosan-based bioactive formulations for the control of powdery mildew in viticulture. Agronomy 2022, 12, 495. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [Green Version]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]
- Sandoval Flores, M.G.; Jiménez Mejía, R.; Santoyo, G.; Alva Murillo, P.N.; López Meza, J.E.; Loeza Lara, P.D. Compósitos de quitosano-ácidos grasos reducen la infección de Botrytis cinerea en fresa en poscosecha. Nova Sci. 2018, 10, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Muñoz, P.; Almenar, E.; Valle, V.D.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria×ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food. Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Manda, V.; Avula, B.; Ali, Z.; Khan, I.; Walker, L.; Khan, S. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014, 80, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Stuppner, H.; Sturm, S.; Konwalinka, G. HPLC analysis of the main oxindole alkaloids from Uncaria tomentosa. Chromatographia 1992, 34, 597–600. [Google Scholar] [CrossRef]
- Tzakou, O.; Mylonas, P.; Hancianu, M.; Poiata, A. Composition and antimicrobial activity of Malabaila aurea Boiss. essential oil. J. Essent. Oil Res. 2008, 20, 270–271. [Google Scholar] [CrossRef]
- Ehsani, A.; Rezaeiyan, A.; Hashemi, M.; Aminzare, M.; Jannat, B.; Afshari, A. Antibacterial activity and sensory properties of Heracleum persicum essential oil, nisin, and Lactobacillus acidophilus against Listeria monocytogenes in cheese. Vet. World 2019, 12, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Rezayan, A.; Ehsani, A. Evaluation of the chemical compounds and antibacterial properties of the aerial parts of Persian Heracleum persicum essence. J. Babol Univ. Med. Sci. 2015, 17, 26–32. [Google Scholar]
- Hamedi, A.; Pasdaran, A.; Pasdaran, A. Antimicrobial activity and analysis of the essential oils of selected endemic edible Apiaceae plants root from Caspian Hyrcanian region (North of Iran). Pharm. Sci. 2019, 25, 138–144. [Google Scholar] [CrossRef]
- İşcan, G.; Demirci, F.; Kürkçüoǧlu, M.; Kıvanç, M.; Can Başer, K.H. The bioactive essential oil of Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Z. Für Nat. C 2003, 58, 195–200. [Google Scholar] [CrossRef]
- Kumar, P.R.; Shreya, B. Antimicrobial activity of Mitragyna parvifolia barks and Butea monosperma leaves extracts against human pathogenic microbial strains. Int. J. Drug Dev. Res. 2011, 3, 141–147. [Google Scholar]
- Padmavathi, R. Antibacterial and antifungal activity of Mitragyna parvifolia leaf extracts in experimental rats. J. Drug Vigil. Altern. Ther. 2021, 1, 14–18. [Google Scholar] [CrossRef]
- Vasmatkar, P.; Dubey, A.; Tyagi, B.; Baral, P.; Tandon, S.; Kadam, A. Antibacterial activity and GC-MS analysis of methanolic extract from stem bark and leaves of Mitragyna parvifolia (Roxb.) Korth. Indo Am. J. Pharm. Res. 2014, 4, 304–311. [Google Scholar]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.H.; Salem, M.F.; Mazrou, K.E.; El-Tras, W.F. Control of citrus molds using bioactive coatings incorporated with fungal chitosan/plant extracts composite. J. Sci. Food Agric. 2016, 96, 1306–1312. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization and antimicrobial activity against Erwinia amylovora, Erwinia vitivora, and Diplodia seriata of a light purple Hibiscus syriacus L. cultivar. Plants 2021, 10, 1876. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Langa-Lomba, N.; Casanova-Gascón, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Characterization and antimicrobial activity of a halophyte from the Asturian coast (Spain): Limonium binervosum (G.E.Sm.) C.E.Salmon. Plants 2021, 10, 1852. [Google Scholar] [CrossRef]
