Effects of Forced-Air Precooling on Postharvest Physiological and Storage Quality of Winged Beans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Respiration Rate and Ethylene Production
2.4. Weight Loss Rate and Quality
2.4.1. Weight Loss Rate
2.4.2. Quality
Peel Color Changes
Texture
Total Soluble Solids (TSS)
Ascorbic Acid
2.5. Decay Rate and Storage Life
2.6. Statistical Analysis
3. Results
3.1. Respiration Rate and Ethylene Production
3.2. Cooling Process of Pods
3.3. Weight Loss Rate and Quality
3.4. Decay Rate and Storage Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harder, D.K.; Smartt, J. Further evidence on the origin of the cultivated winged bean, Psophocarpus tetragonolobus (L.) DC. (Fabaceae): Chromosome numbers and the presence of a host specific fungus. Econ. Bot. 1992, 46, 187–191. [Google Scholar] [CrossRef]
- Erskine, W.; Bala, A.A. Crossing technique in winged bean. Trop. Grain. Legum. Bull. 1976, 6, 32–35. [Google Scholar]
- Eagleton, G. Winged bean in Myanmar, revisted. Econ. Bot 1999, 53, 342–352. [Google Scholar] [CrossRef]
- Tanzi, A.S.; Eagleton, G.E.; Ho, W.K.; Wong, Q.N.; Mayes, S.; Massawe, F. Winged bean (Psophocarpus tetragonolobus (L.) DC.) for food and nutritional security: Synthesis of past research and future direction. Planta 2019, 250, 911–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudrappa, U. Winged Bean Nutrition Facts. 2022. Available online: http://www.nutrition-and-you.com/winged-bean.html (accessed on 28 November 2022).
- Ambuko, J.; Emong’or, E.; Shibairo, S.I.; Chemining’wa, G. Effect of accel (gibberellins (ga4+ 7)+ benzyladenine) on the shelf life of French beans, Phaseolus vulgaris L. Afr. Crop. Sci. J. 2003, 6, 536–540. [Google Scholar]
- Sharma, G.L.; Patel, K.L. Winged Bean; CRC Press: Boca Raton, FL, USA, 2017; pp. 791–798. [Google Scholar]
- Kitinoja, L.; Kader, A.A. Small-Scale Postharvest Handling Practices: A Manual for Horticultural Crops, 4th ed.; Poshtharvest Horticulture Series No. 8E; University of California, Davis Postharvest Technology Research and Information Center: Davis, CA, USA, 2002; p. 260. [Google Scholar]
- Kader, A.A.; Saltveit, M.E. Respiration and gas exchange. In Postharvest Physiology and Pathology of Vegetables; CRC: Boca Raton, FL, USA, 2002; pp. 31–56. [Google Scholar]
- Silva, G.; Champa, W.; Prasanna, P. Effect of Harvest Maturity, Packaging Technique and Storage Temperature on Postharvest Life of Winged Bean (Psophocarpus Tetragonolobus). 2008. Available online: http://repository.rjt.ac.lk/bitstream/handle/123456789/693/107-108.pdf?sequence=1 (accessed on 5 November 2022).
