Effects of Exogenous Salicylic Acid (SA), 6-Benzylaminopurine (6-BA), or Abscisic Acid (ABA) on the Physiology of Rosa hybrida ‘Carolla’ under High-Temperature Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Determination of Physiological and Biochemical Traits
2.2.1. Determination of Chlorophyll (Chl) Content
2.2.2. Determination of Photosynthetic Indices
2.2.3. Determination of Relative Electrical Conductivity (REC) and Malondialdehyde (MDA) Content
2.2.4. Determination of Enzymatic Activity
2.2.5. Determination of Osmotic Substance Contents
2.2.6. Statistical Analysis
3. Results
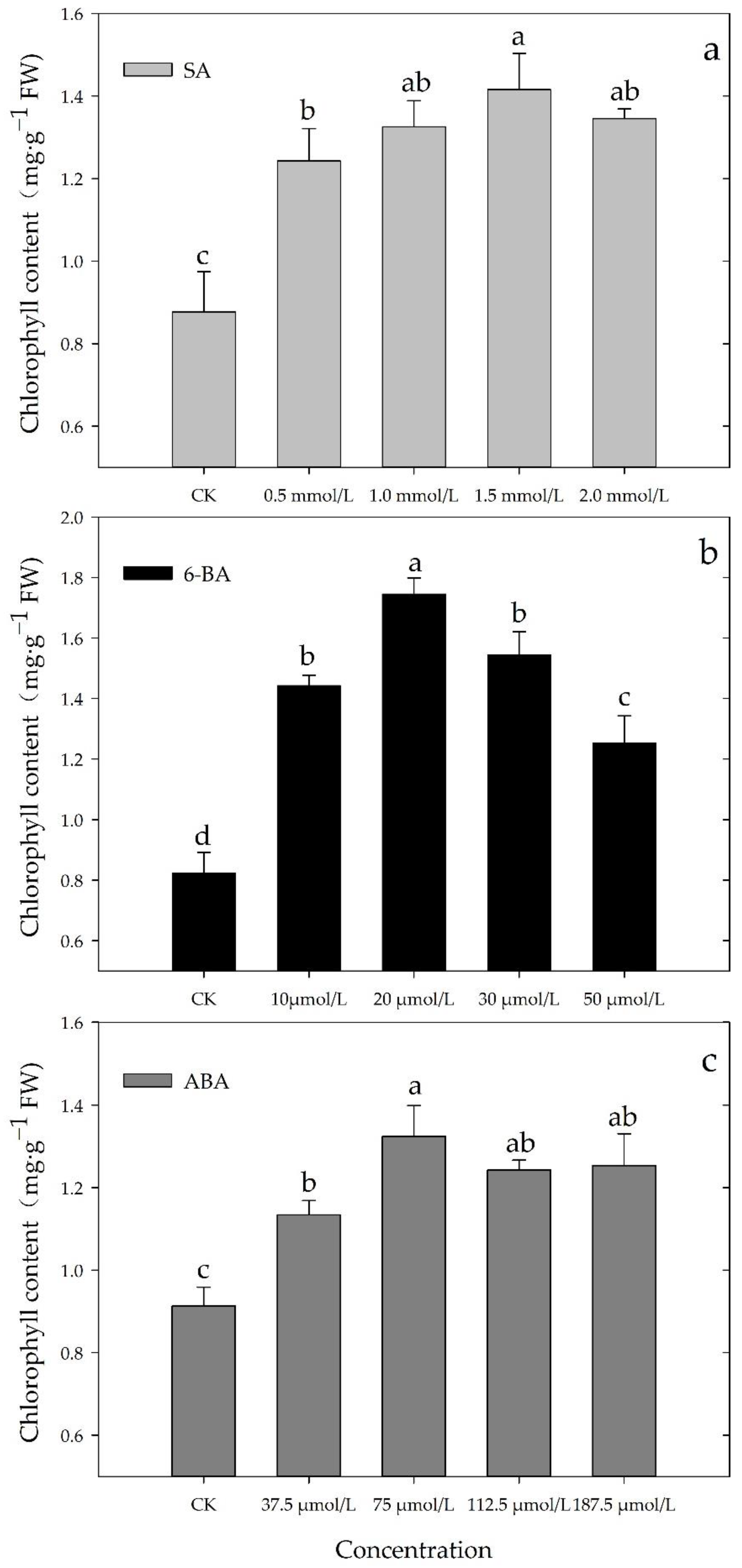
3.1. Effects of Different Concentrations of SA, 6-BA, or ABA on Chl Content of Rosa hybrida ‘Carolla’ under High-Temperatures Stress
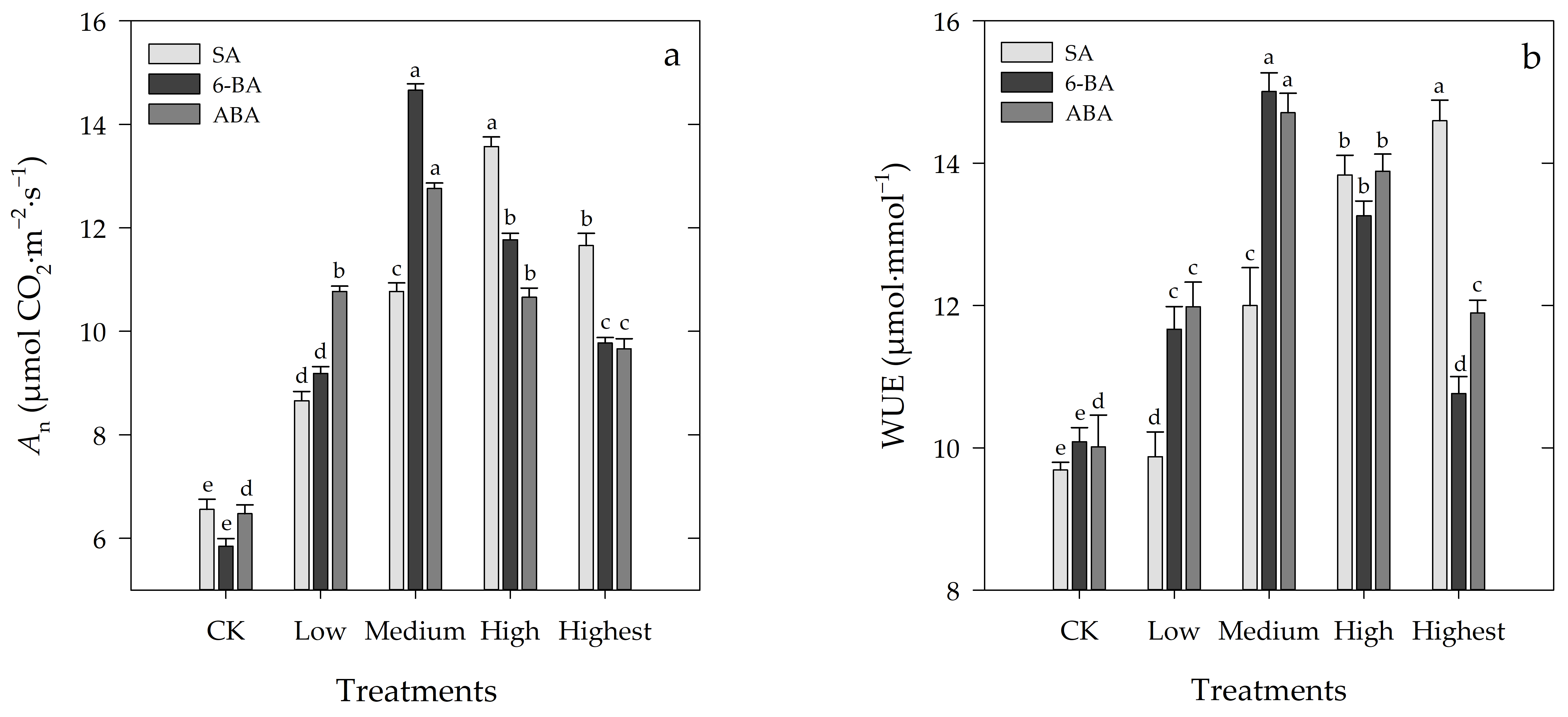
3.2. Effects of Different Concentrations of SA, 6-BA, or ABA on An and WUE of Rosa hybrida ‘Carolla’ under High-Temperature Stress
3.3. Effects of Different Concentrations of SA, 6-BA, or ABA on E and Gs of Rosa hybrida ‘Carolla’ under High-Temperature Stress
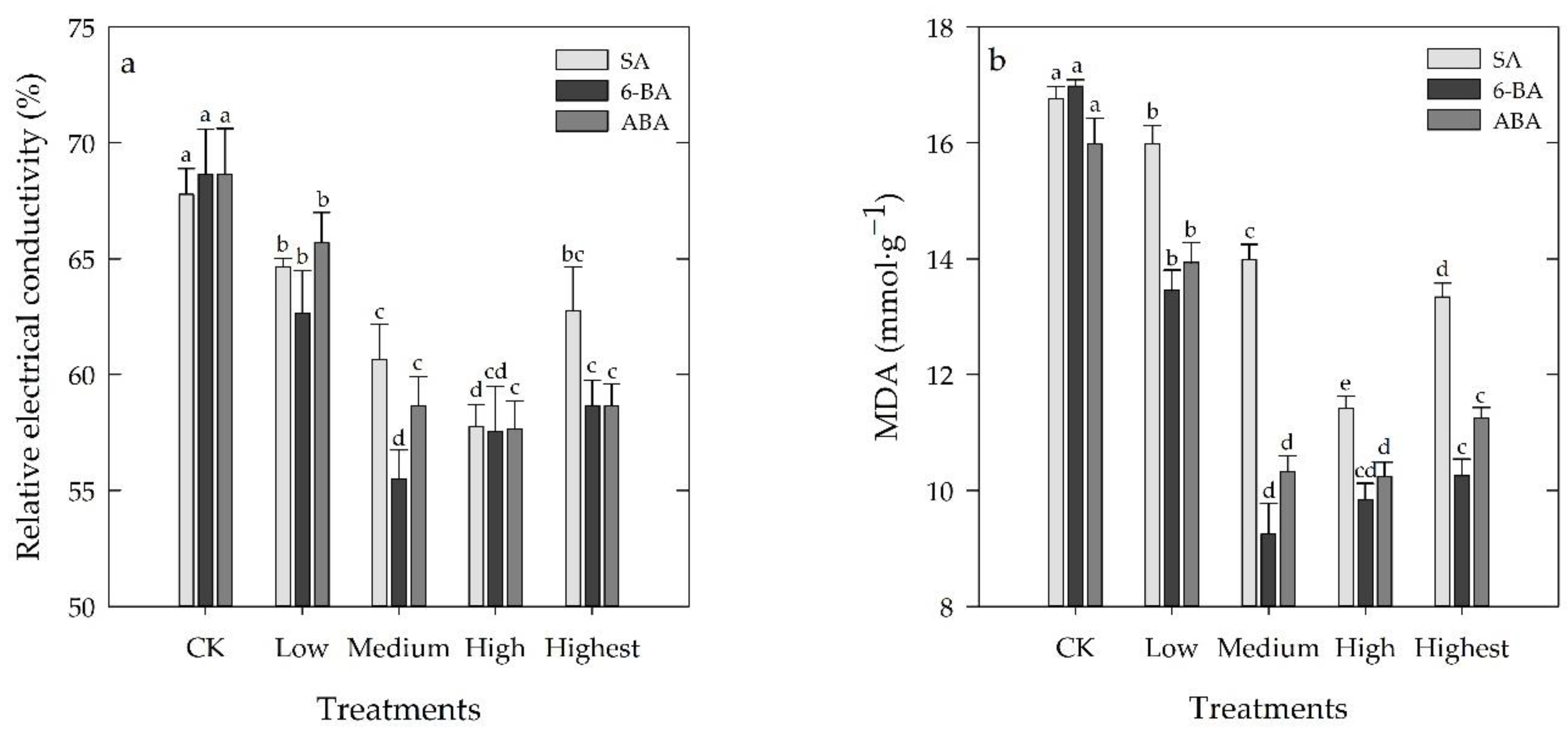
3.4. Effects of Different Concentrations of SA, 6-BA, or ABA on MDA and REC of Rosa hybrida ‘Carolla’ under High-Temperature Stress
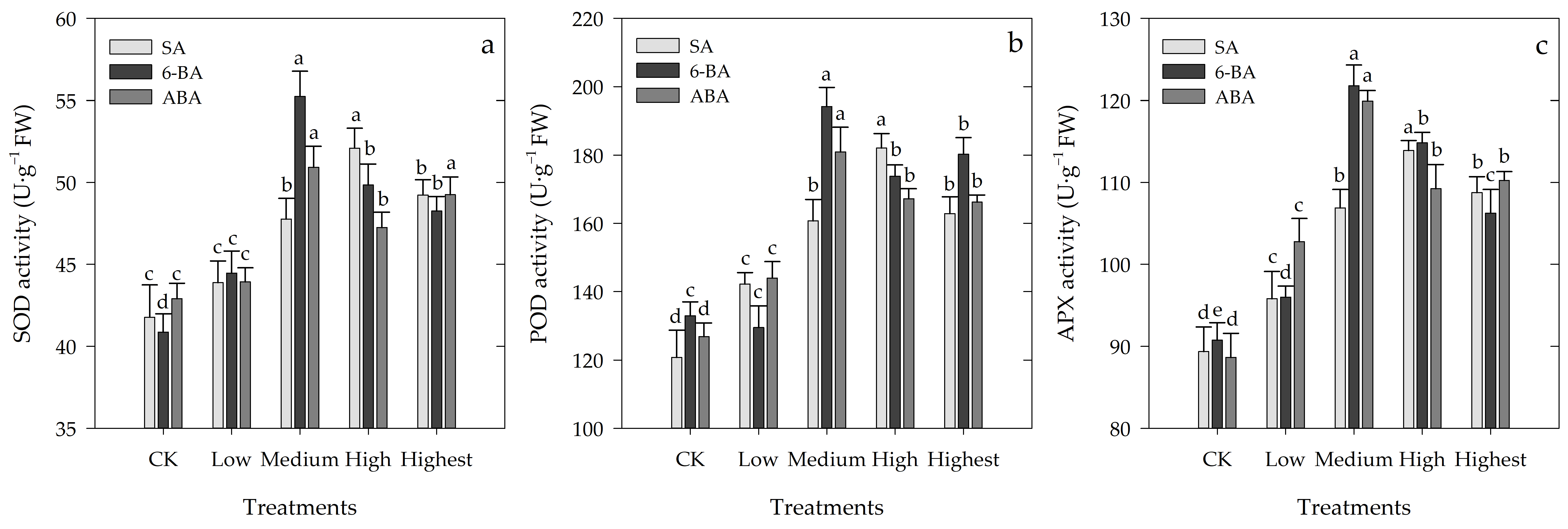
3.5. Effects of Different Concentrations of SA, 6-BA, or ABA on Antioxidant Enzyme Activity of Rosa hybrida ‘Carolla’ under High-Temperature Stress
3.6. Effects of Different Concentrations of SA, 6-BA, or ABA on Osmoregulatory Substances of Rosa hybrida ‘Carolla’ under High-Temperature Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, C.H.; Hu, Y.H.; Qin, J.; Wang, Y.Q.; Zhang, M.L. Research in Effect of High Temperature on Physiological Indexes of Varieties in China Rose. Seed 2008, 6, 31–34+38. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Hurkman, W.J.; Vensel, W.H.; Tanaka, C.K.; Whitehand, L.; Altenbach, S.B. Effect of high temperature on albumin and globulin accumulation in the endosperm proteome of the developing wheat grain. J. Cereal Sci. 2009, 49, 12–23. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Effects of high night temperature and spikelet position on yield-related parameters of rice (Oryza sativa L.) plants. Eur. J. Agron. 2010, 33, 117–123. [Google Scholar] [CrossRef]
- Tan, W.; Meng, Q.W.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004, 55, 485–495. [Google Scholar] [CrossRef]
- Yan, K.; Chen, P.; Shao, H.; Zhang, L.; Xu, G. Effects of Short-Term High Temperature on Photosynthesis and Photosystem II Performance in Sorghum. J. Agron. Crop Sci. 2011, 197, 400–408. [Google Scholar] [CrossRef]
- Sheng, X.; Li, J.; Zhang, X.; Hong, W.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Zhou, L.; Yang, L.; Yao, J.; Huang, Q. Research Progress on High Temperature Stress of Rosa hybrida. North. Hortic. 2021, 10, 124–131. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production Since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Liu, Z.; Xie, L.; Chen, Y.; Wang, H. Research for Response to High Temperature on Part of Morphological and Physiological Indexes in Rose. Heilongjiang Agric. Sci. 2013, 8, 66–72. [Google Scholar]
