Inheritance of Apple (Malus × domestica (L.) Borkh) Resistance against Apple Scab (Venturia inaequalis (Cooke) Wint.) in Hybrid Breeding Material Obtained by Gene Pyramiding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Practice
2.2. Field Evaluation of Apple Scab Severity and Tree Health Status
2.3. Genotyping Using Scab Resistance Gene and Genetic Relatedness Molecular Markers
2.4. Data Analysis
3. Results
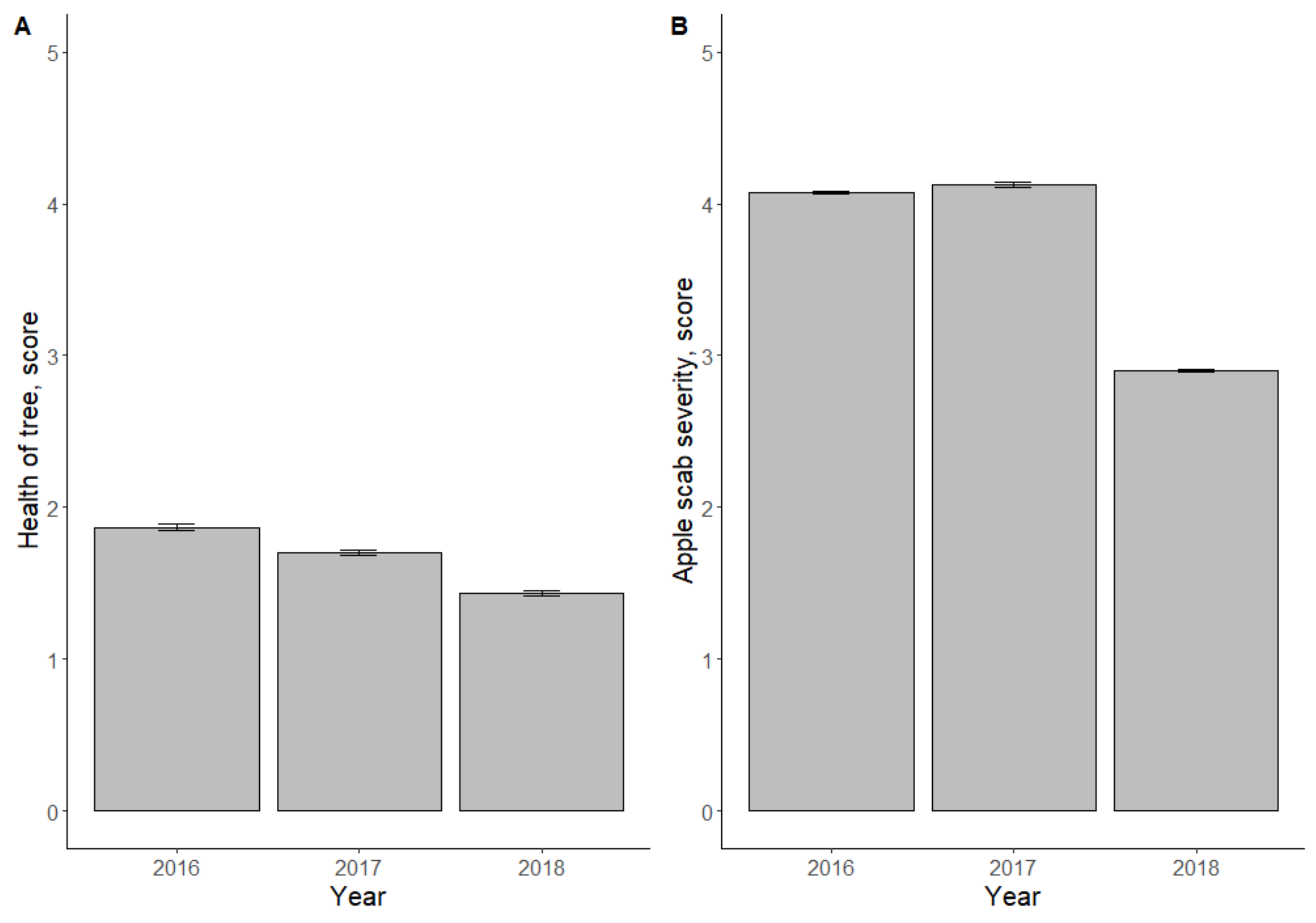
3.1. Resistance of Hybrid Seedling Material against Apple Scab in the Field
3.2. Apple Scab Resistance Specific Gene Identification in Hybrid Seedlings
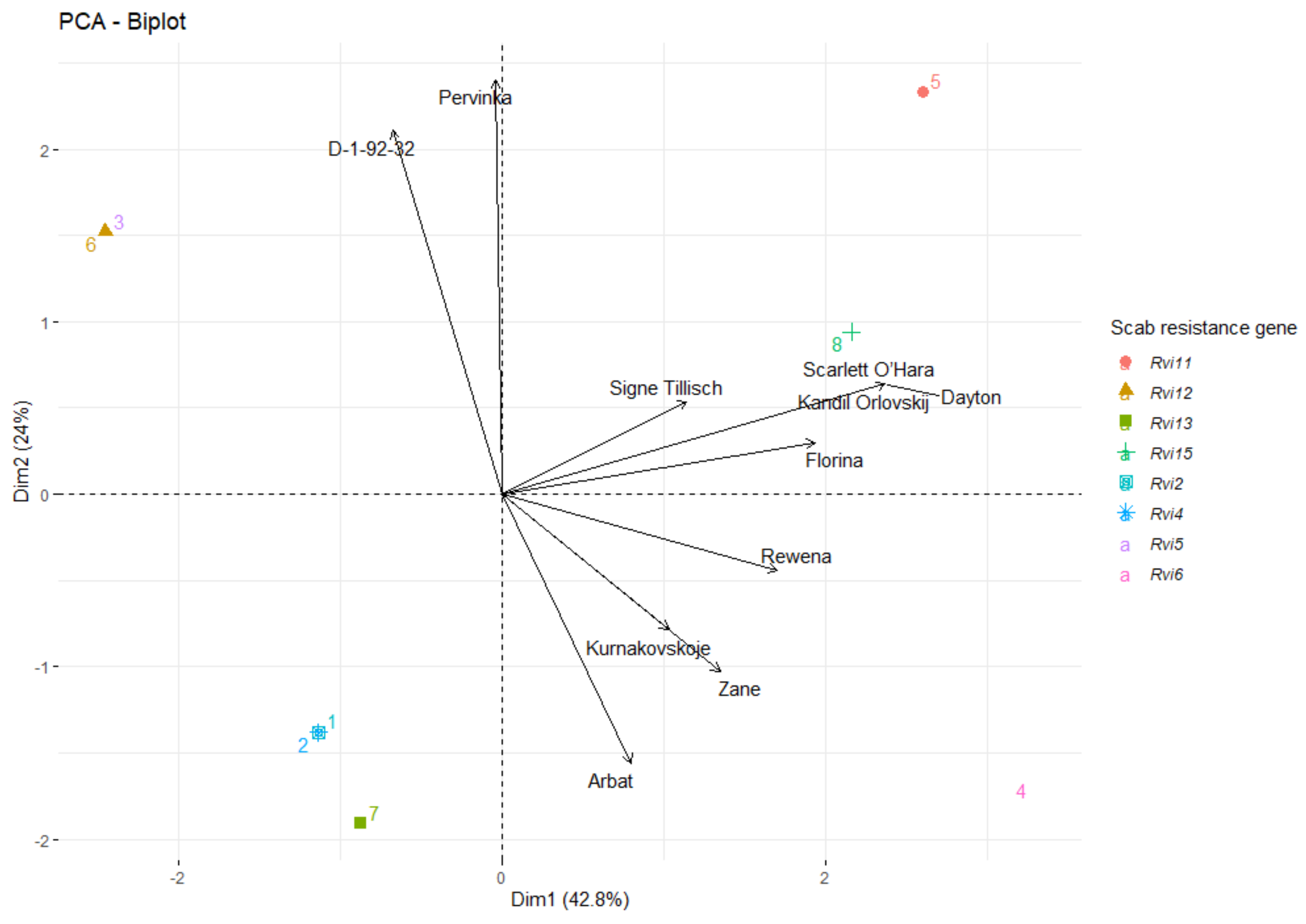
3.3. Genetic Relationship of Parent Plants
4. Discussion
4.1. Relationship between Field Data and Marker Data
4.2. Analysis of Parent Plants
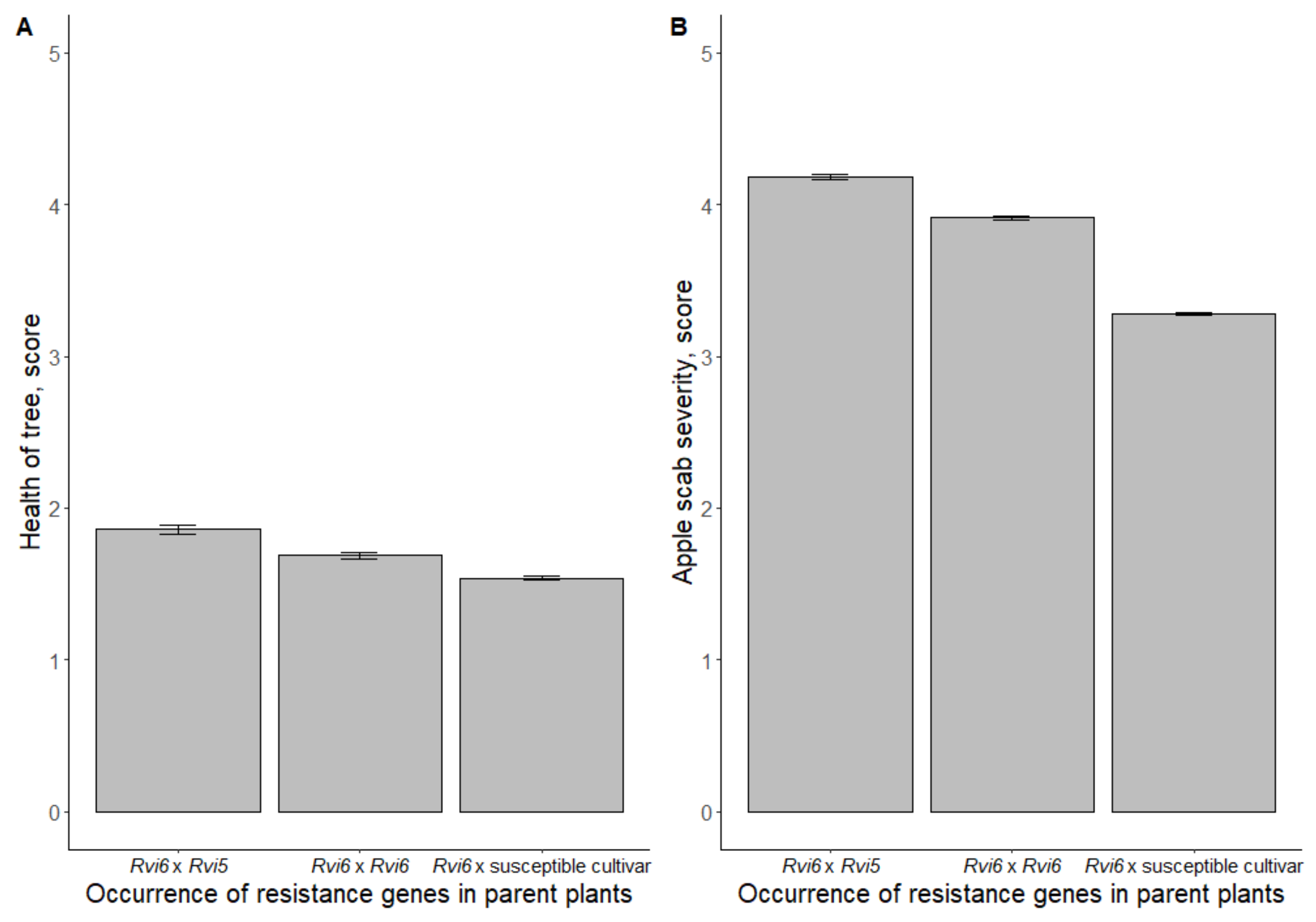
4.3. Effects of Gene Pyramiding
5. Conclusions
- Overall, a combination of field evaluation and molecular studies offers a more measured look into how resistance plays out in a tree’s natural environment and is a viable path for future research.
- Resistance to apple scab is influenced by the overall health of the tree, genetic factors such as the pedigree of the cultivar, genetic composition of parent cultivars, incl. the presence of various resistance genes, whereas the environment alters the way resistance manifests.
- When Rvi6 and Rvi5 are pyramided, they are inherited as separate genes and inheriting both is not the only factor for apple scab resistance since in the field, several factors are at play and can significantly modify resistance outcomes.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, S.A.; Julian, P.; Juniper, B.E. Genetics and the Origin of Apples. Trends Genet. 2002, 18, 426–430. [Google Scholar] [CrossRef]
- Ignatov, A.; Bodishevskaya, A. Malus. In Wild Crop Relatives: Genomic and Breeding Resources: Temperate Fruits; Springer: Berlin/Heidelberg, Germany, 2011; pp. 45–64. ISBN 9783642143878. [Google Scholar]
