Intraspecific and Interstage Similarities in Host-Plant Preference in the Diamondback Moth (Lepidoptera: Plutellidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Species and Plutella xylostella Strains Tested
2.2. Multiple-Choice Oviposition Preference Experiments
2.3. Abaxial vs. Adaxial Preference
2.4. Oviposition Preference Experiments with Sinigrin
2.5. Larval Preference Experiments
2.6. Statistical Analysis
3. Results
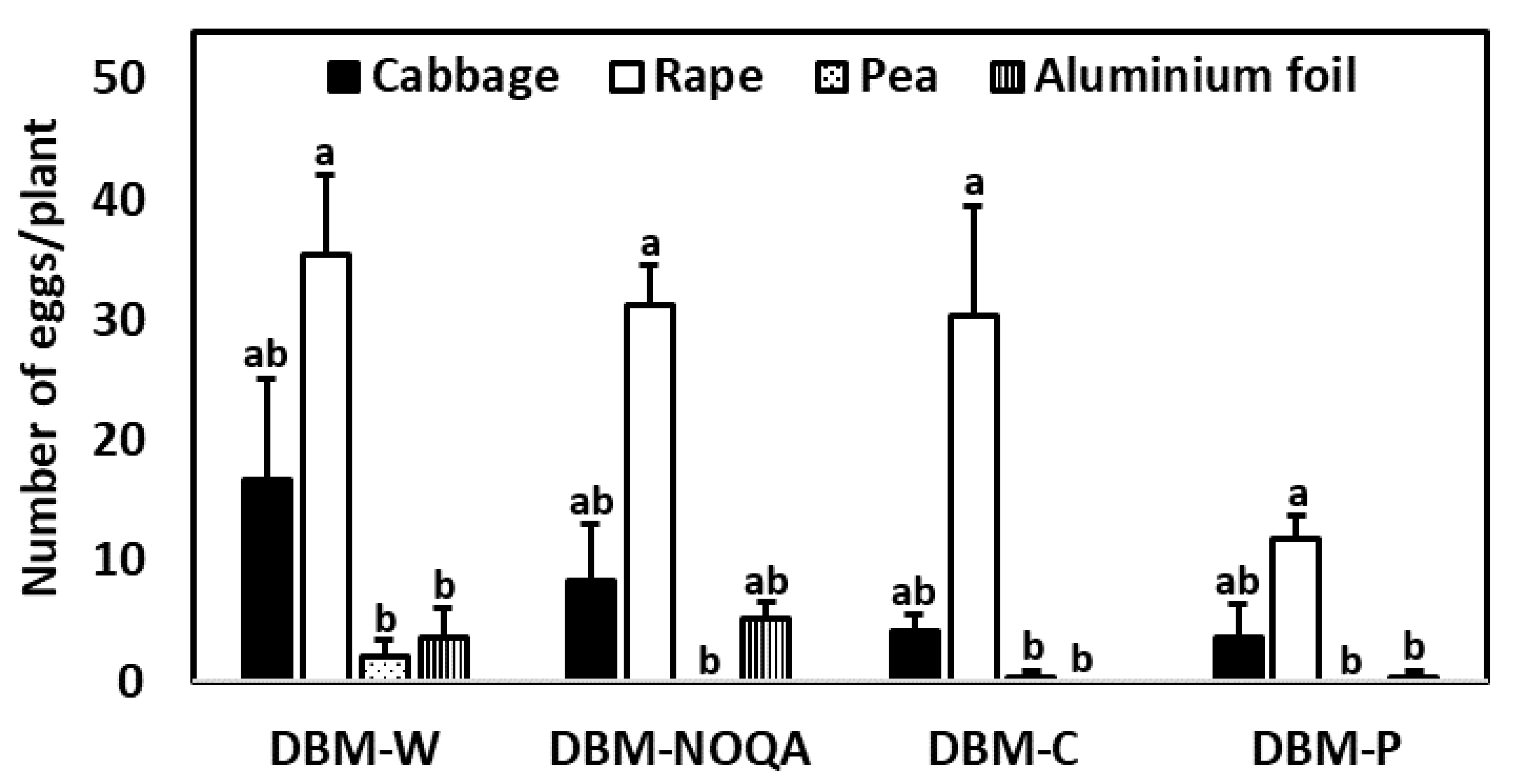
3.1. Multiple-Choice Oviposition Preference Experiments
3.2. Abaxial vs. Adaxial Preference
3.3. Oviposition Preference Experiments with Sinigrin
3.4. Larval Preference Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Pérez, F.R.; Shelton, A.M. Pest management and other agricultural practices among farmers growing cruciferous crops in the central and western highlands of Kenya and the western Himalayas of India. Int. J. Pest Manag. 2006, 52, 303–315. [Google Scholar] [CrossRef]
- Grzywacz, D.; Rossbach, A.; Rauf, A.; Russell, D.A.; Srinivasan, R.; Shelton, A.M. Current control methods for diamondback moth and other Brassica insect pests and the prospects for improved management with lepidopteran-resistant Bt vegetable Brassicas in Asia and Africa. Crop Prot. 2010, 29, 68–79. [Google Scholar] [CrossRef]
- Philips, C.R.; Fu, Z.; Kuhar, T.P.; Shelton, A.M.; Cordero, R.J. Natural history, ecology, and management of diamondback moth (Lepidoptera: Plutellidae), with emphasis on the United States. J. Integr. Pest Manag. 2014, 5, D1–D11. [Google Scholar] [CrossRef]
- Weinberger, K.; Srinivasan, R. Farmers’ management of cabbage and cauliflower pests in India and their approaches to crop protection. J. Asia-Pac. Entomol. 2009, 12, 253–259. [Google Scholar] [CrossRef]
- Sarfraz, M.; Dosdall, L.M.; Keddie, B.A. Diamondback moth-host plant interactions: Implications for pest management. Crop Prot. 2006, 25, 625–639. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Pérez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R. Trap crops and insectary plants in the order Brassicales. Ann. Entomol. Soc. Am. 2019, 112, 318–329. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Gershenzon, J.; Heckel, D.G. Plant glucosinolate content increases susceptibility to diamondback moth (Lepidoptera: Plutellidae) regardless of its diet. J. Pest Sci. 2020, 93, 491–506. [Google Scholar] [CrossRef]
- Talekar, N.S.; Shelton, A.M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Ratzka, A.; Vogel, H.; Kliebenstein, D.J.; Mitchell-Olds, T.; Kroymann, J. Disarming the mustard oil bomb. Proc. Natl. Acad. Sci. USA 2002, 99, 11223–11228. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.J.A.; Wang, C.Z.; Nielsen, J.K.; Gols, R.; Qiu, Y.T. Flavonoids from cabbage are feeding stimulants for diamondback moth larvae additional to glucosinolates: Chemoreception and behaviour. Entomol. Exp. Appl. 2002, 104, 27–34. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Haribal, M.; Gouinguené, S.; Stadler, E. Isothiocyanates stimulating oviposition by the diamondback moth, Plutella xylostella. J. Chem. Ecol. 2006, 32, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sønderby, I.; Halkier, B.; Jander, G.; de Vos, M. Non-volatile intact indole glucosinolates are host R¡recognition cues for ovipositing Plutella xylostella. J. Chem. Ecol. 2009, 35, 1427–1436. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Reichelt, M.; Heckel, D.G. Can sulfur fertilisation increase the effectiveness of trap crops for diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)? Pest Manag. Sci. 2010, 66, 832–838. [Google Scholar]