- Chmelnitsky, I.; Colauzzi, M.; Algom, R.; Zieslin, N. Effects of temperature on phyllody expression and cytokinin content in floral organs of rose flowers. Plant Growth Regul. 2001, 35, 207–214. [Google Scholar] [CrossRef]
- Ji, H.S.; Wan, S.K. Growth, Floral Morphology, and Phytohormone Levels of Flowering Shoots with Bent Peduncle in Greenhouse-grown Cut Rose ‘Beast’. Korean J. Hortic. Sci. Technol. 2013, 31, 714–719. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, C.; Qin, J.; Mo, J. Research for Effects of High Temperature on Several Morphological, Physiological Indexes in China Rose. Seed 2008, 27, 26–29. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, F.; Tan, Y.; Zhang, M.; Xing, W.; Jin, X. Effects of High Temperature Stress on the Physiological Characteristics and Chlorophyll Fluorescence Parameters of Chinese Rose. J. Henan Agric. Sci. 2019, 48, 108–115. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Páel, M. Salicylic acid-mediated abiotic stress tolerance. In Salicylic Acid; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, H.H. Role of salicylic acid in plant abiotic stress. Z. Fur Nat. C 2008, 63, 313–320. [Google Scholar] [CrossRef]
- Horvath, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Jicheng, A.Z. Thermotolerance Related to Antioxidation Induced by SA and Heat Acclimation in Grape Seedlings. Acta Hortic. Sin. 2003, 30, 452–454. [Google Scholar]
- Pan, Q.; Zhan, J.; Liu, H.; Zhang, J.; Chen, J.; Wen, P.; Huang, W. Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Ence 2006, 171, 226–233. [Google Scholar] [CrossRef]
- Vanková, R. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and ABA responses, and ABA biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul. 1997, 23, 79–103. [Google Scholar] [CrossRef]
- Ding, W.; Song, L.; Wang, X.; Bi, Y. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biol. Plant. 2010, 54, 607–613. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 30. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Z.; Zhou, Z.L.; Liu, H.T.; Wang, Y.; Lai, Y.C.; Wang, Y.J.; Mao, F.W.; Zhang, L.P.; Wang, H.C.; Zhang, G.Y. Effects of exogenous salicylic acid on physiological indexes in miniature rose under high temperature stress. J. South. Agric. 2018, 49, 2028–2033. [Google Scholar]
- Kumar, S.; Kaushal, N.; Nayyar, H.; Gaur, P. Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol. Plant. 2012, 34, 1–8. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gray, D.J.; Jiang, L.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Timothy, J.B. Effects of exogenous abscisic acid on fruit quality, antioxidant capacities, and phytochemical contents of southern high bush blueberries. Food Chem. 2012, 132, 1375–1381. [Google Scholar] [CrossRef]