- Cornille, A.; Giraud, T.; Smulders, M.J.M.; Roldán-Ruiz, I.; Gladieux, P. The Domestication and Evolutionary Ecology of Apples. Trends Genet. 2014, 30, 57–65. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Fischer, M. Breeding Apple (Malus × Domestica Borkh) BT—Breeding Plantation Tree Crops: Temperate Species; Gradziel, T.M., Ed.; Springer: New York, NY, USA, 2009; pp. 33–81. ISBN 978-0-387-71203-1. [Google Scholar]
- Belete, T.; Boyraz, N. Critical Review on Apple Scab (Venturia inaequalis) Biology, Epidemiology, Economic Importance, Management and Defense Mechanisms to the Causal Agent. J. Plant Physiol. Pathol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Urrestarazu, J.; Denancé, C.; Ravon, E.; Guyader, A.; Guisnel, R.; Feugey, L.; Poncet, C.; Lateur, M.; Houben, P.; Ordidge, M.; et al. Analysis of the Genetic Diversity and Structure across a Wide Range of Germplasm Reveals Prominent Gene Flow in Apple at the European Level. BMC Plant Biol. 2016, 16, 1–20. [Google Scholar] [CrossRef]
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The Causal Agent of Apple Scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef]
- Jha, G.; Thakur, K.; Thakur, P. The Venturia Apple Pathosystem: Pathogenicity Mechanisms and Plant Defense Responses. J. Biomed. Biotechnol. 2009, 680160. [Google Scholar] [CrossRef]
- Carisse, O.; Dewdney, M. A Review of Non-Fungicidal Approaches for the Control of Apple Scab. Phytoprotection 2002, 83, 1–29. [Google Scholar] [CrossRef]
- Lê Van, A.; Durel, C.E.; le Cam, B.; Caffier, V. The Threat of Wild Habitat to Scab Resistant Apple Cultivars. Plant Pathol. 2011, 60, 621–630. [Google Scholar] [CrossRef]
- Khajuria, Y.P.; Kaul, S.; Wani, A.A.; Dhar, M.K. Genetics of Resistance in Apple against Venturia inaequalis (Wint.) Cke. Tree Genet. Genomes 2018, 14, 16. [Google Scholar] [CrossRef]