- Abou-Jawdah, Y.; Wardan, R.; Sobh, H.; Salameh, A. Antifungal activities of extracts from selected Lebanese wild plants against plant pathogenic fungi. Phytopathol. Mediterr. 2004, 43, 377–386. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Zikeli, F.; Scarascia Mugnozza, G.; Vinciguerra, V.; Tabet, D.; Romagnoli, M.; Woodward, S. Lignin nanoparticles containing essential oils for controlling Phytophthora cactorum diseases. For. Pathol. 2022, 52, e12739. [Google Scholar] [CrossRef]
- Oladejo, O.; Imani, J. Inhibitory effect of CUSTOS, a formulated Allium-based extract, on the growth of some selected plant pathogens. Int. J. Plant Biol. 2022, 13, 44–54. [Google Scholar] [CrossRef]
- Erdoğan, O.; Celik, A.; Zeybek, A. In vitro antifungal activity of mint, thyme, lavender extracts and essential oils on Verticillium dahliae Kleb. Fresenius Environ. Bull. 2016, 25, 4856–4862. [Google Scholar]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. Application of plant extracts to control postharvest gray mold and susceptibility of apple fruits to B. cinerea from different plant hosts. Foods 2020, 9, 1430. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Dambrauskienė, E.; Viškelis, P.; Valiuškaitė, A. Biocontrol of strawberry pathogen Botrytis cinerea using plant extracts and essential oils. Zemdirb. -Agric. 2020, 107, 147–152. [Google Scholar] [CrossRef]
- Righini, H.; Baraldi, E.; García Fernández, Y.; Martel Quintana, A.; Roberti, R. Different antifungal activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299. [Google Scholar] [CrossRef] [Green Version]
- Şesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. Antifungal activity of some plant extracts against Botrytis cinerea Pers. in the blackcurrant crop (Ribes nigrum L.). Acta Sci. Pol., Hortorum Cultus 2015, 14, 29–43. [Google Scholar]
- Salamone, A.; Zizzo, G.V.; Scarito, G. The antimicrobial activity of water extracts from Labiatae. Acta Hortic. 2006, 465–470. [Google Scholar] [CrossRef]
- Minova, S.; Sešķēna, R.; Voitkāne, S.; Metla, Z.; Daugavietis, M.; Jankevica, L. Impact of pine (Pinus sylvestris L.) and spruce (Picea abies (L.) Karst.) bark extracts on important strawberry pathogens. Proc. Latv. Acad. Sciences. Sect. B. Nat. Exact Appl. Sci. 2015, 69, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Onaran, A.; Bayan, Y. Antifungal activity of Liquidambar orientalis L., and Myrtus communis L. against some plant pathogenic fungi. Sci. Papers. Ser. A. Agron. 2016, 59, 360–364. [Google Scholar]
- Lee, Y.-S.; Kim, J.; Shin, S.-C.; Lee, S.-G.; Park, I.-K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.S.; Lee, S.G.; Shin, S.C.; Park, I.K. Fumigant antifungal activity of plant essential oils and components from West Indian bay (Pimenta racemosa) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 272–277. [Google Scholar] [CrossRef]
- Er, Y. In vitro and in vivo antimicrobial activity of propolis extracts against various plant pathogens. J. Plant Dis. Prot. 2021, 128, 693–701. [Google Scholar] [CrossRef]
- Maia, J.N.; Beger, G.; Pereira, W.V.; May De Mio, L.L.; da Silva Silveira Duarte, H. Gray mold in strawberries in the Paraná state of Brazil is caused by Botrytis cinerea and its isolates exhibit multiple-fungicide resistance. Crop Prot. 2021, 140, 105415. [Google Scholar] [CrossRef]