- Huang, X.; Chen, M.H.; Yang, L.T.; Li, Y.R.; Wu, J.M. Effects of Exogenous Abscisic Acid on Cell Membrane and Endogenous Hormone Contents in Leaves of Sugarcane Seedlings under Cold Stress. Sugar Tech. 2015, 17, 59–64. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Z.; Kim, W.S. Effect of drought stress on shoot growth and physiological response in the cut rose ‘charming black’ at different developmental stages. Hortic. Environ. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Shi, L.Y.; Wang, Z.; Kim, W.S. The role of slab water content during supplemental lighting on shoot growth and physiological response of cut rose “Charming Black’. Hortic. Environ. Biotechnol. 2019, 60, 321–328. [Google Scholar] [CrossRef]
- Shi, L.Y.; Wan, S.K. Effect of drought stress during supplemental lighting on diurnal photosynthesis of cut rose ‘Charming Black’. Hortic. Environ. Biotechnol. 2015, 56, 582–587. [Google Scholar] [CrossRef]
- Min, D.; Dong, L.; Shu, P.; Cui, X.; Zhang, X.; Li, F. The application of carbon dioxide and 1-methylcyclopropene to maintain fruit quality of ‘Niuxin’ persimmon during storage-ScienceDirect. Sci. Hortic. 2018, 229, 201–206. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Xu, H. Influence of arbuscular mycorrhiza on lipid peroxidation andantioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 2010, 20, 325–332. [Google Scholar] [CrossRef]
- Erinle, K.O.; Jiang, Z.; Ma, B.; Li, J.; Chen, Y.; Ur-Rehman, K.; Shahla, A.; Zhang, Y. Exogenous calcium induces tolerance to atrazine stress in Pennisetum seedlings and promotes photosynthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicol. Environ. Saf. 2016, 132, 403–412. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Fan, H.M.; Li, T.; Sun, X.; Sun, X.Z.; Zheng, C.S. Effects of humic acid derived from sediments on the postharvest vase life extension in cut chrysanthemum flowers. Postharvest Biol. Technol. 2015, 101, 82–87. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, X.; Wang, W.; Li, K.; Sun, Y. Effects of salt stress on physiological characteristics of two strains of Malus micromalus Mak. with different salt tolerance. J. Fruit Sci. 2021, 38, 335–343. [Google Scholar] [CrossRef]
- Wang, L.J.; Huang, W.D.; Liu, Y.P.; Zhan, J.C. Changes in Salicylic and Abscisic Acid Contents during Heat Treatment and Their Effect on Thermotolerance of Grape Plants. Russ. J. Plant Physiol. 2005, 52, 516–520. [Google Scholar] [CrossRef]
- Papacek, M.; Christmann, A.; Grill, E. Interaction network of ABA receptors in grey poplar. Plant J. Cell Mol. Biol. 2017, 92, 199–210. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic Acid Influences Net Photosynthetic Rate, Carboxylation Efficiency, Nitrate Reductase Activity, and Seed Yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Shi, Q.H.; Bao, Z.Y.; Zhu, Z.J. Effects of Different Treatments of Salicylic Acid on Heat Tolerance, Chlorophyll Fluorescence, and Antioxidant Enzyme Activity in Seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lin, Z.W.; Wu, S.B.; Zhao, Y.J.; Tang, Y.W. Enhancement of Chloroplast Development by 6-Benzylaminopurine in Etiolated Wheat Leaves. Physiol. Mol. Biol. Plants 1982, 8, 45–52. [Google Scholar]
