Cultivars and Fruit Part as Differentiating Factors of Physicochemical Characteristics of Mango Starches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mango Cultivars and Location of the Orchard
2.2. Isolation of Starches from Mango Pulp and Kernel
2.3. Starch Analysis
2.3.1. Chemical Composition
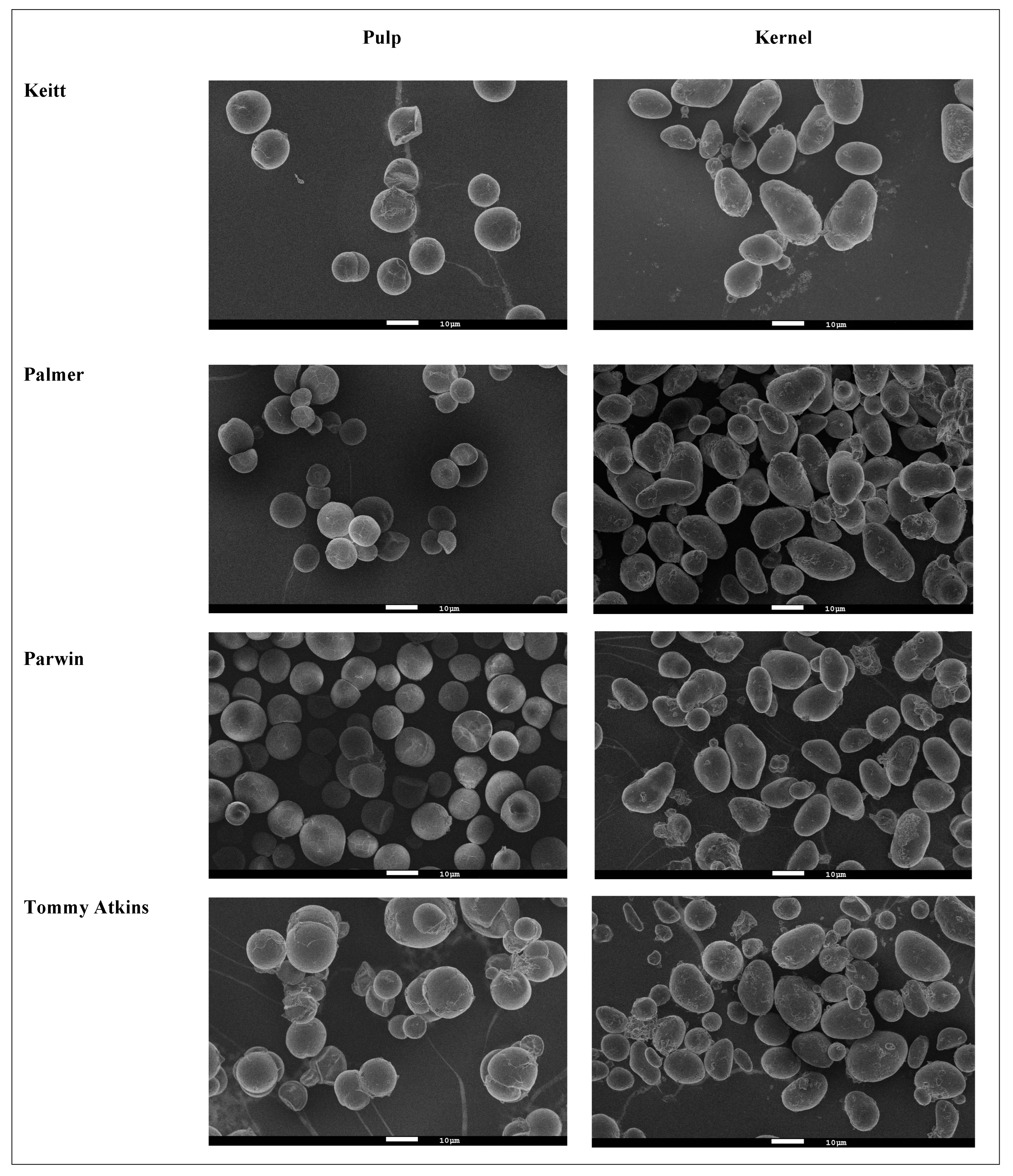
2.3.2. Morphology and Granule Size