- Sullivan, G.H.; Davenport, L.R.; Julian, J.W. Progress in new crops: Precooling: Key factor for assuring quality in new fresh market vegetable crops. Prog. New Crops. 1996, 127, 521–524. [Google Scholar]
- EO, O.; Kunyanga, C.; Kimenju, J.; Okoth, M. Effects of harvest time and duration before cooling on the postharvest quality and shelf-life stability of French bean (Phaseolus vulgaris L.). J. Nutr. 2018, 8, 1000730. [Google Scholar]
- Ambaw, A.; Mukama, M.; Opara, U.L. Analysis of the effects of package design on the rate and uniformity of cooling of stacked pomegranates: Numerical and experimental studies. Comput. Electron. Agric. 2017, 136, 13–24. [Google Scholar] [CrossRef]
- Thompson, J.F.; Mitchell, F.G.; Rumsay, T.R.; Kasmire, R.F.; Crisosto, C.H. Commercial Cooling of Fruits, Vegetables, and Flowers; UCANR Publications: Oakland, CA, USA, 2008; pp. 1–12. [Google Scholar]
- Ryall, A.L.; Pentzer, W.T. Handling, Transportation and Storage of Fruits and Vegetables. Volume 2. Fruits and Tree Nuts; AVI Publishing Co. Inc.: Westport, CT, USA, 1982. [Google Scholar]
- Mercier, S.; Brecht, J.K.; Uysal, I. Commercial forced-air precooling of strawberries: A temperature distribution and correlation study. J. Food Eng. 2019, 242, 47–54. [Google Scholar] [CrossRef]
- Cortbaoui, P.; Goyette, B.; Gariépy, Y.; Charles, M.T.; Raghavan, V.G.S.; Vigneault, C. Forced air cooling system for Zea mays. J. Food Agric. Environ. 2005, 4, 100. [Google Scholar]
- Mittal, T.C.; Sharma, S.R.; Jindal, N. Effect of pre-cooling and packaging materials under ambient condition storage on postharvest quality of white button mushroom. Indian J. Sci. Res. Technol. 2014, 2, 60–72. [Google Scholar]
- Salamat, R.; Ghassemzadeh, H.R.; Ranjbar, F.; Jalali, A.; Mahajan, P.; Herppich, W.B.; Mellmann, J. The effect of additional packaging barrier, air moment and cooling rate on quality parameters of button mushroom (Agaricus bisporus). Food Packag. Shelf Life 2020, 23, 100448. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K. Modified atmosphere packaging of fruits and vegetables for extending shelf-life-A review. Fresh Prod. 2009, 3, 1–31. [Google Scholar]
- Dincer, I. Air flow precooling of individual grapes. J. Food Eng. 1995, 26, 243–249. [Google Scholar] [CrossRef]