- Bus, V.G.M.; Rikkerink, E.H.A.; Caffier, V.; Durel, C.E.; Plummer, K.M. Revision of the Nomenclature of the Differential Host-Pathogen Interactions of Venturia inaequalis and Malus. Annu. Rev. Phytopathol. 2011, 49, 391–413. [Google Scholar] [CrossRef] [Green Version]
- MacHardy, W.; Gadoury, D.; Gessler, C. Parasitic and Biological Fitness of Venturia inaequalis: Relationship to Disease Management Strategies. Plant Dis. 2001, 85, 1036–1051. [Google Scholar] [CrossRef]
- Bastiaanse, H. Genetics of Resistance to Scab Caused by Venturia inaequalis in “President Roulin” and “Geneva” Apple Cultivars. PhD Thesis, Universite de Liege, Gembloux, Belgium, 2015. [Google Scholar]
- Peil, A.; Kellerhals, M.; Höfer, M.; Flachowsky, H. Apple Breeding—From the Origin to Genetic Engineering. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 118–138. [Google Scholar]
- Gianfranceschi, L.; Koller, B.; Seglias, N.; Kellerhals, M.; Gessler, C. Molecular Selection in Apple for Resistance to Scab Caused by Venturia inaequalis. Theor. Appl. Genet. 1996, 93, 199–204. [Google Scholar] [CrossRef]
- Dar, J.A.; Zargar, S.M.; Rather, R.N.; Wani, A.A. Mining New Scab Resistance Alleles in Apple (Malus × Domestica Borkh.) Germplasm of Kashmir: Towards Breeding Scab Free Apple Cultivars. Indian J. Genet. Plant Breed. 2020, 80, 112–114. [Google Scholar] [CrossRef]
- Karapetsi, L.; Nianiou-Obeidat, I.; Zambounis, A.; Osathanunkul, M.; Madesis, P. Molecular Screening of Domestic Apple Cultivars for Scab Resistance Genes in Greece. Czech J. Genet. Plant Breed. 2020, 56, 165–169. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Mushtaq, K.; Mir, J.I.; Amin, M.; Nabi, S.U. Introgression of Scab Resistance Gene Vf (Rvi6) in Commercially Grown Susceptible Cultivar Fuji Azitec of Apple (Malus domestica) Using Marker Assisted Selection. Res. J. Biotechnol. 2020, 15, 50–56. [Google Scholar]