2.3.3. Color
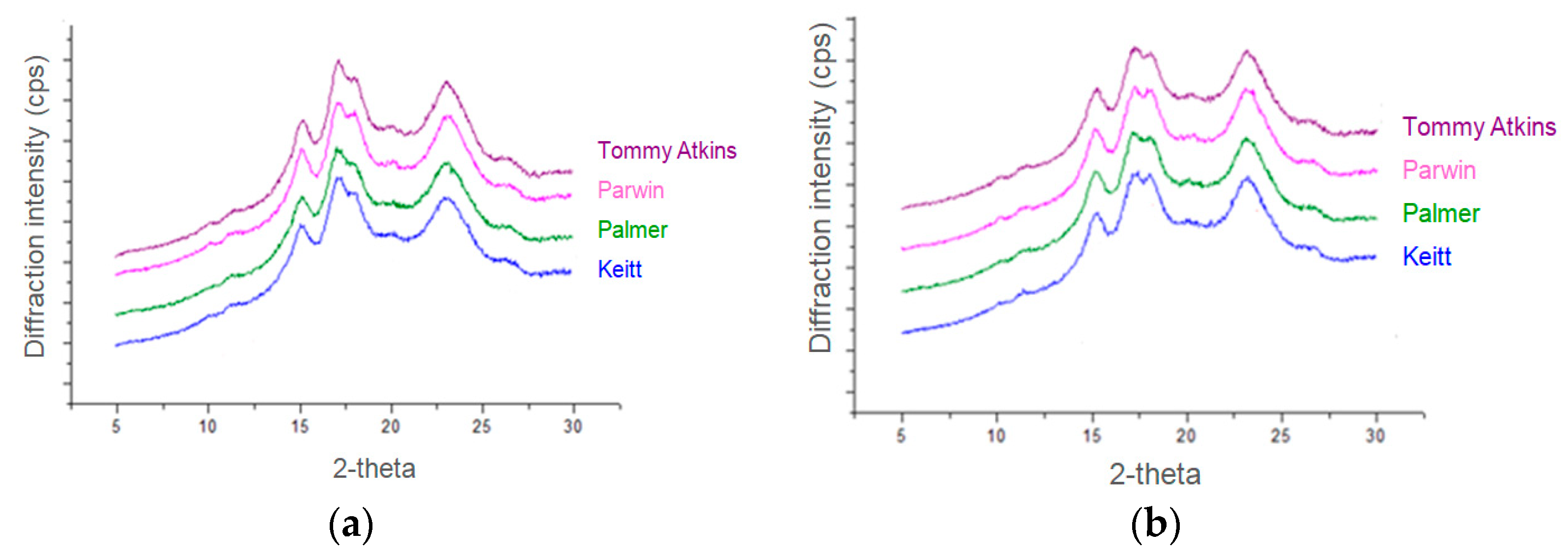
2.3.4. X-ray Diffraction Pattern
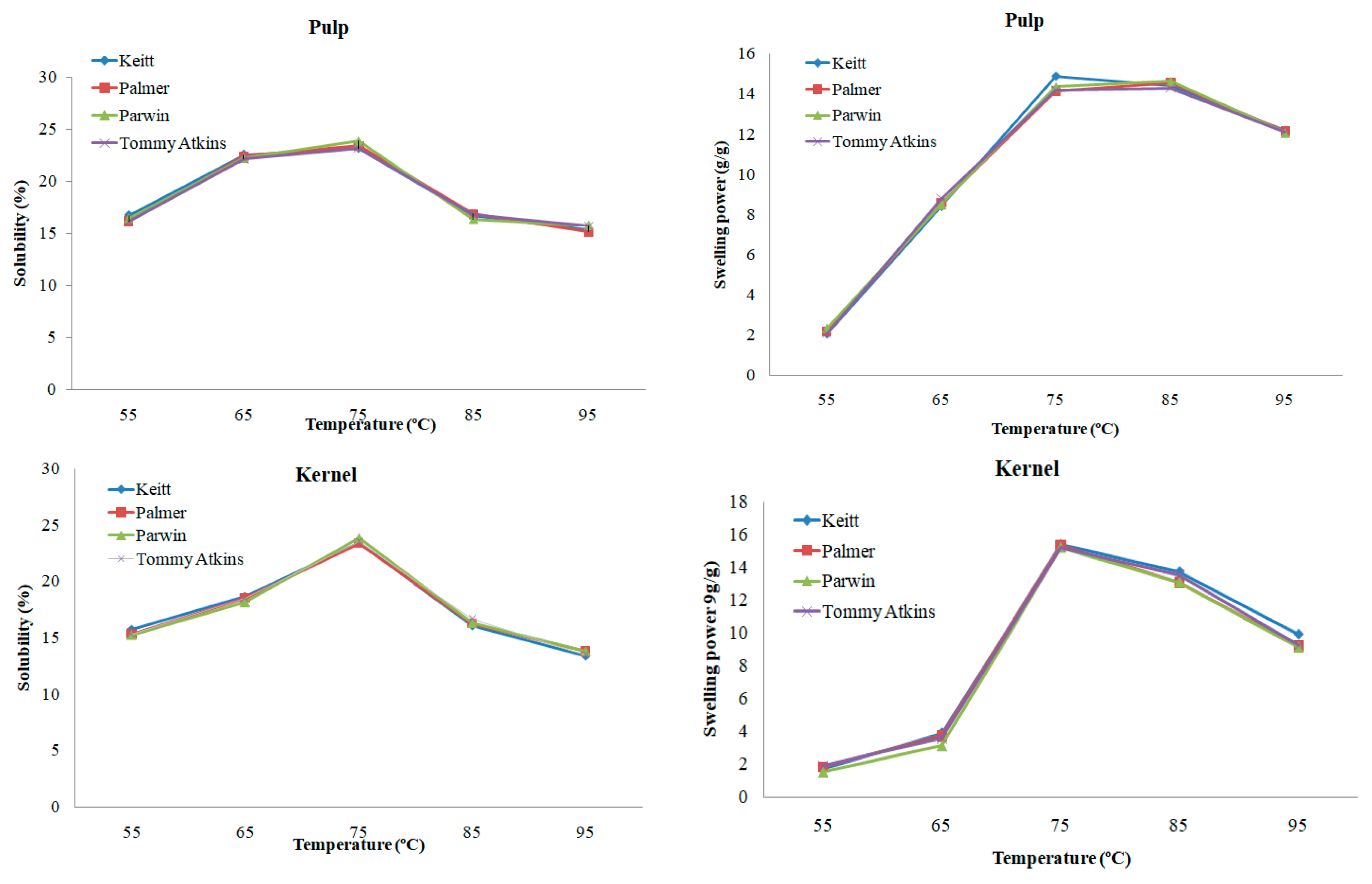
2.3.5. Swelling Power (SP) and Solubility (SS)
2.3.6. Pasting Properties
2.3.7. Thermal Properties
2.4. Data Analysis
3. Results and Discussion
3.1. Chemical Composition, Minerals and Amylose
3.2. Morphology and Granule Size
3.3. Color