- Liu, X.-L.; Zhang, J.; Yan, Q.; Miao, C.-L.; Han, W.-K.; Hou, W.; Yang, K.; Hansson, B.S.; Peng, Y.-C.; Guo, J.-M.; et al. The molecular basis of host selection in a crucifer-specialized moth. Curr. Biol. 2020, 30, 4476–4482.e5. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Bloem, S. Interaction between insect strain and artificial diet in diamondback moth development and reproduction. Entomol. Exp. Appl. 2002, 102, 283–294. [Google Scholar] [CrossRef]
- Gupta, P.D.; Thorsteinson, A.J. Food plant relationships of the diamondback moth (Plutella maculipennis [Curt.]). I. Gustation and olfaction in relation to botanical specificity of the larva. Entomol. Exp. Appl. 1960, 3, 241–250. [Google Scholar] [CrossRef]
- Gupta, P.D.; Thorsteinson, A.J. Food plant relationships of the diamondback moth (Plutella maculipennis [Curt.]). II. Sensory regulation of oviposition of the adult female. Entomol. Exp. Appl. 1960, 3, 305–314. [Google Scholar] [CrossRef]
- Löhr, B.; Gathu, B. Evidence of adaptation of diamondback moth, Plutella xylostella (L.), to pea, Pisum sativum L. Insect Sci. Its Appl. 2002, 22, 161–173. [Google Scholar] [CrossRef]
- Bidart-Bouzat, M.G.; Kliebenstein, D.J. Differential levels of insect herbivory in the field associated with genotypic variation in glucosinolates in Arabidopsis thaliana. J. Chem. Ecol. 2008, 34, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; Broekgaarden, C.; Kabouw, P.; Oude Lenferink, K.; Poelman, E.H.; Vet, L.E.M.; Dicke, M.; van Loon, J.J.A. Relative importance of plant-mediated bottom-up and top-down forces on herbivore abundance on Brassica oleracea. Funct. Ecol. 2011, 25, 1113–1124. [Google Scholar] [CrossRef]
- Mosleh Arany, A.; de Jong, T.; Kim, H.; van Dam, N.; Choi, Y.; Verpoorte, R.; van der Meijden, E. Glucosinolates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effects on a generalist and a specialist herbivore. Chemoecology 2008, 18, 65–71. [Google Scholar] [CrossRef]
- Müller, R.; de Vos, M.; Sun, J.; Sønderby, I.; Halkier, B.; Wittstock, U.; Jander, G. Differential effects of indole and aliphatic glucosinolates on lepidopteran herbivores. J. Chem. Ecol. 2010, 36, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Poelman, E.H.; Van Loon, J.J.A.; Van Dam, N.M.; Vet, L.E.M.; Dicke, M. Performance of specialist and generalist herbivores feeding on cabbage cultivars is not explained by glucosinolate profiles. Entomol. Exp. Appl. 2008, 127, 218–228. [Google Scholar] [CrossRef]
- Sarosh, B.; Wittstock, U.; Halkier, B.; Ekbom, B. The influence of metabolically engineered glucosinolates profiles in Arabidopsis thaliana on Plutella xylostella preference and performance. Chemoecology 2010, 20, 1–9. [Google Scholar] [CrossRef][Green Version]