- MeGuire, R.G. Reporting of objective color measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S. Vitamin C determination by indophenol method. In Food Analysis Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2017; pp. 143–146. [Google Scholar]
- Arah, I.K.; Amaglo, H.; Kumah, E.K.; Ofori, H. Preharvest and postharvest factors affecting the quality and shelf life of harvested tomatoes: A mini review. Int. J. Agron. 2015, 2015, 478041. [Google Scholar] [CrossRef] [Green Version]
- Maassen, A.; Erbersdobler, H.F.; Busch-Stockfisch, M. Preservation of the sensory quality of fresh and frozen vegetables under different storage conditions. Ernahrungs-Umschau 2006, 53, 390–394. [Google Scholar]
- Ismail, S.M.; Ozawa, K.; Khondaker, N.A. Effect of irrigation frequency and timing on tomato yield, soil water dynamics and water use efficiency under drip irrigation. In Proceedings of the 11th International Water Technology Conference, Sharm El-Sheikh, Egypt, 15–18 March 2007; Volume 1, pp. 15–18. [Google Scholar]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling of respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Saltveit, M.E. Commercial Storage of Fruits, Vegetables and Florist and Nursery Crops; Postharvest Technology Centre RIC, Department of Plant Science, University of California: Davis, CA, USA, 2005; 485p. [Google Scholar]
- Zagory, D.; Kader, A.A. Modified atmosphere packaging of fresh produce. Food Technol. 1988, 42, 70–77. [Google Scholar]
- Elansari, A.M.; Mostafa, Y.S. Vertical forced air pre-cooling of orange fruits on bin: Effect of fruit size, air direction, and air velocity. J. Saudi Soc. Agric. Sci. 2020, 19, 92–98. [Google Scholar] [CrossRef]
- Klieber, A.; Jewell, L.; Simbeya, N. Ice or an ice-replacement agent does not improve refrigerated broccoli storage at 1 °C. HortTechnology 1993, 3, 317–318. [Google Scholar] [CrossRef] [Green Version]
- Gillies, S.L.; Toivonen, P.M.A. Cooling method influences the postharvest quality of broccoli. HortScience 1995, 30, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Alibas, I.; Koksal, N. Forced-air, vacuum, and hydro precooling of cauliflower (Brassica oleracea L. var. botrytis cv. Freemont): Part I. Determination of precooling parameters. Food Sci. Technol. 2014, 34, 730–737. [Google Scholar] [CrossRef] [Green Version]