3.4. X-ray Diffraction Pattern and Crystallinity
3.5. Swelling Power and Solubility
3.6. Pasting and Thermal Properties
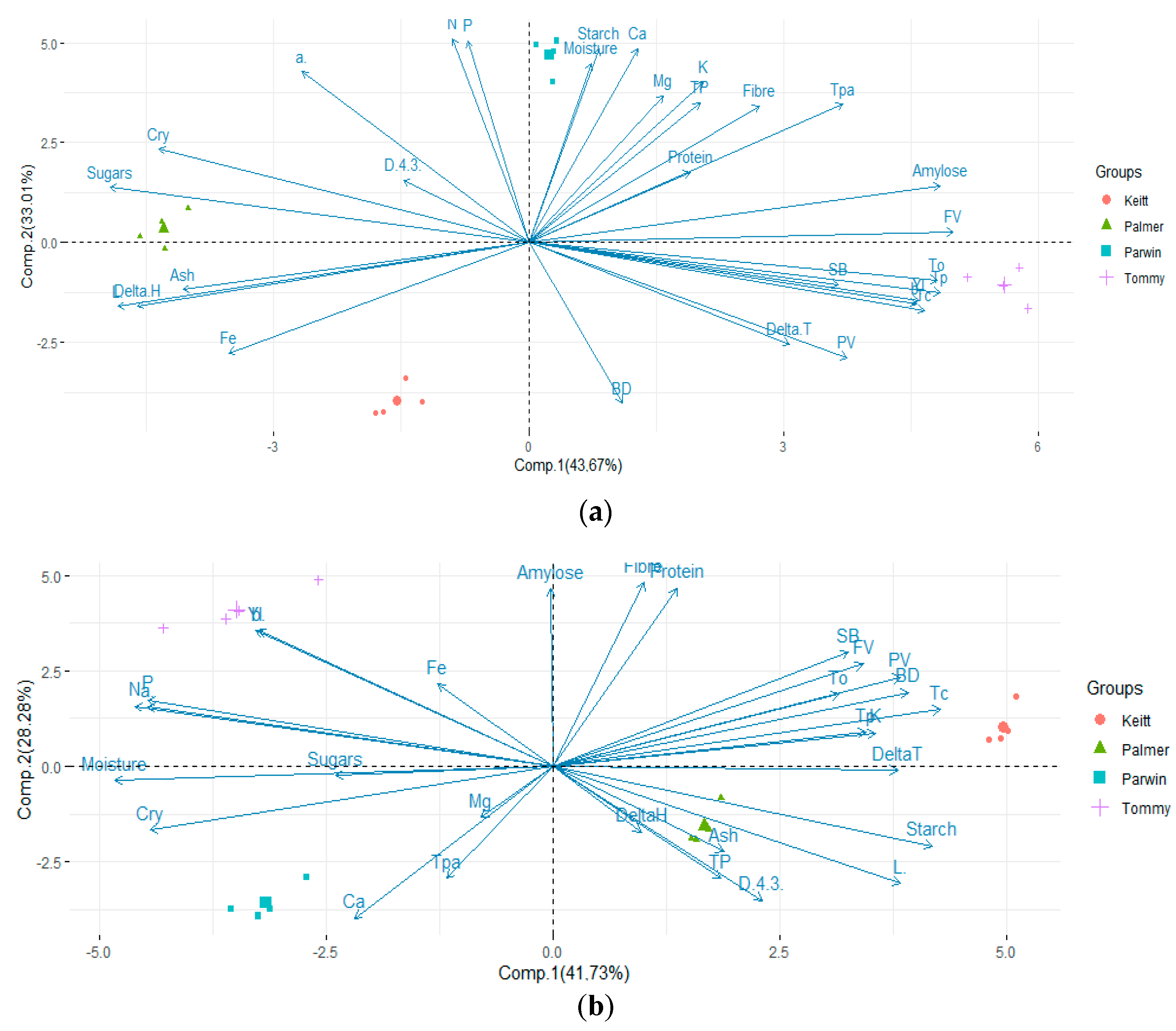
3.7. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mordor Intelligence. Industrial Starches Market-Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/industrial-starches-market (accessed on 1 December 2022).