- Siemens, D.H.; Mitchell-Olds, T. Gluosinolates and herbivory by specialists (Coleoptera: Chrysomelidae, Lepidoptera: Plutellidae): Consequences of concentration and induced resistance. Environ. Entomol. 1996, 25, 1344–1353. [Google Scholar] [CrossRef]
- George, D.R.; Collier, R.; Whitfield, C.; Port, G. Could movement of neonates from non-host plants affect the potential of polyculture to reduce crop colonisation by pest insects? Crop Prot. 2011, 30, 1103–1105. [Google Scholar] [CrossRef]
- Singer, M.C. Preference provides a plethora of problems (don’t panic). Annu. Rev. Entomol. 2021, 66, 1–22. [Google Scholar] [CrossRef]
- Singer, M.C.; Lee, J.R. Discrimination within and between host species by a butterfly: Implications for design of preference experiments. Ecol. Lett. 2000, 3, 101–105. [Google Scholar] [CrossRef]
- Ryan, S.F.; Bidart-Bouzat, M.G. Natal insect experience with Arabidopsis thaliana plant genotypes influences plasticity in oviposition behavior. Entomol. Exp. Appl. 2014, 152, 216–227. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.-F.; Zhang, P.-J.; Wu, Z.-Y.; Liu, S.-S. Experience-induced habituation and preference towards non-host plant odors in ovipositing females of a moth. J. Chem. Ecol. 2008, 34, 330–338. [Google Scholar] [CrossRef]
- Zhang, P.-J.; Lu, Y.; Zalucki, M.; Liu, S.-S. Relationship between adult oviposition preference and larval performance of the diamondback moth, Plutella xylostella. J. Pest Sci. 2012, 85, 247–252. [Google Scholar] [CrossRef]
- Zhang, P.-J.; Liu, S.-S.; Wang, H.; Zalucki, M.P. The influence of early adult experience and larval food restriction on responses toward nonhost plants in moths. J. Chem. Ecol. 2007, 33, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Shi, Z.H.; Hou, Y.M. Host selection behavior and the fecundity of Plutella xylostella (Lepidoptera: Plutellidae) on multiple host plants. J. Insect Sci. 2014, 14, 251. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Reichelt, M.; Gershenzon, J.; Heckel, D.G. Using plant chemistry and insect preference to study the potential of Barbarea (Brassicaceae) as a dead-end trap crop for diamondback moth (Lepidoptera: Plutellidae). Phytochemistry 2014, 98, 137–144. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Reichelt, M.; Gershenzon, J.; Heckel, D.G. Phylloplane location of glucosinolates in Barbarea spp. (Brassicaceae) and misleading assessment of host suitability by a specialist herbivore. New Phytol. 2011, 189, 549–556. [Google Scholar] [CrossRef]
- Reed, D.W.; Pivnick, K.A.; Underhill, E.W. Identification of chemical oviposition stimulants for the diamondback moth, Plutella xylostella, present in three species of Brassicaceae. Entomol. Exp. Appl. 1989, 53, 277–286. [Google Scholar] [CrossRef]
- Bailey, C.D.; Koch, M.A.; Mayer, M.; Mummenhoff, K.; O’Kane, S.L.; Warwick, S.I.; Windham, M.D.; Al-Shehbaz, I.A. Toward a global phylogeny of the Brassicaceae. Mol. Biol. Evol. 2006, 23, 2142–2160. [Google Scholar] [CrossRef]