- Bivolariu (Guzu), G.; Zagrai, I.; Zagrai, L.; Cordea, M.I.; Moldovan, C. Molecular Screening of Some Apple Progenies for Detection Vf Gene Using Marker Assisted Selection. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 2021, 78, 36–40. [Google Scholar] [CrossRef]
- Luo, F.; Evans, K.; Norelli, J.L.; Zhang, Z.; Peace, C. Prospects for Achieving Durable Disease Resistance with Elite Fruit Quality in Apple Breeding. Tree Genet. Genomes 2020, 16, 21. [Google Scholar] [CrossRef]
- Laurens, F.; Aranzana, M.J.; Arus, P.; Bassi, D.; Bink, M.; Bonany, J.; Caprera, A.; Corelli-Grappadelli, L.; Costes, E.; Durel, C.E.; et al. An Integrated Approach for Increasing Breeding Efficiency in Apple and Peach in Europe. Hortic. Res. 2018, 5, 11. [Google Scholar] [CrossRef]
- Patocchi, A.; Wehrli, A.; Dubuis, P.H.; Auwerkerken, A.; Leida, C.; Cipriani, G.; Passey, T.; Staples, M.; Didelot, F.; Philion, V.; et al. Ten Years of VINQUEST: First Insight for Breeding New Apple Cultivars with Durable Apple Scab Resistance. Plant Dis. 2020, 104, 2074–2081. [Google Scholar] [CrossRef]
- Kaufmane, E.; Skrivele, M.; Rubauskis, E.; Strautiņa, S.; Ikase, L.; Lacis, G.; Segliņa, D.; Moročko-Bičevska, I.; Ruisa, S.; Priekule, I. Development of Fruit Science in Latvia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci 2013, 67, 71–83. [Google Scholar] [CrossRef]
- Ikase, L. Results of Fruit Breeding in Baltic and Nordic States. In Proceedings of the 25th Congress of the Nordic Association of Agricultural Scientists (NJF) Nordic View to Sustainable Rural Development, Riga, Latvia, 16–18 June 2015; pp. 31–37. [Google Scholar]
- Lacis, G.; Kota, I.; Ikase, L.; Rungis, D. Molecular Characterization of the Latvian Apple (Malus) Genetic Resource Collection Based on SSR Markers and Scab Resistance Gene Vf Analysis. Plant Genet. Resour. Characterisation Util. 2011, 9, 189–192. [Google Scholar] [CrossRef]