- Sawada, H.; Shim, I.S.; Usui, K.; Kobayashi, K.; Fujihara, S. Adaptive mechanism of Echinochloa crus-galli Beauv. var. formosensis Ohwi under salt stress: Effect of salicylic acid on salt sensitivity. Plant Sci. 2008, 174, 583–589. [Google Scholar] [CrossRef]
- An, Y.; Zhou, P.; Liang, J. Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci. 2014, 65, 274–286. [Google Scholar] [CrossRef]
- Aftab, T.; Masroor, M.; Khan, A.; Idrees, M.; Naeem, M.; Moinuddin. Salicylic acid acts as potent enhancer of growth, photosynthesis and artemisinin production in Artemisia annua L. J. Crop Sci. Biotechnol. 2010, 13, 183–188. [Google Scholar] [CrossRef]
- Salam, A.S.; Cevahir-Oz, G.L.; Gren-Saglam, M. Effect of salicylic acid on pigment, protein content and peroxidase activity in excised sunflower cotyledons. Pak. J. Bot. 2009, 41, 2297–2303. [Google Scholar] [CrossRef]
- Makino, A.; Nakano, H.; Mae, T. Effects of growth temperature on the responses of ribulose-1, 5-bisphosphate carboxylase, electron transport components, and sucrose synthesis enzymes of leaf nitrogen in rice, and their relationships to photosynthesis. Plant Physiol. 1994, 105, 1231–1238. [Google Scholar] [CrossRef]
- Sawan, Z.M.; Mohamed, A.A.; Sakr, R.A.; Tarrad, A.M. Effect of kinetin concentration and methods of application on seed germination, yield components, yield and fiber properties of the Egyptian cotton (Gossypium barbadense). Environ. Exp. Bot. 2000, 44, 59–68. [Google Scholar] [CrossRef]
- Kamalani, A.; Hewage, H.; Yang, J.F.; Di, W.; Ge, F.H.; Guang, F.Y.; Zhu, J.K. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef]
- Long, C.Y.; Hong-Hui, G.U.; Wang, Z.X.; Jiang, X.; Yang, C.Q.; Qin, Y.G.; University, S.A.; University, S.A. Effects of Exogenous Abscisic Acid on the Photosynthesis and Chlorophy II Fluorescence Parameters of Spinach under High Temperature Stress. J. Sichuan Agric. Univ. 2017, 1, 24–30. [Google Scholar] [CrossRef]
- Li, W.T.; Ning, P.; Wang, F.; Cheng, X.M.; Huang, X.X. Effects of exogenous abscisic acid (ABA) on growth and physiological characteristics of Machilus yunnanensis seedlings under drought stress. Ying Yong Sheng Tai Xue 2020, 31, 1543–1550. [Google Scholar]
- Fan, X.R.; Shen, Q.R. Effects of ABA and IAA on the Behavior of Stomata of Rice Crop Cultivated in Aerobic Soil Condition. Sci. Agric. Sin. 2003, 36, 1450–1455. [Google Scholar]
- Atkinson, C.J.; Davies, W.J.; Mansfield, T.A. Changes in Stomatal Conductance in Intact Ageing Wheat Leaves in Response to Abscisic Acid. J. Exp. Bot. 1989, 40, 1021–1028. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Kusumi, K.; Yaeno, T.; Kojo, K.; Hirayama, M.; Hirokawa, D.; Yara, A.; Iba, K. The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiol. Plant. 2010, 128, 651–661. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Shao, H.; Chu, L.; Shao, M.; Cheruth. Higher plant antioxidants and redox signaling under environmental stresses. Comptes Rendus Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Liu, K.G.; Gong, F.R.; Song, Y.P.; Zhang, L.L. Effects of exogenous 6-BA on chlorophyll fluorescence parameters and antioxidant enzyme activities of sweet pepper seedlings under high temperature stress. Acta Agric. Shanghai 2020, 36, 19–25. [Google Scholar]