- Shelton, A.M.; Cooley, R.J.; Kroening, M.K.; Wilsey, W.T.; Eigenbrode, S.D. Comparative analysis of two rearing procedures for diamondback moth (Lepidoptera: Plutellidae). J. Entomol. Sci. 1991, 26, 17–26. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Liu, Y.-B.; Finson, N.; Masson, L.; Heckel, D.G. One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins. Proc. Natl. Acad. Sci. USA 1997, 94, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.J.; Kovaliski, J. Detection of insecticide resistance in Plutella xylostella (L.) (Lepidoptera: Plutellidae) populations in south Australian crucifer crops. Aust. J. Entomol. 1999, 38, 132–134. [Google Scholar] [CrossRef]
- Becerra, J.X.; Noge, K.; Venable, D.L. Macroevolutionary chemical escalation in an ancient plant–herbivore arms race. Proc. Natl. Acad. Sci. USA 2009, 106, 18062–18066. [Google Scholar] [CrossRef]
- Cacho, N.I.; Kliebenstein, D.J.; Strauss, S.Y. Macroevolutionary patterns of glucosinolate defense and tests of defense-escalation and resource availability hypotheses. New Phytol. 2015, 208, 915–927. [Google Scholar] [CrossRef]
- Hopkins, A.D. Economic Investigations of the Scolytid Bark and Timber Beetles of North America; U.S. Department of Agriculture Program of Work for 1917; U.S. Department of Agriculture: Washington, DC, USA, 1916; p. 35.
- Barron, A.B. The life and death of Hopkins’ host-selection principle. J. Insect Behav. 2001, 14, 725–737. [Google Scholar] [CrossRef]
- Maski, D.; Durairaj, D. Effects of charging voltage, application speed, target height, and orientation upon charged spray deposition on leaf abaxial and adaxial surfaces. Crop Prot. 2010, 29, 134–141. [Google Scholar] [CrossRef]
- Palumbo, J.C.; Coates, W.E. Air-assisted electrostatic application of pyrethroid and endosulfan mixtures for sweetpotato whitefly (Homoptera: Aleyrodidae) control and spray deposition in cauliflower. J. Econ. Entomol. 1996, 89, 970–980. [Google Scholar] [CrossRef]
- Kobori, Y.; Amano, H. Effect of rainfall on a population of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Appl. Entomol. Zool. 2003, 38, 249–253. [Google Scholar] [CrossRef]
- Chen, C.; Harvey, J.A.; Biere, A.; Gols, R. Rain downpours affect survival and development of insect herbivores: The specter of climate change? Ecology 2019, 100, e02819. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zalucki, M.P.; Furlong, M.J. Host-plant specific feeding relationships and insect developmental stage modulate the impact of rainfall on diamondback moth larvae. Environ. Entomol. 2019, 48, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Zalucki, M.P.; Furlong, M.J. Diamondback moth egg susceptibility to rainfall: Effects of host plant and oviposition behavior. Entomol. Exp. Appl. 2019, 167, 701–712. [Google Scholar] [CrossRef]
- Müller, C. Resistance at the plant cuticle. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Stuttgart, Germany, 2008; pp. 107–129. [Google Scholar]
- Jermy, T. Evolution of insect/host plant relationships. Am. Nat. 1984, 124, 609–630. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Chew, F.S. Oviposition behavior in Lepidoptera. Annu. Rev. Entomol. 1994, 39, 377–400. [Google Scholar] [CrossRef]
- Städler, E.; Baur, R.; De Jong, R. Sensory basis of host-plant selection: In search of the “fingerprints” related to oviposition of the cabbage root fly. Acta Zool. Acad. Sci. Hung. 2002, 48 (Suppl. 1), 265–280. [Google Scholar]