- Cheng, F.S.; Weeden, N.F.; Brown, S.K.; Aldwinckle, H.S.; Gardiner, S.E.; Bus, V.G. Development of a DNA Marker for V(m), a Gene Conferring Resistance to Apple Scab. Genome 1998, 41, 208–214. [Google Scholar] [CrossRef]
- Vejl, P.; Skupinová, S.; Blažek, J.; Sedlák, P.; Bardová, M.; Drahošová, H.; Blažková, H.; Milec, Z. PCR Markers of Apple Resistance to Scab (Venturia inaequalis CKE.) Controlled by Vf Gene in Czech Apple Breeding. Plant Soil Environ. 2003, 49, 427–432. [Google Scholar] [CrossRef]
- Patocchi, A.; Frei, A.; Frey, J.E.; Kellerhals, M. Towards Improvement of Marker Assisted Selection of Apple Scab Resistant Cultivars: Venturia inaequalis Virulence Surveys and Standardization of Molecular Marker Alleles Associated with Resistance Genes. Mol. Breed. 2009, 24, 337–347. [Google Scholar] [CrossRef]
- Liebhard, R.; Gianfranceschi, L.; Koller, B.; Ryder, C.D.; Tarchini, R.; van de Weg, E.; Gessler, C. Development and Characterisation of 140 New Microsatellites in Apple (Malus × domestica Borkh.). Mol. Breed. 2002, 10, 217–241. [Google Scholar] [CrossRef]
- Vinatzer, B.A.; Patocchi, A.; Tartarini, S.; Gianfranceschi, L.; Sansavini, S.; Gessler, C. Isolation of Two Microsatellite Markers from BAC Clones of the Vf Scab Resistance Region and Molecular Characterization of Scab-Resistant Accessions in Malus Germplasm. Plant Breed. 2004, 123, 321–326. [Google Scholar] [CrossRef]
- Silfverberg-Dilworth, E.; Matasci, C.L.; van de Weg, W.E.; van Kaauwen, M.P.W.; Walser, M.; Kodde, L.P.; Soglio, V.; Gianfranceschi, L.; Durel, C.E.; Costa, F.; et al. Microsatellite Markers Spanning the Apple (Malus × domestica Borkh.) Genome. Tree Genet. Genomes 2006, 2, 202–224. [Google Scholar] [CrossRef]
- Celton, J.M.; Tustin, D.S.; Chagné, D.; Gardiner, S.E. Construction of a Dense Genetic Linkage Map for Apple Rootstocks Using SSRs Developed from Malus ESTs and Pyrus Genomic Sequences. Tree Genet. Genomes 2009, 5, 93–107. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 11 July 2022).
