Minimal Necessary Weed Control Does Not Increase Weed-Mediated Biological Pest Control in Romaine Lettuce (Lactuca sativa L., var. Romana)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Layout
2.2. Data Collection
2.3. Data Analysis
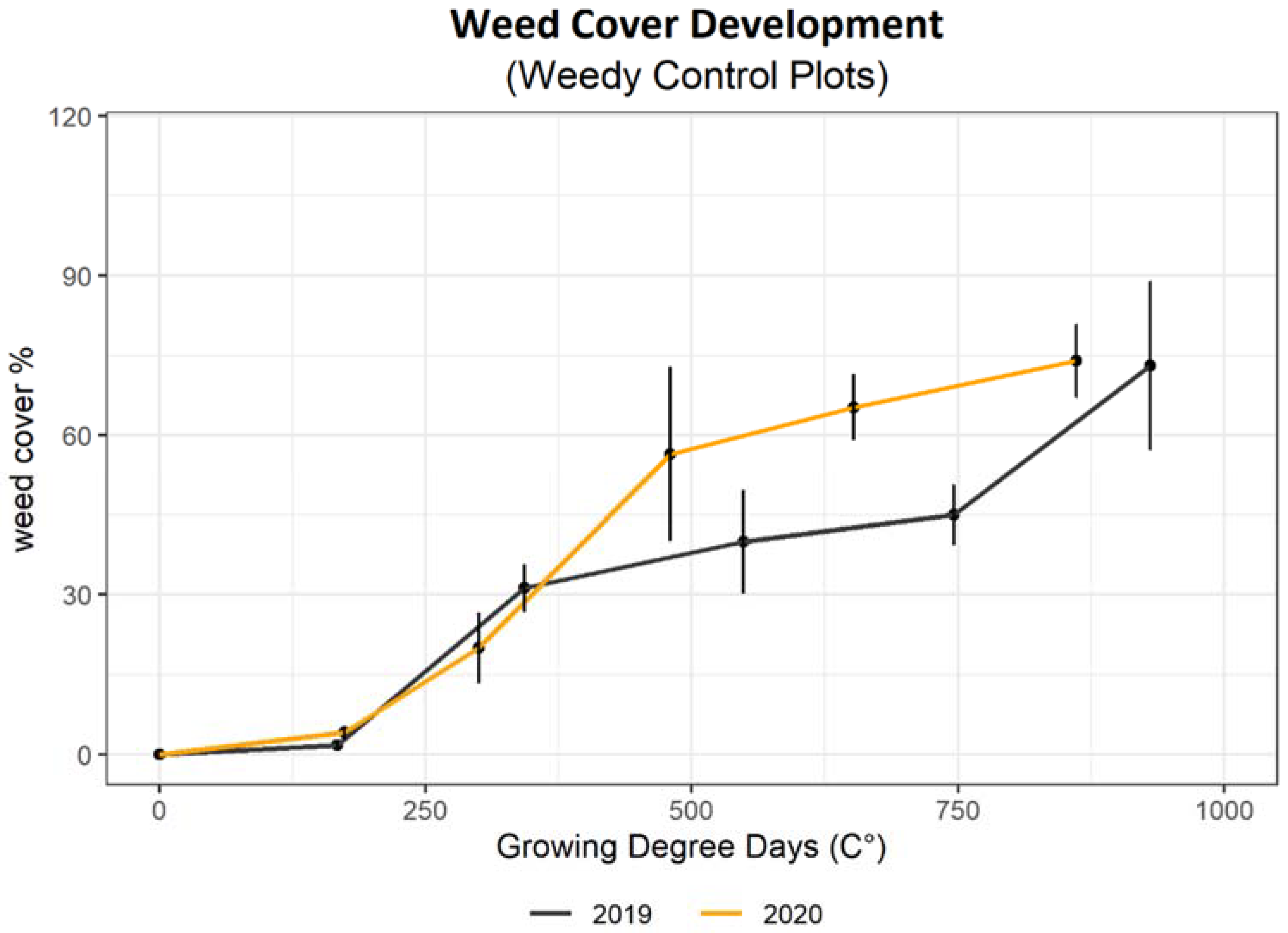
3. Results
3.1. Effect of Weeding Regime on Lettuce Yield
3.2. Effect of Weeding Regime on Lettuce Yield Components
3.3. Effect of Different Weed Groups on Crop Yield, Growth, and Stress Components
3.4. Critical Period of Weed Interference
3.5. Weed Community
3.6. Biological Pest Control Provisioning Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO FAOSTAT. Available online: https://Www.Fao.Org/Faostat/En/#compare (accessed on 17 May 2022).
- FAO FAOSTAT. Available online: https://Www.Fao.Org/Faostat/En/#data/QCL/Visualize (accessed on 17 May 2022).