- Shroff, R.; Schramm, K.; Jeschke, V.; Nemes, P.; Vertes, A.; Gershenzon, J.; Svatoš, A. Quantification of plant surface metabolites by matrix-assisted laser desorption–ionization mass spectrometry imaging: Glucosinolates on Arabidopsis thaliana leaves. Plant J. 2015, 81, 961–972. [Google Scholar] [CrossRef]
- Marazzi, C.; Städler, E. Arabidopsis thaliana leaf-surface extracts are detected by the cabbage root fly (Delia radicum) and stimulate oviposition. Physiol. Entomol. 2004, 29, 192–198. [Google Scholar] [CrossRef]
- Spencer, J.L.; Pillai, S.; Bernays, E.A. Synergism in the oviposition behavior of Plutella xylostella: Sinigrin and wax compounds. J. Insect Behav. 1999, 12, 483–500. [Google Scholar] [CrossRef]
- Roy, N. Behavioral pattern of generalist and specialist insect pests to brassicaceous leaf cuticular n-alkanes and free fatty acids. Arthropod-Plant Interact. 2022, 16, 537–551. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Xiang, Z.-W.; Zhang, S.-Z.; Wu, L.-N.; Liu, T.-X. Adaptations of Plutella xylostella adult females and larvae to waxy host plants. J. Pest Sci. 2022, 95, 203–214. [Google Scholar] [CrossRef]
- Handley, R.; Ekbom, B.; Ågren, J. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecol. Entomol. 2005, 30, 284–292. [Google Scholar] [CrossRef]
- Stamp, N. Enemy-free space via host plant chemistry and dispersion: Assessing the influence of tri-trophic interactions. Oecologia 2001, 128, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, E.; Sasidharan, A.; Ode, P.J.; Venkatesan, R. Oviposition preference and performance of a specialist herbivore is modulated by natural enemies, larval odors, and immune status. J. Chem. Ecol. 2022, 48, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.N.; Pellmyr, O. Evolution of oviposition behavior and host preference in Lepidoptera. Annu. Rev. Entomol. 1991, 36, 65–89. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. J. Agric. Food Chem. 2000, 48, 6025–6029. [Google Scholar] [CrossRef]
- Uefune, M.; Shiojiri, K.; Takabayashi, J. Oviposition of diamondback moth Plutella xylostella females is affected by herbivore-induced plant volatiles that attract the larval parasitoid Cotesia vestalis. Arthropod-Plant Interact. 2016, 11, 1–5. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Jeschke, V.; Kearney, E.E.; Schramm, K.; Kunert, G.; Shekhov, A.; Gershenzon, J.; Vassão, D.G. How glucosinolates affect generalist lepidopteran larvae: Growth, development and glucosinolate metabolism. Front. Plant Sci. 2017, 8, 1995. [Google Scholar] [CrossRef]
- Jeschke, V.; Zalucki, J.M.; Raguschke, B.; Gershenzon, J.; Heckel, D.G.; Zalucki, M.P.; Vassão, D.G. So much for glucosinolates: A generalist does survive and develop on Brassicas, but at what cost? Plants 2021, 10, 962. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Cartea, M.E. Glucosinolate induction and resistance to the cabbage moth, Mamestra brassicae, differs among kale genotypes with high and low content of sinigrin and glucobrassicin. Plants 2021, 10, 1951. [Google Scholar] [CrossRef]
| Family | Species | Common Name | Experiment |
|---|---|---|---|
| Brassicaceae | Aethionema cordifolium DC. | Lebanon stone cress | AA |
| Brassicaceae | Alyssum argenteum All. | Yellow tuft | AA, LP |
| Brassicaceae | Arabidopsis thaliana (L.) Heynh. | Thale cress | AA |