- Kringel, D.H.; Dias, A.R.G.; Zavareze, E.R.; Gandra, E.A. Fruit wastes as promising sources of starch: Extraction, properties, and applications. Starch 2020, 72, 1900200. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.-R. Clean label starch: Production, physicochemical characteristics, and industrial applications. Food Sci. Biotechnol. 2021, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Instituto Brasileiro de Geografia e Estatística. Sidra: Censo Agropecuário. 2020. Available online: https://sidra.ibge.gov.br/tabela/6959 (accessed on 2 November 2022).
- Choudhury, M.M.; Costa, T.S. Perdas na Cadeia de Comercialização da Manga, 1st ed.; Embrapa Semi-Árido: Petrolina, Brazil, 2004; 44p. [Google Scholar]
- Kaur, M.; Punia, S.; Sandhu, S.; Ahmed, J. Impact of high pressure processing on the rheological, thermal and morphological characteristics of mango kernel starch. Int. J. Biol. Macromol. 2019, 140, 149–155. [Google Scholar] [CrossRef]
- Bangar, S.P.; Kumar, M.; Whiteside, W.S. Mango seed starch: A sustainable and eco-friendly alternative to increasing industrial requirements. Int. J. Biol. Macromol. 2021, 183, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Bertof, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service: Washington, DC, USA, 1999; 436p.
- Bello-Pérez, L.A.; Aparicio-Saguila, A.; Méndez-Montealvo, G.; Solorza-Feria, J.; Flores-Huicochea, E. Isolation and partial characterization of mango (Magnifera indica L.) starch: Morphological, physicochemical and functional studies. Plant Foods Hum. Nutr. 2005, 60, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Araujo, T.; Souza, N.; Rodrigues, L.; Lisboa, H.M.; Pasquali, M.; Trindade, G.; Rocha, A.P. Physicochemical, morphological and antioxidant properties of spray-dried mango kernel starch. J. Agric. Res. 2019, 1, 100012. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007; p. 2.
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Associação Brasileira para Pesquisa da Potassa e do Fosfato: Piracicaba, Brazil; p. 308.
- Williams, P.C.; Kuzina, F.D.; Hlynka, L. A rapid calorimetric procedure for estimating the amylose content of starches and flour. Cereal Chem. 1970, 47, 411–420. [Google Scholar]
- Mesquita, C.B.; Leonel, M.; Franco, C.M.L.; Leonel, S.; Garcia, E.L.; Santos, T.P.R. Characterization of banana starches obtained from cultivars grown in Brazil. Int. J. Biol. Macromol. 2016, 89, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.R.; de Carvalho, C.W.P.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rice. Int. J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the relationship between water-satured state and crystallinity by the diffraction method for moistened potato starch. Starch 1983, 35, 407–410. [Google Scholar] [CrossRef]
- Schoch, T.J. Swelling power and solubility of granular starches. In Methods in Carbohydrate Chemistry, 4th ed.; Whistler, R.L., Ed.; Academic Press: New York, NY, USA, 1964; pp. 106–109. [Google Scholar]
- Guo, K.; Lin, L.; Fan, X.; Zhang, L.; Wei, C. Comparison of structural and functional properties of starches from five fruit kernels. Food Chem. 2018, 257, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Saeaurng, K.; Kuakpetoon, D. A comparative study of mango seed kernel starches and other commercial starches: The contribution of chemical fine structure to granule crystallinity, gelatinization, retrogradation, and pasting properties. J. Food Meas. Charact. 2018, 12, 2444–2452. [Google Scholar] [CrossRef]