- Larkindale, J. Protection against Heat Stress-Induced Oxidative Damage in Arabidopsis Involves Calcium, Abscisic Acid, Ethylene, and Salicylic Acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Y.; Cao, W.; Huai, M.; Xu, B.; Huang, B. Effects of Salicylic Acid on Heat Tolerance Associated with Antioxidant Metabolism in Kentucky Bluegrass. Crop Sci. 2005, 45, 988–995. [Google Scholar] [CrossRef]
- Dong, C.; Liang, W.; Cheng, H.; Yu, D.; Lv, D.; Sun, Y.; Miao, C. Plant Lipoxygenases: Advance of the Function in Stress Response. Chin. Agric. Sci. Bull. 2020, 36, 102–107. [Google Scholar]
- Hou, Y.; Meng, K.; Ye, H.; Ban, Q.; Wang, B.; Suo, J.; Lv, J.; Rao, J. The Persimmon 9-lipoxygenase Gene DkLOX3 Plays Positive Roles in Both Promoting Senescence and Enhancing Tolerance to Abiotic Stress. Front. Plant Sci. 2015, 6, 1073. [Google Scholar] [CrossRef]
- Leon-Morcillo, R.J.; Angel, J.; Martin, R.; Vierheilig, H.; Ocampo, J.A.; Garcia-Garrido, J.M. Late activation of the 9-oxylipin pathway during arbuscular mycorrhiza formation in tomato and its regulation by jasmonate signalling. J. Exp. Bot. 2012, 63, 3545–3558. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Misra, N.; Misra, R. Salicylic Acid Changes Plant Growth Parameters and Proline Metabolism in Rauwolfia serpentina Leaves Grown under Salinity Stress. Agric. Environ. Sci. 2012, 12, 1601–1609. [Google Scholar]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Khan, M.; Iqbal, R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav. 2013, 8, e26374. [Google Scholar] [CrossRef]
- Li, K.-N.; Wang, K.-C.; Li, L.; Li, Y.-Q.; Duan, Y.-J. Effects of Ca2+ and SA on physiological and photosynthesis of Platycodon grandiflorum under high temperature stress. China J. Chin. Mater. Med. 2015, 40, 1908–1913. [Google Scholar] [CrossRef]
- Yang, H.; Yan, S.; Chen, H.; Yang, C.; Yang, F.; Liu, Z. Effect of Exogenous Methyl Jasmonate, Calcium and Salicylic Acid on the Heat Tolerance in Phalaenopsis Seedlings Under High Temperature Stress. Chin. Agric. Sci. Bull. 2011, 27, 150–157. [Google Scholar]
- Wu, S.; Jin, X.L.; Zhang, M.H.; Sun, L.X.; Chen, R. Effects of Exogenous Abscisic Acid on Heat Tolerance inTree Peony Seedlings under High Temperature Stress. In Advances in Ornamental Horticulture of China; China Forestry Publishing House: Beijing, China, 2018; pp. 354–360. [Google Scholar]
- Mimouni, H.; Wasti, S.; Manaa, A.; Gharbi, E.; Chalh, A.; Vandoorne, B.; Lutts, S.; Ahmed, H.B. Does Salicylic Acid (SA) Improve Tolerance to Salt Stress in Plants? A Study of SA Effects On Tomato Plant Growth, Water Dynamics, Photosynthesis, and Biochemical Parameters. Omics A J. Integr. Biol. 2016, 20, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Tasgin, E.; Atici, O.; Nalbantoglu, B. Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul. 2003, 41, 231–236. [Google Scholar] [CrossRef]