| Brassicaceae | Arabis caucasica Willd. | Mountain rock cress | AA, LP |
| Brassicaceae | Barbarea vulgaris R.Br. | Wintercress | AA |
| Brassicaceae | Biscutella laevigata L. | Buckler mustard | AA |
| Brassicaceae | Brassica juncea (L.) Czern. | Indian mustard | AA, LP |
| Brassicaceae | Brassica napus L. | Canola | AA |
| Brassicaceae | Brassica oleracea var. capitata L. | Cabbage | AA |
| Brassicaceae | Brassica oleracea var. acephala L. | Glossy collard greens | AA |
| Brassicaceae | Brassica oleracea var. acephala L. | Waxy collard greens | AA |
| Brassicaceae | Bunias orientalis L. | Turkish rocket | AA |
| Brassicaceae | Capsella bursa-pastoris (L.) Medik. | Shepherd’s purse | AA, LP |
| Brassicaceae | Cardamine pratensis L. | Cuckoo flower | AA |
| Brassicaceae | Diplotaxis muralis (L.) DC. | Annual wall rocket | AA, LP |
| Brassicaceae | Eruca sativa Mill. | Arugula, rucola | AA, LP |
| Brassicaceae | Erysimum cheiri (L.) Crantz | Wallflower | AA, LP |
| Brassicaceae | Iberis amara L. | Bitter candytuft | AA, LP |
| Brassicaceae | Lepidium sativum L. | Garden cress | AA; LP |
| Brassicaceae | Neslia paniculata (L.) Desv. | Ball mustard | AA |
| Brassicaceae | Nasturtium officinale W. T. Aiton | Watercress | AA |
| Brassicaceae | Sisymbrium officinale (L.) Scop. | Hedge mustard | AA, LP |
| Caricaceae | Carica papaya L. | Papaya | AA, LP |
| Cleomaceae | Cleome spinosa L. | Spider flower | AA, LP |
| Fabaceae | Pisum sativum L. | Pea | AA, LP |
| Gyrostemonaceae | Codonocarpus cotinifolius (Desf.) F.Muell. | Bell-fruit tree | AA |
| Limnanthaceae | Limnanthes douglasii R. Br. | Douglas’ meadowfoam | AA |
| Moringaceae | Moringa oleifera Lam. | Drumstick tree | AA, LP |
| Resedaceae | Reseda odorata L. | Common mignonette | AA, LP |
| Tropaeolaceae | Tropaeolum majus L. | Garden nasturtium | AA, LP |
| % Abaxial Oviposition as Mean ± SE, Test Statistic, and p-Value | |||
|---|---|---|---|
| DBM-C | DBM-G88 | DBM-P | |
| A. cordifolium | 11.15 ± 4.41, z = 2.70, p = 0.003 * | 11.98 ± 4.75, z = 2.63, p = 0.004 * | 8.71 ± 4.15, z = 2.84, p = 0.002 * |
| A. argenteum | 4.87 ± 2.23, z = 3.12, p ≤ 0.001 * | 4.11 ± 1.29, z = 3.19, p ≤ 0.001 * | 1.40 ± 0.95, z = 3.39, p ≤ 0.001 * |
| A. thaliana | 53.41 ± 1.66, z = 0.95, p = 0.172 | 50.00 ± 1.25, z = 0.00, p = 0.500 | 50.33 ± 0.11, z = 0.09, p = 0.464 |
| A. thaliana, detached | 57.34 ± 4.47, z = 0.92, p = 0.178 | - | - |
| A. caucasica | 60.83 ± 4.67, z = 0.76, p = 0.223 | 52.72 ± 7.43, z = 0.21, p = 0.418 | 31.12 ± 8.16, z = 1.32, p = 0.094 |
| B. vulgaris | 50.38 ± 11.71, z = 0.00, p = 0.500 | 52.22 ± 3.68, z = 0.14, p = 0.445 | 58.84 ± 4.61, z = 0.62, p = 0.266 |
| B. laevigata | 56.33 ± 4.28, z = 0.34, p = 0.416 | 56.99 ± 3.04, z = 0.49, p = 0.314 | 43.55 ± 5.82, z = 0.42, p = 0.339 |
| B. juncea | 52.09 ± 6.11, z = 0.14, p = 0.445 | 64.76 ± 2.64, z = 0.24, p = 0.403 | 37.10 ± 3.20, z = 0.21, p = 0.418 |
| B. napus | 53.06 ± 8.35, z = 0.21, p = 0.418 | 44.99 ± 5.25, z = 0.35, p = 0.365 | 38.24 ± 8.10, z = 0.83, p = 0.203 |
| B. oleracea (cabba.) | 32.90 ± 11.86, z = 1.18, p = 0.119 | 35.33 ± 11.43, z = 1.04, p = 0.149 | 39.68 ± 15.71, z = 0.49, p = 0.312 |