- Lu, X.; Ma, R.; Zhan, J.; Wang, F.; Tian, Y. The role of protein and its hydrolysates in regulating the digestive properties of starch: A review. Trends Food Sci. Technol. 2022, 125, 54–65. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Fan, X.; Wang, Q.; Wang, P.; Zhang, Y.; Cui, L.; Yuan, J.; Yu, Y. Effect of disaccharides of different composition and linkage on corn and waxy corn starch retrogradation. Food Hydrocoll. 2016, 61, 531–536. [Google Scholar] [CrossRef]
- Cai, J.; Chao, C.; Niu, B.; Copeland, L.; Yu, J.; Wang, S.; Wang, S. New insight into the interactions among starch, lipid and protein in model systems with different starches. Food Hydrocoll. 2021, 112, 106323. [Google Scholar] [CrossRef]
- Noda, T.; Takigawa, S.; Matsuura-Endo, C.; Kim, S.-J.; Hashimoto, N.; Yamauchi, H.; Hanashiro, I.; Takeda, Y. Physicochemical properties and amylopectin structures of large, small, and extremely small potato starch granules. Carbohydr. Polym. 2005, 60, 245–251. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Norulaini, N.; Omar, M.A.K.; Yamauchi, H.; Noda, T. Correlations of the composition, minerals, and RVA pasting properties of various potato starches. Starch 2007, 59, 269–276. [Google Scholar] [CrossRef]
- Noda, T.; Matsuura-Endo, C.; Ishiguro, K. Preparation of iron-fortified potato starch and its properties. J. Food Sci. Technol. 2018, 55, 1360–1365. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; Jane, J.-L.; Bello-Perez, L.A. Physicochemical characteristics of starches from unripe fruits of mango and banana. Starch 2009, 61, 291–299. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Agama-Acevedo, E.; Ramos-Lopez, G.; Bello-Perez, L.A. Unripe mango kernel starch: Partial characterization. Food Hydrocoll. 2020, 101, 105512. [Google Scholar] [CrossRef]
- Fernandes, D.S.; Santos, T.P.R.; Fernandes, A.M.; Leonel, M. Harvest time optimization leads to the production of native cassava starches with different properties. Int. J. Biol. Macromol. 2019, 132, 710–721. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of amylose content on morphological, functional and emulsification properties of OSA modified corn starch. Food Hydrocoll. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Kalaivendan, R.G.T.; Mishra, A.; Eazhumalai, G.; Annapure, U.S. Effect of atmospheric pressure non-thermal pin to plate plasma on the functional, rheological, thermal, and morphological properties of mango seed kernel starch. Int. J. Biol. Macromol. 2022, 196, 63–71. [Google Scholar] [CrossRef]
- Shahrim, N.A.; Sarifuddin, N.; Ismail, H. Extraction and characterization of starch from mango seeds. J. Phy. Conf. Series 2018, 1082, 012019. [Google Scholar] [CrossRef]
- Lagunes-Delgado, C.; Agama-Acevedo, E.; Patiño-Rodríguez, O.; Martinez, M.M.; Bello-Pérez, L.A. Recovery of mango starch from unripe mango juice. LWT-Food Sci. Technol. 2022, 153, 112514. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Hasjim, J.; Gidley, M.J. Physicochemical and structural properties of maize and potatostarches as a function of granule size. J. Agric. Food Chem. 2011, 59, 10151–10161. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, P.; Zhu, J.; Hou, H.; Li, X.; Cui, B. Physicochemical properties of corn starch affected by the separation of granule shells. Int. J. Biol. Macromol. 