- Smouse, R.P.P.; Peakall, R. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar]
- Masny, S. Occurrence of Venturia inaequalis Races in Poland Able to Overcome Specific Apple Scab Resistance Genes. Eur. J. Plant Pathol. 2017, 147, 313–323. [Google Scholar] [CrossRef]
- Papp, D.; Singh, J.; Gadoury, D.; Khan, A. New North American Isolates of Venturia inaequalis Can Overcome Apple Scab Resistance of Malus floribunda 821. Plant Dis. 2020, 104, 649–655. [Google Scholar] [CrossRef]
- Baumgartner, I.O.; Patocchi, A.; Frey, J.E.; Peil, A.; Kellerhals, M. Breeding Elite Lines of Apple Carrying Pyramided Homozygous Resistance Genes Against Apple Scab and Resistance Against Powdery Mildew and Fire Blight. Plant Mol. Biol. 2015, 33, 1573–1583. [Google Scholar] [CrossRef]
- Laloi, G.; Vergne, E.; Durel, C.E.; le Cam, B.; Caffier, V. Efficiency of Pyramiding of Three Quantitative Resistance Loci to Apple Scab. Plant Pathol. 2017, 66, 412–422. [Google Scholar] [CrossRef]
- Gessler, C.; Patocchi, A.; Sansavini, S.; Tartarini, S.; Gianfranceschi, L. Venturia inaequalis Resistance in Apple. Crit. Rev. Plant Sci. 2006, 25, 473–503. [Google Scholar] [CrossRef]
- Calenge, F.; Faure, A.; Goerre, M.; Gebhardt, C.; van de Weg, W.E.; Parisi, L.; Durel, C.E. Quantitative Trait Loci (QTL) Analysis Reveals Both Broad-Spectrum and Isolate-Specific QTL for Scab Resistance in an Apple Progeny Challenged with Eight Isolates of Venturia inaequalis. Phytopathology 2004, 94, 370–379. [Google Scholar] [CrossRef]
- Crosby, J.A.; Janick, J.; Pecknold, P.C.; Korban, S.S.; O’Connor, P.A.; Ries, S.M.; Goffreda, J.; Voordeckers, A. Breeding Apples for Scab Resistance: 1945–1990. Acta Hortic. 1992, 317, 43–70. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Bhat, K.; Mir, J.; Mir, M.; Nabi, S.U.; Bhat, H.A.; Shafi, W.; Zaffer, S.; Jan, S.; Raja, W.H. Phenotypic and Molecular Screening for Disease Resistance of Apple Cultivars and Selections against Apple Scab (Venturia inaequalis) Phenotypic and Molecular Screening for Diseases Resistance of Apple Cultivars and Selections against Apple Scab (Venturia). Int. J. Chem. Stud. 2017, 5, 1107–1113. [Google Scholar]