| B. oleracea (g. co.) | 17.62 ± 3.31, z = 2.22, p = 0.013 * | 21.20 ± 3.80, z = 2.01, p = 0.022 * | 30.10 ± 5.39, z = 1.39, p = 0.083 |
| B. oleracea (w. co.) | 4.17 ± 4.17, z = 3.19, p ≤ 0.001 * | 19.53 ± 9.99, z = 2.08, p = 0.019 * | 10.00 ± 6.12, z = 2.53, p = 0.006 * |
| B. orientalis | 44.50 ± 9.38, z = 0.42, p = 0.339 | 31.49 ± 10.47, z = 1.32, p = 0.094 | 48.83 ± 6.77, z = 0.06, p = 0.475 |
| C. bursa-pastoris | 40.71 ± 16.36, z = 0.57, p = 0.285 | n/a | 81.67 ± 8.22, z = 1.81, p = 0.035 ** |
| C. pratensis | 47.50 ± 4.61, z = 0.14, p = 0.445 | 45.37 ± 7.23, z = 0.24, p = 0.403 | 52.67 ± 3.60, z = 0.21, p = 0.418 |
| C. papaya | 55.88 ± 8.82, z = 0.24, p = 0.405 | 59.22 ± 7.30, z = 0.51, p = 0.305 | 34.77 ± 5.04, z = 0.73, p = 0.231 |
| C. spinosa | 33.46 ± 6.85, z = 1.08, p = 0.141 | 28.50 ± 7.69, z = 1.45, p = 0.073 | 25.53 ± 9.47, z = 1.66, p = 0.048 * |
| C. cotinifolius | 38.95 ± 8.37, z = 0.62, p = 0.267 | 56.02 ± 10.57, z = 0.34, p = 0.367 | 40.04 ± 8.81, z = 0.63, p = 0.263 |
| D. muralis | 49.53 ± 7.85, z = 0.00, p = 0.500 | 48.85 ± 3.11, z = 0.07, p = 0.472 | 41.46 ± 5.55, z = 0.62, p = 0.266 |
| E. sativa | 36.16 ± 3.12, z = 0.97, p = 0.166 | 32.24 ± 3.96, z = 1.25, p = 0.106 | 28.85 ± 6.63, z = 1.45, p = 0.073 |
| E. cheiri | 69.29 ± 2.96, z = 1.20, p = 0.115 | 50.46 ± 6.89, z = 0.00, p = 0.500 | 23.80 ± 6.18, z = 1.80, p = 0.036 * |
| I. amara | 22.12 ± 4.74, z = 1.94, p = 0.020 * | 25.69 ± 6.98, z = 1.66, p = 0.048 * | 13.34 ± 5.97, z = 2.56, p = 0.005 * |
| L. sativum | 71.84 ± 4.52, z = 1.52, p = 0.064 | 54.46 ± 2.66, z = 0.28, p = 0.391 | 26.93 ± 8.21, z = 1.59, p = 0.055 |
| L. douglasii | 24.15 ± 1.33, z = 1.80, p = 0.036 * | 36.00 ± 1.51, z = 0.97, p = 0.166 | 26.90 ± 5.39, z = 1.59, p = 0.055 |
| M. oleifera | 46.43 ± 3.57, z = 0.16, p = 0.436 | n/a | n/a |
| N. officinale | 58.54 ± 5.58, z = 0.39, p = 0.348 | 58.79 ± 1.83, z = 0.15, p = 0.442 | 47.10 ± 5.05, z = 0.34, p = 0.366 |
| N. paniculata | 60.00 ± 30.55, z = 0.62, p = 0.266 | 73.21 ± 1.79, z = 0.92, p = 0.179 | n/a |
| p. sativum | n/a | n/a | 0.00 ± 0.00, z = 2.00, p = 0.023 * |
| R. odorata | 48.33 ± 25.87, z = 0.10, p = 0.461 | 43.75 ± 25.77, z = 0.45, p = 0.325 | 7.87 ± 4.56, z = 2.38, p = 0.009 * |
| S. officinale | 35.31 ± 5.99, z = 1.04, p = 0.149 | 25.45 ± 3.72, z = 1.66, p = 0.048 * | 33.07 ± 6.74, z = 1.18, p = 0.119 |
| T. majus | 75.93 ± 14.46, z = 1.27, p = 0.100 | n/a | 83.65 ± 12.71, z = 2.15, p = 0.016 ** |
| DBM-C | DBM-P | |||||
|---|---|---|---|---|---|---|
| Sinigrin Concentration (M) | Lower Concentration | Higher Concentration | Test Statistic and p-Value | Lower Concentration | Higher Concentration | Test Statistic and p-Value |
| 10−3 vs. 10−2 | 35.5 ± 9.0 a | 64.5 ± 9.0 b | z = 1.70, p = 0.045 | 25.0 ± 5.5 a | 75.0 ± 5.5 b | z = 2.92, p = 0.002 |
| 10−4 vs. 10−3 | 26.6 ± 7.5 a | 73.4 ± 1.7 b | z = 2.29, p = 0.011 | 15.5 ± 9.8 a | 84.5 ± 9.8 b | z = 3.09, p = 0.001 |
| 10−5 vs. 10−4 | 28.5 ± 7.5 a | 71.5 ± 7.5 b | z = 1.82, p = 0.034 | 22.5 ± 11.1 a | 77.5 ± 11.1 b | z = 2.33, p = 0.010 |
| 10−6 vs. 10−5 | 15.4 ± 7.8 a | 84.6 ± 7.8 b | z = 1.70, p = 0.045 | 26.8 ± 10.8 a | 73.2 ± 10.8 b | z = 1.74, p = 0.041 |
| 10−7 vs. 10−6 | 22.9 ± 11.1 a | 77.1 ± 11.1 b | z = 2.03, p = 0.021 | 0.0 ± 0.0 a | 100.0 ± 0.0 b | z = 2.45, p = 0.007 |