- Baggio, J.S.; Peres, N.A.; Amorim, L. Sensitivity of Botrytis cinerea isolates from conventional and organic strawberry fields in Brazil to azoxystrobin, iprodione, pyrimethanil, and thiophanate-methyl. Plant Dis. 2018, 102, 1803–1810. [Google Scholar] [CrossRef] [Green Version]
- Harper, L.A.; Paton, S.; Hall, B.; McKay, S.; Oliver, R.P.; Lopez-Ruiz, F.J. Fungicide resistance characterized across seven modes of action in Botrytis cinerea isolated from Australian vineyards. Pest Manag. Sci. 2021, 78, 1326–1340. [Google Scholar] [CrossRef]
- Kim, B.-S.; Ahn, J.-W. Identification and fungicide responses of Phytophthora cactorum isolated from lily growing Daekwallyong alpine area. Korean J. Pestic. Sci. 2002, 6, 42–44. [Google Scholar]
- Ramírez-Gil, J.G.; Morales-Osorio, J.G. Proposal for integrated management of verticillium wilt disease in avocado cultivar Hass crops. Agronomy 2021, 11, 1932. [Google Scholar] [CrossRef]
- Tawil, M.; Barhoum, B.; Muhrez, K. Effect of some fungicides on Verticillium dahliae Kleb. The causal agent of olive tree wilt disease.(in vitro). Tishreen Univ. J. Res. Sci. Stud. 2020, 42, 4242. [Google Scholar]
- Mihajlovic, M.; Rekanovic, E.; Hrustic, J.; Grahovac, M.; Tanovic, B. In vitro and in vivo toxicity of fungicides and biofungicides for the control of verticillium and fusarium wilt of pepper. Pestic. I Fitomedicina 2021, 36, 23–34. [Google Scholar] [CrossRef]
- Utkhede, R.S. Effects of fungicides on apple crown rot caused by Phytophthora cactorum. Pestic. Sci. 1984, 15, 241–246. [Google Scholar] [CrossRef]
- Tsipouridis, K.; Thomidis, T. Effectiveness of metalaxyl, fosetyl-Al, dimethomorph, and cymoxanil against Phytophthora cactorum and P. citrophthora of peach tree. Phytopathol. Mediterr. 2001, 40, 253–259. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Feliziani, E.; Landi, L.; Romanazzi, G. Preharvest treatments with chitosan and other alternatives to conventional fungicides to control postharvest decay of strawberry. Carbohydr. Polym. 2015, 132, 111–117. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. The control of Botrytis fruit rot in strawberry using combined treatments of chitosan with Zataria multiflora or Cinnamomum zeylanicum essential oil. J. Food Sci. Technol. 2015, 52, 7441–7448. [Google Scholar] [CrossRef]






| Wavenumber (cm−1) | Assignment |
|---|---|
| 3185 | Bonded O‒H stretching (cellulose, hemicellulose, lignin) |
| 2922 | –CH2 asymmetric stretching of alkyls (cutine, wax, pectin) |
| 2362 | CN (alkaloids) |
| 2343 | C−H stretching |
| 1653 | O−H−O scissors-bending/C=N/amide |
| 1560 | aromatic C−H stretching/COO− symmetric stretching (esters) |
| 1520 | Aromatic skeletal |
| 1394 | C−H bending |
| 1258 | Guaiacyl units |
| 1046 | C–O−H stretching/C–O deformation/O−H out-of-plane bending |
| 992 | CH2 groups in cellulose |
| 924 | β-Glycosidic linkages (glucose units of cellulose chains) |
| 870 | Aromatic C−H stretching/methyl double bonds |
| 817 | C–C−H deformation |
| 764 | COO− deformation (esters)/C−H aromatics |
| Pathogen | COS | U. tomentosa | Octyl Isobutyrate | COS− U. tomentosa | COS−Octyl Isobutyrate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 | EC90 | EC50 | EC90 | EC50 | EC90 | EC50 | EC90 | EC50 | EC90 | |
| B. cinerea | 236.2 | 1426.3 | 185.7 | 482.3 | 153.6 | 322.3 | 40.2 | 82.3 | 76.7 | 159.1 |
| P. cactorum | 200.8 | 592.8 | 103.3 | 162.8 | 91.5 | 171.9 | 29.2 | 38.3 | 73.6 | 89.3 |
| V. dahliae | 601.7 | 1321.2 | 185.7 | 482.3 | 142.4 | 471.2 | 32.0 | 87.5 | 116.5 | 248.9 |
| Pathogen | COS–U. tomentosa | COS–Octyl Isobutyrate | ||