- Giancotti, P.R.F.; Machado, M.H.; Yamauti, M.S. Período Total de Prevenção a Interferência Das Plantas Daninhas Na Cultura Da Alface Cultivar Solaris. Semina Ciênc. Agrár. 2011, 31, 1299. [Google Scholar] [CrossRef]
- Ben-Issa, R.; Gomez, L.; Gautier, H. Companion Plants for Aphid Pest Management. Insects 2017, 8, 112. [Google Scholar] [CrossRef]
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator Habitat Enhancement: Benefits to Other Ecosystem Services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Nelson, E.H.; Hogg, B.N.; Mills, N.J.; Daane, K.M. Syrphid Flies Suppress Lettuce Aphids. BioControl 2012, 57, 819–826. [Google Scholar] [CrossRef]
- Bugg, R.L.; Colfer, R.G.; Chaney, W.E.; Smith, H.A.; Cannon, J. Flower Flies (Syrphidae) and Other Biological Control Agents for Aphids in Vegetable Crops; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2008; ISBN 978-1-60107-529-1. [Google Scholar]
- Smith, H.A.; Chaney, W.E. A Survey of Syrphid Predators of Nasonovia Ribisnigri in Organic Lettuce on the Central Coast of California. J. Econ. Entomol. 2007, 100, 39–48. [Google Scholar] [CrossRef]
- Fonseca, M.M.; Lima, E.; Lemos, F.; Venzon, M.; Janssen, A. Non-Crop Plant to Attract and Conserve an Aphid Predator (Coleoptera: Coccinellidae) in Tomato. Biol. Control 2017, 115, 129–134. [Google Scholar] [CrossRef]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing Arthropod-Mediated Ecosystem Services in Agricultural Landscapes: The Role of Native Plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Jimé, E.; Carmona, N.; Daza, M.C.; Escobar, S.; Vi´, V.; Galindo, V.; et al. Does Plant Diversity Benefit Agroecosystems? A Synthetic Review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Adeux, G.; Vieren, E.; Carlesi, S.; Bàrberi, P.; Munier-Jolain, N.; Cordeau, S. Mitigating Crop Yield Losses through Weed Diversity. Nat. Sustain. 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Bàrberi, P.; Bocci, G.; Carlesi, S.; Armengot, L.; Blanco-Moreno, J.M.; Sans, F.X. Linking Species Traits to Agroecosystem Services: A Functional Analysis of Weed Communities. Weed Res. 2018, 58, 76–88. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Datta, A. The Critical Period for Weed Control: Revisiting Data Analysis. Weed Sci. 2015, 63, 188–202. [Google Scholar] [CrossRef]
- Nascimento, l.R.; Barreto, H.G.; Chaves, P.P.N.; Erasmo, E.A.L.; Momenté, V.G. Effects of the Different Periods of Weed Interference on Lettuce in Protected Cultivation. Rev. Bras. Tecnol. Apl. Nas Ciênc. Agrár. 2013, 6, 75–82. [Google Scholar] [CrossRef]
- Parry, S.; Shrestha, A. Effects of Weed-Free Periods on Organic Romaine Lettuce Production. J. Crop Improv. 2018, 32, 124–139. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD Value and Grain Yield Can Be Affected by Cultivar, Environment and Soil Nitrogen Content in Wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Le Bourgeois, T.; Merlier, H. Adventrop: Les Adventices d’Afrique Soudano-Sahélienne. In Adventrop: Les Adventices d’Afrique Soudano-Sahélienne; CIRAD-CA: Montpellier, France, 1995; pp. 13–20. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2021. Available online: http://florianhartig.github.io/DHARMa/ (accessed on 17 May 2022).
- Lenth, V.R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.6.2-1; 2021; Available online: https://github.com/rvlenth/emmeans (accessed on 17 May 2022).
- Ritz, C.; Baty, F.; Streibig, J.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Borrelli, K.; Koenig, R.T.; Jaeckel, B.M.; Miles, C.A. Yield of Leafy Greens in High TunnelWinter Production in the NorthwestUnited States. Hortscience 2013, 48, 183–188. [Google Scholar] [CrossRef]
- Bocci, G. TR8: An R Package for Easily Retrieving Plant Species Traits. Methods Ecol. Evol. 2015, 6, 347–350. [Google Scholar] [CrossRef]
- Klotz, S.; Kühn, I.; Durka, W.; Briemle, G. BIOLFLOR: Eine Datenbank Mit Biologisch-Ökologischen Merkmalen Zur Flora von Deutschland; Bundesamt für Naturschutz: Bonn, Germanym, 2002; Volume 38. [Google Scholar]
- Julve, P.B. Index Botanique, Écologique et Chorologique de La Flore de France, Version 2015; Programme Catminat: 1998; Available online: http://perso.wanadoo.fr/philippe.julve/catminat.htm (accessed on 17 May 2022).