- Raskin, I. Role of Salicylic Acid in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Nazari, F.; Maleki, M.; Rasouli, M. Effect of Salicylic Acid on Changes in Superoxide Dismutase Enzyme Activity, Protein, Proline, and Some Photosynthetic Pigments in Grape (Vitis vinifera L.) Bidane Ghermez and Bidane Sefid Cultivars at Two Growth Stages. Erwerbs-Obstbau 2022, 1–9. [Google Scholar] [CrossRef]
- Demiralay, M.; Salam, A.; Kadiolu, A. Salicylic acid delays leaf rolling by inducing antioxidant enzymes and modulating osmoprotectant content in Ctenanthe setosa under osmotic stress. Sci. Technol. Res. Counc. Turk. 2013, 37, 49–59. [Google Scholar] [CrossRef]
- Heuer, B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003, 165, 693–699. [Google Scholar] [CrossRef]
- Bourbouloux, A.; Raymond, P.; Delrot, S. Effects of salicylic acid on sugar and amino acid uptake. J. Exp. Bot. 1998, 49, 239–427. [Google Scholar] [CrossRef]
- Yuan, Z.; Cong, G.; Zhang, J. Effects of exogenous salicylic acid on polysaccharides production of Dendrobium officinale. S. Afr. J. Bot. 2014, 95, 78–84. [Google Scholar] [CrossRef]
- Luo, Y.; Su, Z.; Bi, T.; Cui, X.; Lan, Q. Salicylic acid improves chilling tolerance by affecting antioxidant enzymes and osmoregulators in sacha inchi (Plukenetia volubilis). Braz. J. Bot. 2014, 37, 357–363. [Google Scholar] [CrossRef]
- Rottmann, T.; Zierer, W.; Subert, C.; Sauer, N.; Stadler, R. STP10 encodes a high-affinity monosaccharide transporter and is induced under low-glucose conditions in pollen tubes of Arabidopsis. J. Exp. Bot. 2016, 67, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.D.; Zhang, P.; Li, W.Q. The Effects of 6-BA on the Fruit Development and Transportation of Carbon and Nitrogen Assimilates in Grape. Acta Hortic. Sin. 2002, 29, 303–306. [Google Scholar]
- Chakraborty, U.; Tongden, C. Evaluation of heat acclimation and salicylic acid treatments as potent inducers of thermotolerance in Cicer arietinum L. Curr. Sci. 2005, 89, 384–389. [Google Scholar]
- Naeem, M.; Traub, J.R.; Athar, H.; Loescher, W. Exogenous calcium mitigates heat stress effects in common bean: A coordinated impact of photoprotection of PSII, up-regulating antioxidants, and carbohydrate metabolism. Acta Physiol. Plant. 2020, 42. [Google Scholar] [CrossRef]
- Lehmann, J.; Atzorn, R.; Brückner, C.; Reinbothe, S.; Leopold, J.; Wasternack, C.; Parthier, B. Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta 1995, 197, 156–162. [Google Scholar] [CrossRef]
- Xin, Z.; Zhou, X.; Pilet, P. Level changes of jasmonic, abscisic, and indole-3yl-acetic acids in maize under desiccation stress. J. Plant Physiol. 1997, 151, 120–124. [Google Scholar] [CrossRef]
- Hao, J.; Yin, Y.; Fei, S.Z. Brassinosteroid signaling network: Implications on yield and stress tolerance. Plant Cell Rep. 2013, 32, 1017–1030. [Google Scholar] [CrossRef]
- Wu, X.X.; Zha, D.S.; Zhu, Z.W.; Xu, S. Effects of Exogenous 24-Epibrassinolide on Plant Growth and Antioxidant System in Eggplant Seedlings under High Temperature Stress. Plant Physiol. J. 2013, 49, 929–934. [Google Scholar] [CrossRef]