- Liebhard, R.; Koller, B.; Patocchi, A.; Kellerhals, M.; Pfammatter, W.; Jermini, M.; Gessler, C. Mapping Quantitative Field Resistance against Apple Scab in a “Fiesta” x “Discovery” Progeny. Phytopathology 2003, 93, 493–501. [Google Scholar] [CrossRef]
- Patocchi, A.; Walser, M.; Tartarini, S.; Broggini, G.A.L.; Gennari, F.; Sansavini, S.; Gessler, C. Identification by Genome Scanning Approach (GSA) of a Microsatellite Tightly Associated with the Apple Scab Resistance Gene Vm. Genome 2005, 48, 630–636. [Google Scholar] [CrossRef]
- Soufflet-Freslon, V.; Gianfranceschi, L.; Patocchi, A.; Durel, C.E. Inheritance Studies of Apple Scab Resistance and Identification of Rvi14, a New Major Gene That Acts Together with Other Broad-Spectrum QTL. Genome 2008, 51, 657–667. [Google Scholar] [CrossRef]
- Bastiaanse, H.; Bassett, H.C.M.; Kirk, C.; Gardiner, S.E.; Deng, C.; Groenworld, R.; Chagné, D.; Bus, V.G.M. Scab Resistance in “Geneva” Apple Is Conditioned by a Resistance Gene Cluster with Complex Genetic Control. Mol. Plant Pathol. 2016, 17, 159–172. [Google Scholar] [CrossRef]
- Galli, P.; Broggini, G.A.L.; Gessler, C.; Patocchi, A. Phenotypic Characterization of the Rvi15 (Vr2) Apple Scab Resistance. J. Plant Pathol. 2010, 92, 219–226. [Google Scholar] [CrossRef]
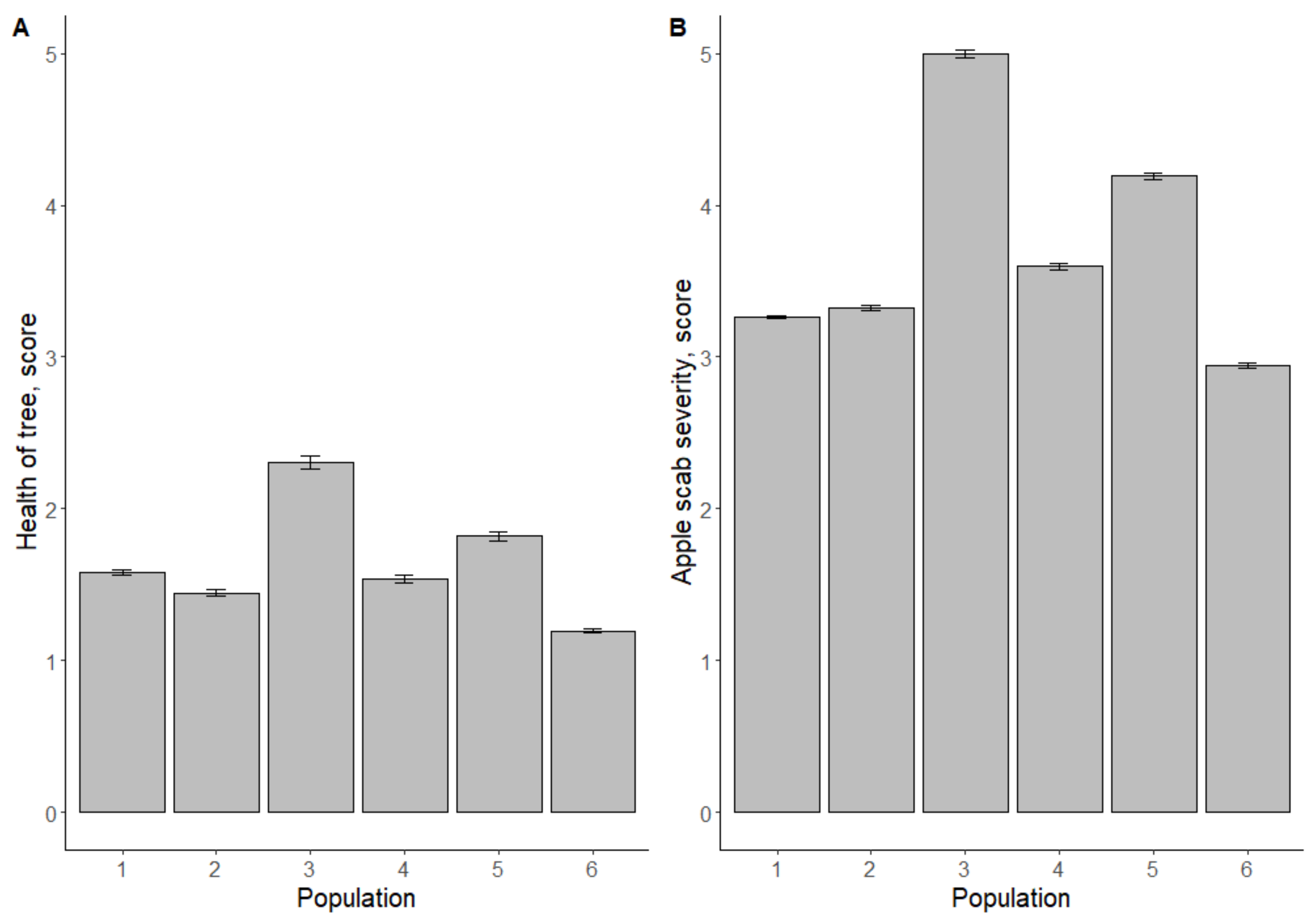
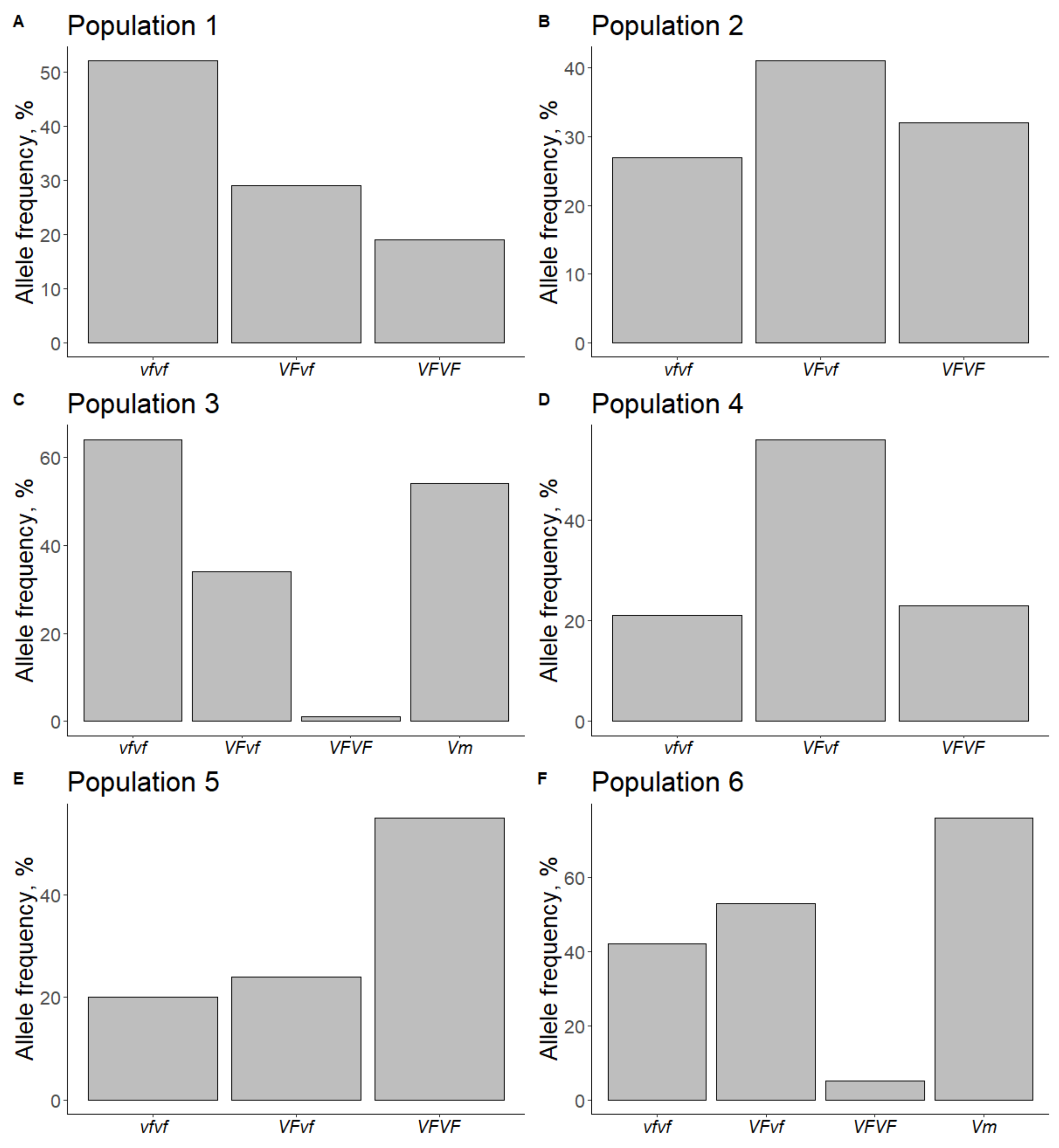


| Population Number | Population Code | Combination of Resistance Genes | Parent Plants | Number of Hybrids |
|---|---|---|---|---|
| 1 | MA-2 | Rvi6 × susceptible cultivar | ‘Arbat’ × ‘Signe Tillisch’ | 274 |
| 2 | CO-3A | Rvi6 × susceptible cultivar | ‘Arbat’ × ‘Zane’ | 119 |
| 3 | VM-5 | Rvi6 × Rvi5 | ‘Dayton’ × ‘Pervinka’ | 135 |
| 4 | VF-3 | Rvi6 × Rvi6 | ‘Kandil Orlovskij’ × ‘Florina’ | 117 |
| 5 | VF-4 | Rvi6 × Rvi6 | ‘Kurnakovskoje’ × ‘Rewena’ | 132 |
| 6 | VM-2 | Rvi6 × Rvi5 | ‘Scarlett O’Hara’ × D-1-92-32 | 85 |