- Fitter, A.H.; Peat, H.J. The Ecological Flora Database. J. Ecol. 1994, 82, 415. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A Database of Life-History Traits of the Northwest European Flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Evans, S.P.; Blankenship, E.E.; Van Acker, R.C.; Lindquist, J.L. Critical Period for Weed Control: The Concept and Data Analysis. Weed Sci. 2002, 50, 773–786. [Google Scholar] [CrossRef]
- Da Silva, A.F.T.; Avelino, R.C.; Da Silva Brito, L.P.; Dos Anjos, J.C.R.; Da Silva Júnior, J.V.; Beckmann-Cavalcante, M.Z. Growth and Yield of Lettuce Cultivars under Organic Fertilization and Different Environments. Comun. Sci. 2017, 8, 265–274. [Google Scholar] [CrossRef][Green Version]
- de Resende, G.M.; Alvarenga, M.A.R.; Yuri, J.E.; Souza, R.J. de Yield and Postharvest Quality of Winter Growing Crisphead Lettuce as Affected by Doses of Nitrogen and Molybdenum. Hortic. Bras. 2010, 28, 441–445. [Google Scholar] [CrossRef]
- Casadei, E.; Bacha, A.L.; Rodrigues, J.S.; Santos, R.T.S.; Alves, P.L.C.A.; Filho, A.B.C. Redroot Pigweed Interference with Lettuce Crop. Planta Daninha 2020, 38, 1–8. [Google Scholar] [CrossRef]
- Galon, L.; Forte, C.T.; Giacomini, J.P.; Reichert, F.W.; Scariot, M.A.; David, F.A.; Perin, G.F. Habilidade Competitiva de Alface Com Azevém. Planta Daninha 2016, 34, 239–248. [Google Scholar] [CrossRef]
- Bretagnolle, V.; Gaba, S. Weeds for Bees? A Review. Agron. Sustain. Dev. 2015, 35, 891–909. [Google Scholar] [CrossRef]
- Eraud, C.; Cadet, E.; Powolny, T.; Gaba, S.; Bretagnolle, F.; Bretagnolle, V. Weed Seeds, Not Grain, Contribute to the Diet of Wintering Skylarks in Arable Farmlands of Western France. Eur. J. Wildl. Res. 2015, 61, 151–161. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The Role of Weeds in Supporting Biological Diversity within Crop Fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The Biology of Canadian Weeds. 130. Amaranthus Retroflexus L., A. Powellii S. Watson and A. Hybridus L. Can. J. Plant Sci. 2004, 84, 631–668. [Google Scholar] [CrossRef]
- Ögür, E.; Ünlü, L.; Karaca, M. Chenopodium Album L.: A New Host Plant of Tuta Absoluta Povolny (Lep.: Gelechiidae). Türkiye Entomoloji Bül. 2014, 4, 61–65. [Google Scholar] [CrossRef][Green Version]
- Fried, G.; Kazakou, E.; Gaba, S. Trajectories of Weed Communities Explained by Traits Associated with Species’ Response to Management Practices. Agric. Ecosyst. Environ. 2012, 158, 147–155. [Google Scholar] [CrossRef]
- Leoni, F.; Lazzaro, M.; Carlesi, S.; Moonen, A.-C. Legume Ecotypes and Commercial Cultivars Differ in Performance and Potential Suitability for Use as Permanent Living Mulch in Mediterranean Vegetable Systems. Agronomy 2020, 10, 1836. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.C. Landscape Complexity and Field Margin Vegetation Diversity Enhance Natural Enemies and Reduce Herbivory by Lepidoptera Pests on Tomato Crop. BioControl 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.-C. Augmenting Flower Trait Diversity in Wildflower Strips to Optimise the Conservation of Arthropod Functional Groups for Multiple Agroecosystem Services. J. Insect Conserv. 2014, 18, 713–728. [Google Scholar] [CrossRef]



| Weeding Regime | Marketable Lettuce Mean Fresh Biomass (g/m2) |
|---|---|
| Weedy control | 29.99 ± 7.19 a |
| Weedy 0–40 DAT | 155.20 ± 22.89 ab |
| Weedy 0–30 DAT | 143.14 ± 15.31 ab |