2020, 164, 242–252. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Wei, Y.; Chen, J.; Shan, S.; Cao, F.; Chen, G.; Zou, Y. Amylose content and starch granule size in rice grains are affected by growing season. Phyton-Int. J. Exp. Bot. 2019, 88, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch-composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydrate Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Torres-Leon, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Paramasivam, S.K.; Saravanan, A.; Narayanan, S.; Shiva, K.N.; Ravi, I.; Mayilvaganan, M.; Raman Pushpa, R.; Uma, S. Exploring differences in the physicochemical, functional, structural, and pasting properties of banana starches from dessert, cooking, and plantain cultivars (Musa spp). Int. J. Biol. Macromol. 2021, 191, 1056–1067. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the crystallinity degree of starch having different types of crystal structure after mechanical pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars. LWT-Food Sci. Technol. 2019, 110, 197–206. [Google Scholar] [CrossRef]
- Chakraborty, I.N.P.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An insight into the gelatinization properties influencing the starches used in food industry: A review. Food Bioprocess Technol. 2022, 15, 1195–1223. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, F.; Douglas Gof, H.; Li, Y. Study on starch-protein interactions and their efects on physicochemical and digestible properties of the blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef]

| Variables (g/100 g) | Keitt | Palmer | Parwin | Tommy Atkins | |
|---|---|---|---|---|---|
| Moisture | Pulp | 8.66 ± 0.14 Ba | 8.67 ± 0.07 Ba | 10.93 ± 0.02 Aa | 8.91 ± 0.02 Bb |
| Kernel | 7.76 ± 0.17 Cb | 8.94 ± 0.01 Ba | 9.90 ± 0.03 Ab | 9.87 ± 0.05 Aa | |
| Dry basis | |||||
| Ash | Pulp | 0.03 ± 0.00 Ca | 0.04 ± 0.00 Ba | 0.05 ± 0.00 Aa | 0.03 ± 0.00 Ca |
| Kernel | 0.03 ± 0.00 Aa | 0.03 ± 0.00 Ab | 0.03 ± 0.00 Ab | 0.03 ± 0.00 Aa | |
| Fiber | Pulp | 0.13 ± 0.04 Bb | 0.19 ± 0.02 Ab | 0.20 ± 0.01 Ab | 0.21 ± 0.03 Ab |
| Kernel | 0.29 ± 0.02 Aa | 0.27 ± 0.04 Aa | 0.26 ± 0.02 Aa | 0.31 ± 0.02 Aa | |
| Lipids | Pulp | 0.44 ± 0.01 Bb | 0.44 ± 0.02 Bb | 0.75 ± 0.08 Ab | 0.41 ± 0.06 Bb |
| Kernel | 3.74 ± 0.02 Ca | 4.26 ± 0.07 Aa | 3.97 ± 0.06 Ba | 4.36 ± 0.08 Aa | |
| Protein | Pulp | 0.47 ± 0.06 Bb | 0.68 ± 0.06 Ab | 0.61 ± 0.06 Ab | 0.71 ± 0.02 Ab |
| Kernel | 1.57 ± 0.14 Aa | 1.53 ± 0.04 Aa | 1.48 ± 0.03 Aa | 1.61 ± 0.04 Aa | |
| Sugars | Pulp | 0.79 ± 0.02 Bb | 0.97 ± 0.03 Ab | 0.85 ± 0.04 Ab | 0.66 ± 0.02 Bb |
| Kernel | 2.57 ± 0.10 Ba | 3.22 ± 0.03 Aa | 2.97 ± 0.05 Aa | 3.12 ± 0.06 Aa | |
| Starch | Pulp | 97.94 ± 0.08 Aa | 97.64 ± 0.21 Aa | 98.09 ± 0.15 Aa | 98.03 ± 0.18 Aa |
| Kernel | 90.41 ± 0.08 Ab | 89.46 ± 0.11 Ab | 89.12 ± 0.17 Ab | 89.84 ± 0.11 Ab | |
| Amylose | Pulp | 17.17 ± 0.09 Bb | 16.20 ± 0.12 Cb | 18.44 ± 0.04 Ab | 18.95 ± 0.01 Ab |
| Kernel | 27.38 ± 0.13 Aa | 25.82 ± 0.10 Ba | 25.91 ± 0.03 Ba | 27.98 ± 0.04 Aa | |
| Minerals (g/Kg) | |||||
| P | Pulp | 0.04 ± 0.00 Cb | 0.13 ± 0.01 Bb | 0.32 ± 0.02 Aa | 0.06 ± 0.00 Cb |
| Kernel | 0.16 ± 0.01 Ca | 0.16 ± 0.01 Ca | 0.28 ± 0.01 Bb | 0.35 ± 0.02 Aa | |
| N | Pulp | 0.02 ± 0.01 Cb | 0.32 ± 0.01 Aa | 0.27 ± 0.01 Ba | 0.06 ± 0.01 Cb |