- Zainal, B.; Ding, P.; Ismail, I.S.; Saari, N. Physico-chemical and microstructural characteristics during postharvest storage of hydrocooled rockmelon (Cucumis melo L. reticulatus cv. Glamour). Postharvest Biol. Technol. 2019, 152, 89–99. [Google Scholar] [CrossRef]
- Sargent, S.A.; Ritenour, M.A.; Brecht, J.K.; Bartz, J.A. Handling, Cooling and Sanitation Techniques for Maintaining Postharvest Quality; University of Florida IFAS Extension: Gainesville, FL, USA, 2007; pp. 97–109. [Google Scholar]
- Alique, R.; Martínez, M.A.; Alonso, J. Metabolic response to two hydrocooling temperatures in sweet cherries cv Lapins and cv Sunburst. J. Sci. Food Agric. 2006, 86, 1847–1854. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, G.B.; Fawole, O.A.; Verboven, P.; Zhang, X.R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K. Postharvest prcooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- Sena, E.D.O.A.; da Silva, P.S.O.; de Araujo, H.G.S.; de Aragao Batista, M.C.; Matos, P.N.; Sargent, S.A.; Junior, L.F.G.O.; Carnelossi, M.A.G. Postharvest quality of cashew apple after hydrocooling and cold room. Postharvest Biol. Technol. 2019, 155, 65–71. [Google Scholar] [CrossRef]
- Choi, J.H.; Yim, S.H.; Cho, K.S.; Kim, M.S.; Park, Y.S.; Jung, S.K.; Choi, H.S. Fruit quality and core breakdown of ‘Wonhwang’ pears in relation to harvest date and pre-storage cooling. Sci. Hortic. 2015, 188, 1–5. [Google Scholar] [CrossRef]
- Han, Q.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Precooling and ozone treatments affects postharvest quality of black mulberry (Morus nigra) fruits. Food Chem. 2017, 221, 1947–1953. [Google Scholar] [CrossRef]
- Rab, A.; Rehman, H.; Haq, I.; Sajid, M.; Nawab, K.; Ali, K. Harvest stages and pre-cooling influence the quality and storage life of tomato fruit. J. Anim. Plant Sci. 2013, 23, 1347–1352. [Google Scholar]