| Weedy 0–20 DAT | 262.26 ± 20.88 b |
| Weedy 0–10 DAT | 218.15 ± 20.94 b |
| Weed–free control | 245.84 ± 26.08 b |
| Weedy control | 29.99 ± 7.19 a |
| Weed–free 0–10 DAT | 171.49 ± 17.54 ab |
| Weed–free 0–20 DAT | 267.40 ± 19.20 b |
| Weed–free 0–30 DAT | 227.22 ± 20.65 b |
| Weed–free 0–40 DAT | 278.12 ± 23.06 b |
| Weed-free control | 245.84 ± 26.08 b |
| Weeding Regime | Lettuce Chlorophyll Content (SPAD Values) | Lettuce Circumference (cm) |
|---|---|---|
| Weed-free control | 29.47 ± 0.48 b | 59.85 ± 1.44 c |
| Weed-free 0–10 DAT | 29.10± 0.55 b | 53.83 ± 1.19 b |
| Weed-free 0–20 DAT | 30.12 ± 0.56 b | 55.16 ± 1.33 bc |
| Weed-free 0–30 DAT | 28.91 ± 0.68 b | 57.69 ± 1.47 bc |
| Weed-free 0–40 DAT | 28.95 ± 0.63 b | 57.92 ± 1.50 bc |
| Weedy control | 26.19 ± 0.44 a | 46.36 ± 1.28 a |
| Weeding Regime | Lettuce Chlorophyll Content (SPAD Values) | Lettuce Circumference (cm) | |||
|---|---|---|---|---|---|
| 2019 | 2020 | 25 DAT | 35 DAT | 45 DAT | |
| Weed-free control | 31.17 ± 0.74 b | 27.73 ± 0.49 bc | 53.08 ± 2.6 n.s. | 60.80 ± 2.16 c | 65.86 ± 2.18 c |
| Weedy 0–10 DAT | 31.48 ± 0.96 b | 27.16 ± 0.38 bc | 48.63 ± 2.68 n.s. | 58.10 ± 2.62 c | 61.57 ± 2.20 bc |
| Weedy 0–20 DAT | 30.89 ± 0.78 b | 28.92 ± 0.63 c | 52.38 ± 2.29 n.s. | 56.08 ± 2.56 bc | 62.43 ± 2.50 bc |
| Weedy 0–30 DAT | 31.93 ± 1.04 b | 25.71 ± 0.62 ab | 46.68 ± 2.75 n.s. | 45.14 ± 2.15 a | 53.68 ± 2.22 ab |
| Weedy 0–40 DAT | 29.66 ± 1.19 b | 24.34 ± 0.52 a | 50.63± 2.14 n.s. | 53.47 ± 2.58 abc | 52.10 ± 2.15 a |
| Weedy control | 26.82 ± 0.72 a | 25.57 ± 0.52 ab | 46.97 ± 2.23 n.s. | 46.8 ± 1.97 ab | 45.30 ± 2.48 a |
| Lettuce Stress/Growth Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Dry Total Biomass | Fresh Marketable Biomass | Chlorophyll Content (SPAD Values) | Plant Height | Head Circumference | |||
| Weeds | Biomass | Grasses | −0.52 *** | −0.37 ** | −0.14 | −0.03 | −0.39 ** |
| Cyperus spp. | 0.12 | −0.01 | −0.18 | 0.27 * | −0.05 | ||
| Broadleaved | −0.24 | −0.45 ** | −0.12 | 0.30 * | −0.56 *** | ||
| Total | −0.46 ** | −0.55 *** | −0.19 | 0.24 | −0.64 *** | ||
| Cover | Grasses | −0.52 *** | −0.33 * | −0.19 | −0.06 | −0.37 ** | |
| Cyperus spp. | 0.15 | 0.09 | −0.21 | 0.25 | 0.03 | ||
| Broadleaved | −0.27 * | −0.47 ** | −0.10 | 0.14 | −0.57 *** | ||
| Total | −0.49 *** | −0.50 *** | −0.25 | 0.12 | −0.61 *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virili, A.; Moonen, A.-C. Minimal Necessary Weed Control Does Not Increase Weed-Mediated Biological Pest Control in Romaine Lettuce (Lactuca sativa L., var. Romana). Horticulturae 2022, 8, 787. https://doi.org/10.3390/horticulturae8090787
Virili A, Moonen A-C. Minimal Necessary Weed Control Does Not Increase Weed-Mediated Biological Pest Control in Romaine Lettuce (Lactuca sativa L., var. Romana). Horticulturae. 2022; 8(9):787. https://doi.org/10.3390/horticulturae8090787
Chicago/Turabian StyleVirili, Alessandra, and Anna-Camilla Moonen. 2022. "Minimal Necessary Weed Control Does Not Increase Weed-Mediated Biological Pest Control in Romaine Lettuce (Lactuca sativa L., var. Romana)" Horticulturae 8, no. 9: 787. https://doi.org/10.3390/horticulturae8090787
APA StyleVirili, A., & Moonen, A.-C. (2022). Minimal Necessary Weed Control Does Not Increase Weed-Mediated Biological Pest Control in Romaine Lettuce (Lactuca sativa L., var. Romana). Horticulturae, 8(9), 787. https://doi.org/10.3390/horticulturae8090787