- Dat, J.F.; Lopez-Delgado, H.; Foyer, C.H.; Scott, I.M. Parallel Changes in H2O2 and Catalase during Thermotolerance Induced by Salicylic Acid or Heat Acclimation in Mustard Seedlings 1. Plant Physiol. 1998, 116, 1351–1357. [Google Scholar] [CrossRef] [Green Version]
- López-Delgado, H.; Mora-Herrera, M.E.; Zavaleta-Mancera, H.A.; Cadena-Hinojosa, M.; Scott, I.M. Salicylic acid enhances heat tolerance and potato virus X (PVX) elimination during thermotherapy of potato microplants. Am. J. Potato Res. 2004, 81, 171–176. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.H. Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul. 2006, 48, 137–144. [Google Scholar] [CrossRef]
- Liu, K.G.; Zhu, Y.L.; Hao, T.; Gong, F.R.; Song, Y.P. Effect of Foliar-spraying 6-BA on the Growth and Physiological and Biochemical Indexes of Sweet Pepper Seedlings under High Temperature Stress. Acta Bot. Boreali-Occident. Sin. 2014, 34, 2508–2514. [Google Scholar]
- Li, X.L.; Ji, L.L.; Hua, Z.R. Exogenous Abscisic Acid on Heat Resistance of Rhododendron lapponicum in Qinling Mountain. Guizhou Agric. Sci. 2018, 46, 33–36. [Google Scholar]


| Plant Name | Applied PGRs Concentration | Parameters Studied | Response * | Reference |
|---|---|---|---|---|
| Mustard (Sinapis alba L.) | 100 µM SA | H2O2 content and catalase activity | − | [93] |
| 45 °C | ||||
| Kentucky bluegrass (Poa pratensis L.) | 0.25 mmol/100 mL SA | O2− generating rate and H2O2 | − | [64] |
| 46 °C | SOD and CAT activity | + | ||
| Cicer arietinum L. | 100 µM SA | Catalase (CAT) activities | − | [87] |
| Peroxidase (POX), ascorbate peroxidase (APOX) | + | |||
| Winter wheat (Triticum aestivum cv.) | 0.01 mM SA | Ice nucleation activity | + | [77] |
| 10/5 and 5/3 °C | ||||
| Cucumber (Cucumis sativa L.) | 1 mM SA | Electrolyte leakage, H2O2, and thiobarbituric acid-reactive substances (TBARS) | − | [45] |
| 40 °C | Fv/Fm, the photosystem II electron transport (ΦPS II), SOD, CAT, GPX, APX, and GR activity | + | ||
| Solanum tuberosum L. | 10−5 M SA | Catalase activity | − | [94] |
| 42 °C | H2O2 content | + | ||
| Pea (Pisum sativun L.) | 150 µM SA | MDA content, leaf injury, and the synthesis of heat-shock proteins (HSP 70 and Hsp 17.6) | − | [22] |
| 45 °C | ||||
| Grapevine (Vitis vinifera L.) | 100 µmol/L SA | CAT activity | − | [95] |
| 38 °C | Heat killing time, POD, APX, SOD activity, H2O2, GR, AsA, and GSH | + | ||
| Platycodon grandiflorum | 1.5 mmol/L SA | Relative conductivity | − | [73] |
| 35/25 °C (day/night) | SOD, CAT activity, proline, soluble protein, chlorophyll, carotenoid, leaf photosynthesis, ASA, and GSH content | + | ||
| Phalaenopsis ‘Red Sky’ | 80 µmol/L SA | CAT, the relative electrical conductivity, and MDA content | − | [74] |
| 40 °C | SOD, POD activity, proline, and soluble protein | + | ||
| Miniature rose ‘Golden Coast’ | 2.0 mmol/L SA | The degradation rate of chlorophyll, MDA content, and relative conductivity | − | [27] |
| 36 °C | Free proline (Pro) content, superoxide dismutase (SOD) activity, peroxidase (POD) activity, ascorbate peroxidase (APX) activity | + | ||