- Brosnan, T.; Sun, D.W. Precooling techniques and applications for horticultural products—A review. Int. J. Refrig. 2001, 24, 154–170. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Physical and chemical quality characteristics of strawberries after storage are reduced by a short delay to cooling. Postharvest Biol. Technol. 1995, 6, 17–28. [Google Scholar] [CrossRef]
- Liang, Y.S.; Wongmetha, O.; Wu, P.S.; Ke, L.S. Influence of hydrocooling on browning and quality of litchi cultivar Feizixiao during storage. Int. J. Refrig. 2013, 36, 1173–1179. [Google Scholar] [CrossRef]
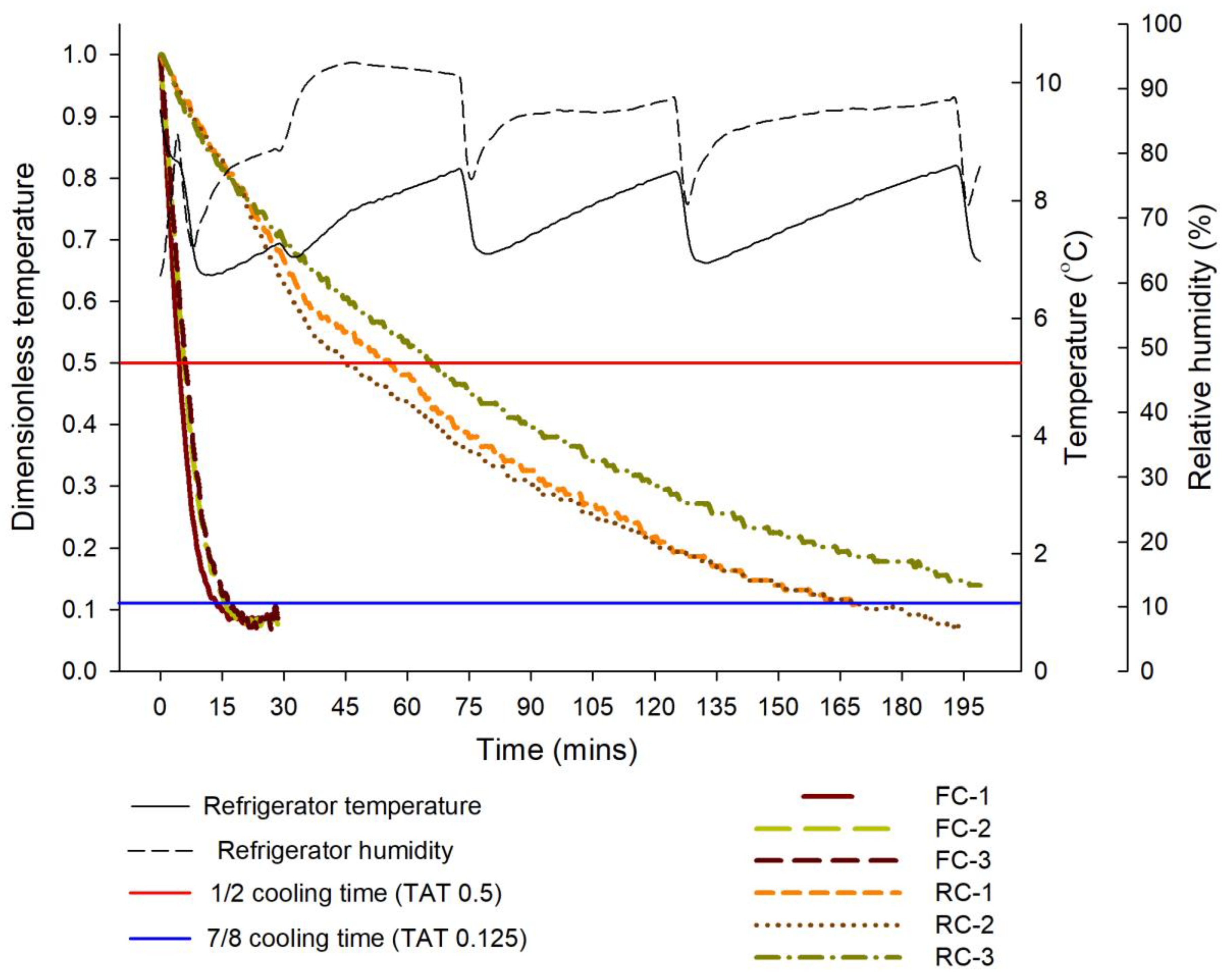

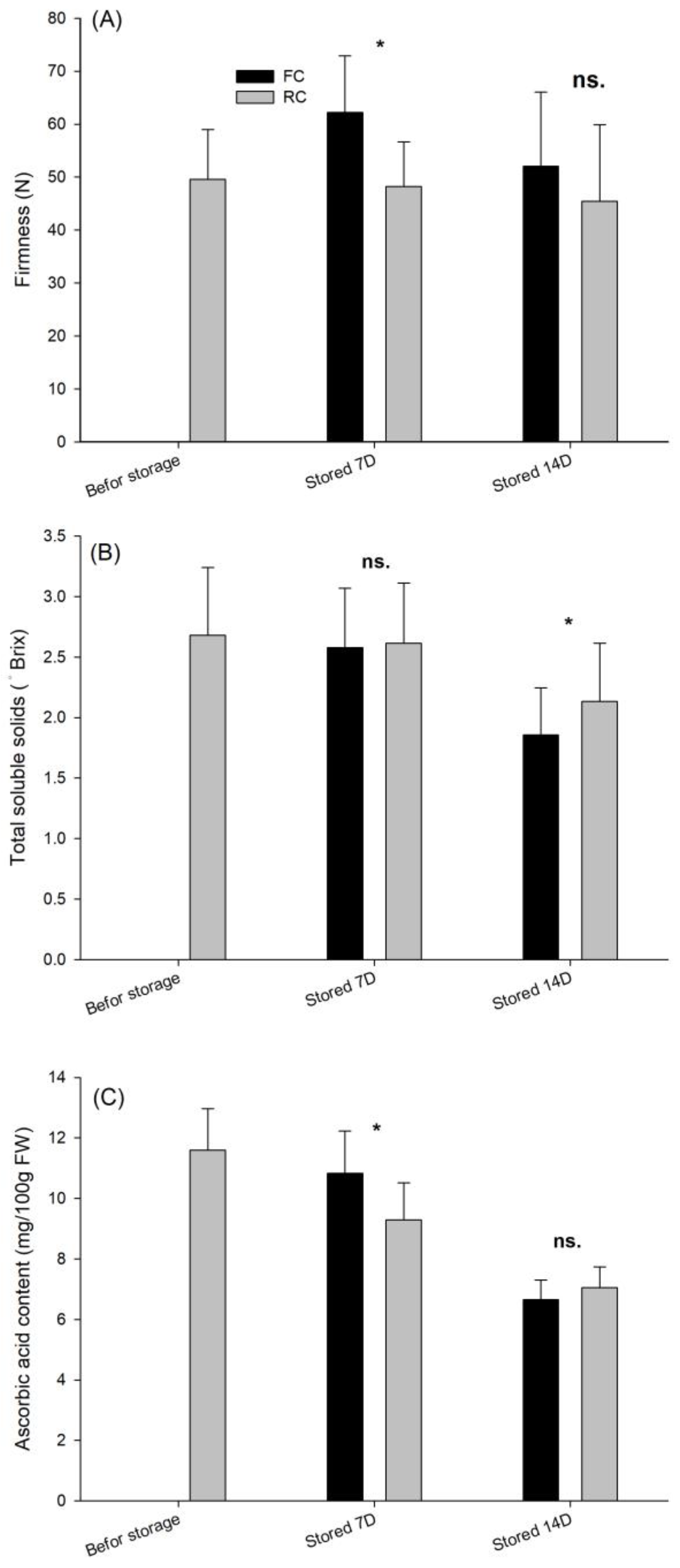


| Temperature (°C) | Respiration Rate (mg CO2 kg−1·h−1) | Ethylene Production (μL C2H4 kg−1·h−1) |
|---|---|---|
| 5 | 10.60 ± 4.16 | 0.23 ± 0.05 |
| 10 | 18.81 ± 3.17 | 0.25 ± 0.07 |
| 15 | 25.70 ± 5.47 | 0.30 ± 0.07 |
| 25 | 41.07 ± 10.60 | 0.42 ± 0.09 |
| Storage at 12 °C | Treatment | Color | ||
|---|---|---|---|---|
| L | Hue Angle (h) | Chroma (θ) | ||
| Before storage | 48.6 ± 4.8 | 109.1 ± 3.7 | 39.6 ± 6.2 | |
| 7 days | FC | 46.6 ± 6.7 b z | 105.1 ± 4.3 b | 34.9 ± 3.9 b |
| RC | 49.7 ± 3.9 a | 111.0 ± 3.0 a | 39.8 ± 5.4 a | |
| 14 days | FC | 43.2 ± 6.7 a | 102.8 ± 4.8 a | 36.3 ± 4.0 a |
| RC | 43.4 ± 6.5 a | 102.5 ± 6.2 a | 36.7 ± 4.2 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Hsu, M.-C.; Liao, J.-Z.; Wei, Z.-W.; Chung, H.-Y.; Liang, Y.-S. Effects of Forced-Air Precooling on Postharvest Physiological and Storage Quality of Winged Beans. Horticulturae 2023, 9, 45. https://doi.org/10.3390/horticulturae9010045
Lee Y-C, Hsu M-C, Liao J-Z, Wei Z-W, Chung H-Y, Liang Y-S. Effects of Forced-Air Precooling on Postharvest Physiological and Storage Quality of Winged Beans. Horticulturae. 2023; 9(1):45. https://doi.org/10.3390/horticulturae9010045
Chicago/Turabian StyleLee, Ying-Che, Min-Chi Hsu, Jia-Zhu Liao, Zhao-Wei Wei, Hsin-Ying Chung, and Yu-Shen Liang. 2023. "Effects of Forced-Air Precooling on Postharvest Physiological and Storage Quality of Winged Beans" Horticulturae 9, no. 1: 45. https://doi.org/10.3390/horticulturae9010045
APA StyleLee, Y.-C., Hsu, M.-C., Liao, J.-Z., Wei, Z.-W., Chung, H.-Y., & Liang, Y.-S. (2023). Effects of Forced-Air Precooling on Postharvest Physiological and Storage Quality of Winged Beans. Horticulturae, 9(1), 45. https://doi.org/10.3390/horticulturae9010045