| LPI, Test Statistic, and p-Value | OPI, Test Statistic, and p-Value | |
|---|---|---|
| A. argenteum | 0.31 ± 0.11, z = 3.20, p ≤ 0.001 * | 0.08 ± 0.02, z = 2.11, p = 0.018 * |
| A. caucasica | 0.87 ± 0.12, z = 0.38, p = 0.352 | 0.43 ± 0.05, z = 0.98, p = 0.164 |
| B. juncea | 4.14 ± 0.69, z = 2.52, p = 0.012 ** | 1.71 ± 0.25, z = 0.59, p = 0.278 |
| C. bursa-pastoris | 0.17 ± 0.01, z = 3.80, p ≤ 0.001 * | 0.03 ± 0.03, z = 2.30, p = 0.011 * |
| C. papaya | 0.23 ± 0.06, z = 2.41, p = 0.008 * | 0.05 ± 0.05, z = 2.25, p = 0.012 * |
| C. spinosa | 0.25 ± 0.05, z = 2.15, p = 0.032 * | 0.09 ± 0.05, z = 2.06, p = 0.020 * |
| D. muralis | 1.71 ± 0.34, z = 0.85, p = 0.197 | 1.51 ± 0.17, z = 0.49, p = 0.312 |
| E. sativa | 1.42 ± 0.21, z = 0.57, p = 0.285 | 1.35 ± 0.25, z = 0.29, p = 0.384 |
| E. cheiri | 0.69 ± 0.10, z = 1.34, p = 0.090 | 0.22 ± 0.18, z = 1.71, p = 0.043* |
| I. amara | 0.49 ± 0.09, z = 1.41, p = 0.079 | 0.72 ± 0.46, z = 0.78, p = 0.217 |
| L. sativum | 1.69 ± 0.18, z = 1.21, p = 0.114 | 4.28 ± 1.74, z = 1.18, p = 0.120 |
| M. oleifera | 0.22 ± 0.06, z = 2.55, p = 0.005 * | 0 ± 0, z = 2.45, p = 0.007 * |
| p. sativum | 0.17 ± 0.01, z = 2.69, p = 0.004 * | 0 ± 0, z = 2.45, p = 0.007 * |
| R. odorata | 0.24 ± 0.05, z = 2.27, p = 0.011 * | 0.36 ± 0.30, z = 1.47, p = 0.071 |
| S. officinale | 3.10 ± 0.66, z = 2.09, p = 0.018 ** | 6.67 ± 2.04, z = 1.67, p = 0.048 ** |
| T. majus | 1.33 ± 0.22, z = 0.50, p = 0.308 | 0.04 ± 0.04, z = 2.16, p = 0.016 * |
| A | |||||
|---|---|---|---|---|---|
| p-Value and Correlation Coefficient of Spearman’s Rho Correlation | |||||
| OPI | S | HA | HB | GCI | |
| LPI | p ≤ 0.001; 0.864 | p = 0.007; 0.641 | p = 0.010; 0.624 | p = 0.063; 0.476 | p = 0.010; 0.623 |
| OPI | - | p = 0.011; 0.614 | p = 0.002; 0.723 | p = 0.086; 0.443 | p = 0.005; 0.668 |
| B | |||||
| p-Value and Correlation Coefficient of Spearman’s Rho Correlation | |||||
| TOT | AO | BEN | IN | AS | |
| LPI | p = 0.015; 0.594 | p = 0.016; 0.590 | p = 0.673; −0.115 | p = 0.137; 0.388 | p = 0.064; 0.473 |
| OPI | p = 0.002; 0.716 | p = 0.024; 0.561 | p = 0.630; −0.131 | p = 0.033; 0.533 | p = 0.049; 0.498 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badenes-Pérez, F.R.; Heckel, D.G. Intraspecific and Interstage Similarities in Host-Plant Preference in the Diamondback Moth (Lepidoptera: Plutellidae). Horticulturae 2023, 9, 39. https://doi.org/10.3390/horticulturae9010039
Badenes-Pérez FR, Heckel DG. Intraspecific and Interstage Similarities in Host-Plant Preference in the Diamondback Moth (Lepidoptera: Plutellidae). Horticulturae. 2023; 9(1):39. https://doi.org/10.3390/horticulturae9010039
Chicago/Turabian StyleBadenes-Pérez, Francisco Rubén, and David G. Heckel. 2023. "Intraspecific and Interstage Similarities in Host-Plant Preference in the Diamondback Moth (Lepidoptera: Plutellidae)" Horticulturae 9, no. 1: 39. https://doi.org/10.3390/horticulturae9010039
APA StyleBadenes-Pérez, F. R., & Heckel, D. G. (2023). Intraspecific and Interstage Similarities in Host-Plant Preference in the Diamondback Moth (Lepidoptera: Plutellidae). Horticulturae, 9(1), 39. https://doi.org/10.3390/horticulturae9010039