|---|---|---|---|---|
| EC50 | EC90 | EC50 | EC90 | |
| B. cinerea | 5.17 | 8.76 | 2.43 | 3.30 |
| P. cactorum | 4.67 | 6.67 | 1.71 | 2.98 |
| V. dahliae | 8.87 | 8.08 | 1.38 | 1.92 |
| Commercial Fungicide | Pathogen | Radial Growth of Mycelium (mm) | Inhibition (%) | ||||
|---|---|---|---|---|---|---|---|
| Rd/10 | Rd * | Rd × 10 | Rd/10 | Rd * | Rd × 10 | ||
| Azoxystrobin | B. cinerea | 12 | 51 | 0 | 84 | 32 | 100 |
| P. cactorum | 6 | 0 | 0 | 92 | 100 | 100 | |
| V. dahliae | 26 | 24 | 0 | 65.3 | 68 | 100 | |
| Mancozeb | B. cinerea | 0 | 0 | 0 | 100 | 100 | 100 |
| P. cactorum | 0 | 0 | 0 | 100 | 100 | 100 | |
| V. dahliae | 0 | 0 | 0 | 100 | 100 | 100 | |
| Metalaxyl | B. cinerea | 45 | 21 | 0 | 40 | 72 | 100 |
| P. cactorum | 0 | 0 | 0 | 100 | 100 | 100 | |
| V. dahliae | 41 | 36 | 0 | 45.3 | 52 | 100 | |
| Fosetyl-Al | B. cinerea | 38 | 0 | 0 | 49.3 | 100 | 100 |
| P. cactorum | 64 | 0 | 0 | 14.7 | 100 | 100 | |
| V. dahliae | 36 | 0 | 0 | 52 | 100 | 100 | |
| Coating | Severity (0−5) |
|---|---|
| Distilled water (control) | 4.4 ± 0.7 a |
| COS−U. tomentosa 100 µg·mL−1 | 3.5 ± 0.8 b |
| COS−U. tomentosa 500 µg·mL−1 | 1.7 ± 0.8 c |
| COS−U. tomentosa 1000 µg·mL−1 | 0.5 ± 0.5 d |
| Pathogen | Natural Product | Effective Concentration/MIC (µg·mL−1) | Ref. |
|---|---|---|---|
| B. cinerea | U. tomentosa bark extract COS−U. tomentosa | MIC = 375 MIC = 93.75 | This work |
| Pimenta dioica PE Cinnamomum cassia PE Laurus nobilis PE | MIC = 2200 MIC = 600 MIC = 3000 | [46] | |
| Syzygium aromaticum PE S. aromaticum EO L. nobilis PE L. nobilis EO Rosmarinus officinalis PE R. officinalis EO | MIC = 600 MIC = 1200 MIC > 2000 MIC > 2000 MIC > 2000 MIC > 2000 | [47] | |
| Anabaena sp. Ecklonia sp. Jania sp. | MIC = 2500 MIC = 5000 MIC = 10,000 | [48] | |
| Achillea millefolium Allium sativum Artemisia dracunculus Hyssopus officinalis Mentha sp. R. officinalis Satureja hortensis Tagetes patula Valeriana officinalis | MIC > 20,000 MIC = 20,000 MIC > 20,000 MIC < 5000 MIC = 20,000 MIC > 20,000 MIC = 10,000 MIC > 20,000 MIC > 20,000 | [49] | |
| Origanum vulgare EO Thymus capitatus EO O. dictamnus EO O. majorana EO Lavandula angustifolia EO R. officinalis EO Salvia fruticosa EO M. pulegium EO | EC50 = 50 EC50 = 83 EC50 = 67 EC50 = 143 EC50 = 223 EC50 = 606 EC50 ≤ 1000 EC50 = 216 | [42] | |
| Micromeria nervosa PE Origanum syriacum PE Inula viscosa PE Plumbago maritime PE | MIC = 500 MIC = 60 MIC > 2 × 106 MIC = 1 × 106 | [41] | |
| O. heracleoticum PE Salvia officinalis PE R. officinalis PE | MIC > 5 × 105 MIC > 5 × 105 MIC > 5 × 105 | [50] | |
| Pinus sylvestris bark P. abies bark | MIC = 20,000 MIC = 20,000 | [51] | |
| Liquidambar orientalis PE Myrtus communis PE | MIC > 4 × 105 MIC = 400 | [52] | |
| P. cactorum | U. tomentosa bark extract COS−U. tomentosa | MIC = 187.5 MIC = 39.05 | This work |
| Allium-based extract | MIC = 100 | [44] | |
| O. heracleoticum PE S. officinalis PE R. officinalis PE | MIC > 5 × 105 MIC > 5 × 105 MIC > 5 × 105 | [50] | |
| P. sylvestris bark P. abies bark | MIC = 100 MIC = 100 | [51] | |
| T. serpyllum EO | EC50 = 20.45 | [43] | |
| Eucalyptus citriodora EO Melaleuca quinquenervia EO Leptospermum pertersonii EO | MIC > 28,000 MIC > 28,000 MIC = 28,000 | [53] | |
| Polylepis. racemosa EO Junierus oxycedrus EO Cymbopogon nardus EO Pelargonium graveolens EO Cuminum cyminum EO Myrristica fragrans EO C. martini EO M. pulegium EO M. spicata EO T. vulgaris EO | MIC > 28,000 MIC > 28,000 MIC > 28,000 MIC = 28,000 MIC > 28,000 MIC > 28,000 MIC = 28,000 n.a. n.a. MIC = 14,000 | [54] | |