| Sweet pepper (Capsicum annum L.) | 10 μmol/L 6-BA | O2− production rate, MDA content, and relative electric conductivity | − | [62] |
| 40 °C/30 °C (day/night) | SOD, POD, APX activity, chlorophyll content, and chlorophyll fluorescence parameters | + | ||
| Sweet pepper cultivar ‘P13201’ | 10 μmol/L 6-BA | O2− production rate, MDA content, and relative conductivity | − | [96] |
| 40 °C/30 °C (day/night) | SOD, POD, APX activity, non-photochemical fluorescence quenching (NPQ), the actual photochemical yield of PSII (yield), actual photochemical efficiency of PSII (ΦPSII) chlorophyll a, chlorophyll b, and total chlorophyll | + | ||
| Chickpea (Cicer arietinum L.) | 2.5 µM ABA | Electrolyte leakage, 2,3,5-triphenyl tetrazolium chloride (TTC), malondialdehyde, and hydrogen peroxide | − | [28] |
| 40/35 °C | Shoot length, survival rate, endogenous ABA, proline, glycine betaine, trehalose, and chlorophyll | + | ||
| Reed (Phragmites communis Trin.) | 10 µM ABA | H2O2 and MDA content | − | [25] |
| 45 °C | Superoxide dismutase, catalase, ascorbate peroxidase, and peroxidase | + | ||
| Sugarcane varieties GT 28 and YL 6 | 100 µM ABA | MDA and GA3 content | − | [31] |
| 0 °C | Proline, ABA, and the ratio of ABA/GA3 | + | ||
| Lucerne (alfalfa, Medicago sativa L.) | 0.1 mM ABA | Electrolyte leakage, stomatal conductance | − | [48] |
| 38 °C | Leaf water potential | + | ||
| Paeonia ostii ‘Fengdan’ | 40 mg/L ABA | Leakage rate of electrolyte and MDA content | − | [75] |
| 40 °C | SOD activity, soluble protein, chlorophyll, proline, and soluble sugar | + | ||
| Rhododendron lapponicum | 10 mg/L ABA | The degradation rate of chlorophyll | − | [97] |
| 37 °C/25 °C (day/night) | SOD, POD, and CAT activity | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Shen, Y.; Wang, H.; He, S.; Kim, W.S.; Shang, W.; Wang, Z.; Shi, L. Effects of Exogenous Salicylic Acid (SA), 6-Benzylaminopurine (6-BA), or Abscisic Acid (ABA) on the Physiology of Rosa hybrida ‘Carolla’ under High-Temperature Stress. Horticulturae 2022, 8, 851. https://doi.org/10.3390/horticulturae8090851
Wang K, Shen Y, Wang H, He S, Kim WS, Shang W, Wang Z, Shi L. Effects of Exogenous Salicylic Acid (SA), 6-Benzylaminopurine (6-BA), or Abscisic Acid (ABA) on the Physiology of Rosa hybrida ‘Carolla’ under High-Temperature Stress. Horticulturae. 2022; 8(9):851. https://doi.org/10.3390/horticulturae8090851
Chicago/Turabian StyleWang, Kaixuan, Yuxiao Shen, Han Wang, Songlin He, Wan Soon Kim, Wenqian Shang, Zheng Wang, and Liyun Shi. 2022. "Effects of Exogenous Salicylic Acid (SA), 6-Benzylaminopurine (6-BA), or Abscisic Acid (ABA) on the Physiology of Rosa hybrida ‘Carolla’ under High-Temperature Stress" Horticulturae 8, no. 9: 851. https://doi.org/10.3390/horticulturae8090851
APA StyleWang, K., Shen, Y., Wang, H., He, S., Kim, W. S., Shang, W., Wang, Z., & Shi, L. (2022). Effects of Exogenous Salicylic Acid (SA), 6-Benzylaminopurine (6-BA), or Abscisic Acid (ABA) on the Physiology of Rosa hybrida ‘Carolla’ under High-Temperature Stress. Horticulturae, 8(9), 851. https://doi.org/10.3390/horticulturae8090851