| Kernel | 0.14 ± 0.00 Ca | 0.17 ± 0.01 Cb | 0.27 ± 0.02 Ba | 0.35 ± 0.02 Aa | |
| K | Pulp | 0.88 ± 0.01 Ba | 0.78 ± 0.01 Ca | 1.32 ± 0.01 Aa | 0.99 ± 0.02 Ba |
| Kernel | 0.48 ± 0.00 Ab | 0.51 ± 0.01 Ab | 0.37 ± 0.01 Bb | 0.43 ± 0.02 Bb | |
| Ca | Pulp | 0.08 ± 0.01 Cb | 0.12 ± 0.01 Bb | 0.17 ± 0.01 Ab | 0.13 ± 0.02 Ba |
| Kernel | 0.15 ± 0.01 Ba | 0.17 ± 0.01 Ba | 0.22 ± 0.02 Aa | 0.15 ± 0.02 Ba | |
| Mg | Pulp | 0.05 ± 0.00 Bb | 0.04 ± 0.00 Bb | 0.09 ± 0.00 Aa | 0.06 ± 0.00 Bb |
| Kernel | 0.08 ± 0.00 Aa | 0.08 ± 0.00 Aa | 0.09 ± 0.00 Aa | 0.08 ± 0.00 Aa | |
| Fe | Pulp | 0.006 ± 0.00 Aa | 0.006 ± 0.00 Aa | 0.004 ± 0.00 Ba | 0.002 ± 0.00 Cb |
| Kernel | 0.004 ± 0.00 Bb | 0.005 ± 0.00 Ab | 0.004 ± 0.00 Ba | 0.004 ± 0.00 Ba | |
| Keitt | Palmer | Parwin | Tommy Atkins | ||
|---|---|---|---|---|---|
| D [4, 3] (µm) | Pulp | 15.79 ± 0.01 Cb | 16.24 ± 0.01 Bb | 16.07 ± 0.01 Bb | 16.70 ± 0.01 Ab |
| Kernel | 24.40 ± 0.01 Aa | 19.75 ± 0.01 Ba | 24.39 ± 0.01 Aa | 25.33 ± 0.01 Aa | |
| L* | Pulp | 88.24 ± 0.7 Aa | 86.88 ± 0.5 Ba | 81.70 ± 0.2 Ca | 78.15 ± 0.3 Ca |
| Kernel | 80.32 ± 0.9 Ab | 80.60 ± 0.3 Ab | 78.88 ± 0.6 Aa | 75.88 ± 0.3 Bb | |
| a* | Pulp | 3.41 ± 0.1 Cb | 3.71 ± 0.1 Bb | 4.08 ± 0.2 Ab | 3.16 ± 0.1 Cb |
| Kernel | 5.23 ± 0.2 Aa | 5.19 ± 0.1 Ba | 5.49 ± 0.1 Aa | 5.60 ± 0.1 Aa | |
| b* | Pulp | 0.55 ± 0.05 Bb | 0.35 ± 0.02 Bb | 0.11 ± 0.0 Cb | 2.89 ± 0.13 Ab |
| Kernel | 2.72 ± 0.1 Ba | 2.18 ± 0.13 Ca | 2.99 ± 0.12 Ba | 4.51 ± 0.1 Aa | |
| Yellowness index | Pulp | 0.89 ± 0.06 Bb | 0.58 ± 0.12 Cb | 0.19 ± 0.03 Db | 5.28 ± 0.11 Ab |
| Kernel | 4.84 ± 0.04 Ba | 3.86 ± 0.14 Ca | 5.41 ± 0.13 Ba | 8.49 ± 0.12 Aa | |
| Crystallinity (%) | Pulp | 31.70 ± 0.12 Aa | 32.24 ± 0.20 Aa | 32.35 ± 0.27 Aa | 30.31 ± 0.26 Ba |
| Kernel | 28.37 ± 0.03 Cb | 29.35 ± 0.34 Bb | 30.45 ± 0.38 Ab | 29.84 ± 0.13 Aa |
| Keitt | Palmer | Parwin | Tommy Atkins | ||
|---|---|---|---|---|---|
| Solubility (%) | |||||
| 55 °C | Pulp | 16.76 ± 0.15 Aa | 16.20 ± 0.12 Aa | 16.43 ± 0.09 Aa | 16.19 ± 0.18 Aa |
| Kernel | 15.76 ± 0.13 Ab | 15.34 ± 0.11 Ab | 15.26 ± 0.14 Ab | 15.29 ± 0.10 Ab | |
| 65 °C | Pulp | 22.58 ± 0.22 Aa | 22.45 ± 0.19 Aa | 22.25 ± 0.16 Aa | 22.16 ± 0.13 Aa |
| Kernel | 18.70 ± 0.15 Ab | 18.51 ± 0.13 Ab | 18.13 ± 0.09 Ab | 18.52 ± 0.17 Ab | |
| 75 °C | Pulp | 23.19 ± 0.26 Aa | 23.41 ± 0.21 Aa | 23.86 ± 0.14 Aa | 23.18 ± 0.20 Aa |
| Kernel | 23.44 ± 0.17 Aa | 23.35 ± 0.16 Aa | 23.86 ± 0.14 Aa | 23.51 ± 0.11 Aa | |
| 85 °C | Pulp | 16.70 ± 0.13 Aa | 16.91 ± 0.15 Aa | 16.36 ± 0.09 Aa | 16.80 ± 0.08 Aa |
| Kernel | 16.07 ± 0.22 Aa | 16.26 ± 0.24 Aa | 16.29 ± 0.27 Aa | 16.63 ± 0.18 Aa | |
| 95 °C | Pulp | 15.39 ± 0.12 Aa | 15.18 ± 0.13 Aa | 15.74 ± 0.12 Aa | 15.73 ± 0.15 Aa |
| Kernel | 13.42 ± 0.07 Ab | 13.78 ± 0.03 Ab | 13.84 ± 0.08 Ab | 13.46 ± 0.04 Ab | |
| Swelling power (g/g) | |||||
| 55 °C | Pulp | 2.06 ± 0.05 Aa | 2.18 ± 0.01 Aa | 2.25 ± 0.03 Aa | 2.09 ± 0.04 Aa |
| Kernel | 1.74 ± 0.02 Ab | 1.85 ± 0.04 Ab | 1.52 ± 0.07 Bb | 1.89 ± 0.09 Ab | |
| 65 °C | Pulp | 8.40 ± 0.13 Aa | 8.57 ± 0.18 Aa | 8.51 ± 0.22 Aa | 8.80 ± 0.32 Aa |
| Kernel | 3.79 ± 0.20 Ab | 3.73 ± 0.17 Ab | 3.22 ± 0.21 Ab | 3.57 ± 0.19 Ab | |
| 75 °C | Pulp | 14.86 ± 0.15 Ab | 14.12 ± 0.14 Ab | 14.35 ± 0.13 Ab | 14.21 ± 0.08 Ab |
| Kernel | 15.39 ± 0.11 Aa | 15.34 ± 0.09 Aa | 15.20 ± 0.12 Aa | 15.21 ± 0.05 Aa | |