| V. dahliae | U. tomentosa bark extract COS−U. tomentosa | MIC = 500 MIC = 93.75 | This work |
| O. heracleoticum PE S. officinalis PE R. officinalis PE | MIC > 5 × 105 MIC > 5 × 105 MIC > 5 × 105 | [50] | |
| Propolis | MIC > 60,000 | [55] | |
| M. piperita EO/PE T. vulgaris EO/PE Lavandula angustufolia EO/PE | MIC = 16 / > 1 × 105 MIC = 8 / > 1 × 105 MIC = 32 / > 1 × 105 | [45] |
| Synthetic Fungicide | Pathogen | Provenance of Isolate | Effective Concentration/MIC (µg·mL−1) | Ref. |
|---|---|---|---|---|
| Azoxystrobin | B. cinerea | Strawberry | EC50 ≥ 100 | [56] |
| EC50 ≥ 71.9 | [57] | |||
| Grapevine | EC50 ≥ 50 | [58] | ||
| P. cactorum | Strawberry | MIC ≥ 100 | [59] | |
| V. dahliae | Avocado tree | MIC ≥ 40,000 | [60] | |
| Olive tree | MIC = 1000 | [61] | ||
| Pepper | EC50= 71.95 | [62] | ||
| Mancozeb | P. cactorum | Apple tree | MIC = 100 | [63] |
| Strawberry | MIC = 100 | [59] | ||
| Metalaxyl | P. cactorum | Apple tree | MIC = 50 | [63] |
| Peach tree | MIC = 1 × 105 | [64] | ||
| Strawberry | MIC = 100 | [59] | ||
| Fosetyl-Al | P. cactorum | Apple tree | MIC = 1000 | [63] |
| Peach tree | MIC = 1.5 × 106 | [64] | ||
| V. dahliae | Olive tree | MIC = 5000 | [61] |
| Application | Natural Product | Storage Conditions | Severity (0–5) | Ref. |
|---|---|---|---|---|
| Postharvest | Chitosan acetate (1% w/v) | 4 days at 20 ± 1 °C, 95–98% RH | 3.1 | [16] |
| Chitosan chloride (1% w/v) | 3.2 | |||
| Chitosan formate (1% w/v) | 3.4 | |||
| Chitosan glutamate (1% w/v) | 3.4 | |||
| Commercial chitosan (1% w/v) | 3.5 | |||
| Abies sibirica extract (1% v/v) | 7 days at 0 ± 1 °C, 95–98% RH, followed by 3 days of shelf life at 20 ± 1 °C, 95–98% RH | 2.2 | ||
| Oligosaccharides (1% v/v) | 3.4 | |||
| Chitosan (1% w/v) | 2.7 | |||
| Ca+organic acids (1% v/v) | 3.4 | |||
| Urtica dioica extract (1% w/v) | 2.9 | |||
| Soybean lecitin (1% v/v) | 3.2 | |||
| Chitosan NP (1500 μg·mL−1) | 7 days at 4 °C, followed by 2 days at 20 °C | 2.6 | [65] | |
| Chitosan NP-Zataria multiflora (1500 μg·mL−1) | 1.5 | |||
| Cinnamomum zeylanicum EO (1500 μg·mL−1) | 3.2 | [67] | ||
| Zataria multiflora EO (1500 μg·mL−1) | 3.5 | |||
| Chitosan + C. zeylanicum (1500 μg·mL−1) | 2.4 | |||
| Chitosan + Z. multiflora (1500 μg·mL−1) | 1.5 | |||
| Preharvest | Chitosan 0.5% | 7 days at 0.5 ± 1 °C, followed by 4 days at 20 ± 1 °C and 95–98% RH | 2.1−3.0 * | [66] |
| Chitosan 1% | 2.0−2.8 * | |||
| Fir extract/organic acids and Ca (10 mg·mL−1) | 2.0−3.5 * | |||
| Laminarin 1% | 2.3−3.3 * | |||
| Laminarin + Saccharomyces spp. extract (1 + 3 mg·mL−1) | 2.0−3.1 * | |||
| Laminarin + Polygonum spp. extract (1 + 3 mg·mL−1) | 1.8−3.0 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark Extract of Uncaria tomentosa L. for the Control of Strawberry Phytopathogens. Horticulturae 2022, 8, 672. https://doi.org/10.3390/horticulturae8080672
Sánchez-Hernández E, Martín-Ramos P, Martín-Gil J, Santiago-Aliste A, Hernández-Navarro S, Oliveira R, González-García V. Bark Extract of Uncaria tomentosa L. for the Control of Strawberry Phytopathogens. Horticulturae. 2022; 8(8):672. https://doi.org/10.3390/horticulturae8080672
Chicago/Turabian StyleSánchez-Hernández, Eva, Pablo Martín-Ramos, Jesús Martín-Gil, Alberto Santiago-Aliste, Salvador Hernández-Navarro, Rui Oliveira, and Vicente González-García. 2022. "Bark Extract of Uncaria tomentosa L. for the Control of Strawberry Phytopathogens" Horticulturae 8, no. 8: 672. https://doi.org/10.3390/horticulturae8080672