| Score | Definition of the Symptoms | Proportion of Affected Organs (%) |
|---|---|---|
| 0/n | No observation (missing plant) | - |
| 1 | No visible symptom | 0% |
| 2 | One or very few lesions detectable on scrutiny of the tree | 0 to 1% |
| 3 | Immediately apparent lesions in general clustered in few parts of the tree | 1 to 5% |
| 4 | intermediate | ±15% |
| 5 | Numerous lesions widespread over a large part of the tree | ±25% |
| 6 | intermediate | ±35% |
| 7 | Severe infection with half of the leaves badly infected by multiple lesions | ±50% |
| 8 | intermediate | ±75% |
| 9 | Tree completely affected with (nearly) all the leaves badly infected by multiple lesions | >90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelmene, K.; Kārkliņa, K.; Ikase, L.; Lācis, G. Inheritance of Apple (Malus × domestica (L.) Borkh) Resistance against Apple Scab (Venturia inaequalis (Cooke) Wint.) in Hybrid Breeding Material Obtained by Gene Pyramiding. Horticulturae 2022, 8, 772. https://doi.org/10.3390/horticulturae8090772
Zelmene K, Kārkliņa K, Ikase L, Lācis G. Inheritance of Apple (Malus × domestica (L.) Borkh) Resistance against Apple Scab (Venturia inaequalis (Cooke) Wint.) in Hybrid Breeding Material Obtained by Gene Pyramiding. Horticulturae. 2022; 8(9):772. https://doi.org/10.3390/horticulturae8090772
Chicago/Turabian StyleZelmene, Kristīne, Katrīna Kārkliņa, Laila Ikase, and Gunārs Lācis. 2022. "Inheritance of Apple (Malus × domestica (L.) Borkh) Resistance against Apple Scab (Venturia inaequalis (Cooke) Wint.) in Hybrid Breeding Material Obtained by Gene Pyramiding" Horticulturae 8, no. 9: 772. https://doi.org/10.3390/horticulturae8090772
APA StyleZelmene, K., Kārkliņa, K., Ikase, L., & Lācis, G. (2022). Inheritance of Apple (Malus × domestica (L.) Borkh) Resistance against Apple Scab (Venturia inaequalis (Cooke) Wint.) in Hybrid Breeding Material Obtained by Gene Pyramiding. Horticulturae, 8(9), 772. https://doi.org/10.3390/horticulturae8090772