| 85 °C | Pulp | 14.41 ± 0.10 Aa | 14.54 ± 0.09 Aa | 14.63 ± 0.15 Aa | 14.29 ± 0.16 Aa |
| Kernel | 13.72 ± 0.09 Ab | 13.07 ± 0.07 Ab | 13.08 ± 0.12 Ab | 13.51 ± 0.11 Ab | |
| 95 °C | Pulp | 12.16 ± 0.08 Aa | 12.14 ± 0.15 Aa | 12.07 ± 0.10 Aa | 12.11 ± 0.13 Aa |
| Kernel | 9.94 ± 0.05 Ab | 9.24 ± 0.07 Ab | 9.16 ± 0.11 Ab | 9.24 ± 0.09 Ab | |
| Keitt | Palmer | Parwin | Tommy Atkins | ||
|---|---|---|---|---|---|
| Pasting properties | |||||
| Peak viscosity (cP) | Pulp | 3619 ± 0.74 Ab | 3171 ± 0.54 Bb | 3373 ± 2.50 Ba | 3648 ± 0,40 Ab |
| Kernel | 4081 ± 1.28 Aa | 4040 ± 0.77 Aa | 3554 ± 0.04 Ba | 3884 ± 0.35 Aa | |
| Breakdown (cP) | Pulp | 2553 ± 1.47 Aa | 2132 ± 0.60 Bb | 2205 ± 2.94 Ba | 2332 ± 3.13 Aa |
| Kernel | 2352 ± 2.65 Aa | 2344 ± 1.12 Aa | 1850 ± 1.53 Ba | 2140 ± 2.08 Ba | |
| Final viscosity (cP) | Pulp | 1606 ± 1.32 Bb | 1615 ± 0.57 Bb | 1706 ± 1.26 Bb | 1964 ± 2.46 Ab |
| Kernel | 2778 ± 0.10 Aa | 2733 ± 0.62 Aa | 2685 ± 0.24 Aa | 2736 ± 3.18 Aa | |
| Set Back (cP) | Pulp | 540 ± 1.96 Bb | 576 ± 0.86 Bb | 538 ± 0.33 Bb | 648 ± 0.00 Ab |
| Kernel | 1048 ± 1.21 Aa | 1037 ± 1.16 Aa | 981 ± 3.39 Ba | 1031 ± 5.63 Aa | |
| Peak Time (min) | Pulp | 3.10 ± 0.00 Bb | 3.30 ± 0.00 Ab | 3.30 ± 0.00 Ab | 3.30 ± 0.00 Ab |
| Kernel | 4.16 ± 1.13 Aa | 4.16 ± 1.13 Aa | 4.16 ± 1.13 Aa | 4.10 ± 0.00 Aa | |
| Pasting temperature (°C) | Pulp | 69.42 ± 0.15 Bb | 69.82 ± 0.96 Ab | 71.07 ± 0.05 Ab | 71.02 ± 0.00 Ab |
| Kernel | 74.32 ± 0.05 Aa | 74.65 ± 0.76 Aa | 74.65 ± 0.66 Aa | 74.37 ± 0.05 Aa | |
| Thermal properties | |||||
| To (°C) | Pulp | 64.57 ± 0.07 Bb | 63.70 ± 0.11 Cb | 64.55 ± 0.62 Bb | 65.75 ± 0.43 Ab |
| Kernel | 67.73 ± 0.40 Aa | 66.75 ± 0.05 Ba | 66.90 ± 0.09 Ba | 67.03 ± 0.11 Ba | |
| Tp (°C) | Pulp | 68.22 ± 0.05 Bb | 66.71 ± 0.29 Cb | 67.99 ± 0.56 Bb | 69.57 ± 0.47 Ab |
| Kernel | 72.31 ± 0.0 Aa | 71.39 ± 0.05 Ba | 71.57 ± 0.15 Ba | 71.47 ± 0.22 Ba | |
| Tc (°C) | Pulp | 72.37 ± 0.14 Bb | 70.34 ± 0.34 Cb | 71.79 ± 0.43 Bb | 73.40 ± 0.34 Ab |
| Kernel | 77.22 ± 0.41 Aa | 76.29 ± 0.12 Ba | 76.05 ± 0.0 Ba | 76.22 ± 0.17 Ba | |
| ΔT (°C) | Pulp | 7.80 ± 0.07 Ab | 6.64 ± 0.22 Cb | 7.24 ± 0.19 Bb | 7.65 ± 0.09 Ab |
| Kernel | 9.49 ± 0.82 Aa | 9.54 ± 0.17 Aa | 9.15 ± 0.08 Ba | 9.19 ± 0.05 Ba | |
| ΔH (J/g) | Pulp | 12.60 ± 0.24 Aa | 13.13 ± 0.69 Aa | 11.26 ± 0.89 Bb | 10.63 ± 0.20 Cb |
| Kernel | 12.58 ± 0.55 Ba | 13.56 ± 0.88 Aa | 12.70 ± 1.04 Ba | 12.54 ± 0.17 Ba | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lossolli, N.A.B.; Leonel, M.; Leonel, S.; Izidoro, M.; de Paula, G.V.; dos Santos, T.P.R.; de Oliveira, L.A. Cultivars and Fruit Part as Differentiating Factors of Physicochemical Characteristics of Mango Starches. Horticulturae 2023, 9, 69. https://doi.org/10.3390/horticulturae9010069
Lossolli NAB, Leonel M, Leonel S, Izidoro M, de Paula GV, dos Santos TPR, de Oliveira LA. Cultivars and Fruit Part as Differentiating Factors of Physicochemical Characteristics of Mango Starches. Horticulturae. 2023; 9(1):69. https://doi.org/10.3390/horticulturae9010069
Chicago/Turabian StyleLossolli, Nathalia Aparecida Barbosa, Magali Leonel, Sarita Leonel, Maiqui Izidoro, Gustavo Veiga de Paula, Thais Paes Rodrigues dos Santos, and Luciana Alves de Oliveira. 2023. "Cultivars and Fruit Part as Differentiating Factors of Physicochemical Characteristics of Mango Starches" Horticulturae 9, no. 1: 69. https://doi.org/10.3390/horticulturae9010069





