Potential of Suaeda nudiflora and Suaeda fruticosa to Adapt to High Salinity Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Sampling Site
2.2. Collection and Analysis of the Samples
2.3. Soil Analysis
2.4. Determination of Soluble Ions in Plants
2.5. Determination of Osmo-Protective Compounds
2.6. Determination of Total Phenolic Content (TPC) and Antioxidant Activity
2.7. Determination of Metal Contents and Bioaccumulation Factor in Plants
- (BAF) Leaves: [Metal in leaves tissue]/[Metal in soils]
- (BAF) Stem: [Metal in stem tissue]/[Metal in soils]
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Experimental Design and Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Accumulation of Soluble Ions in Plants
3.3. Accumulation of Osmo-Protective Compounds
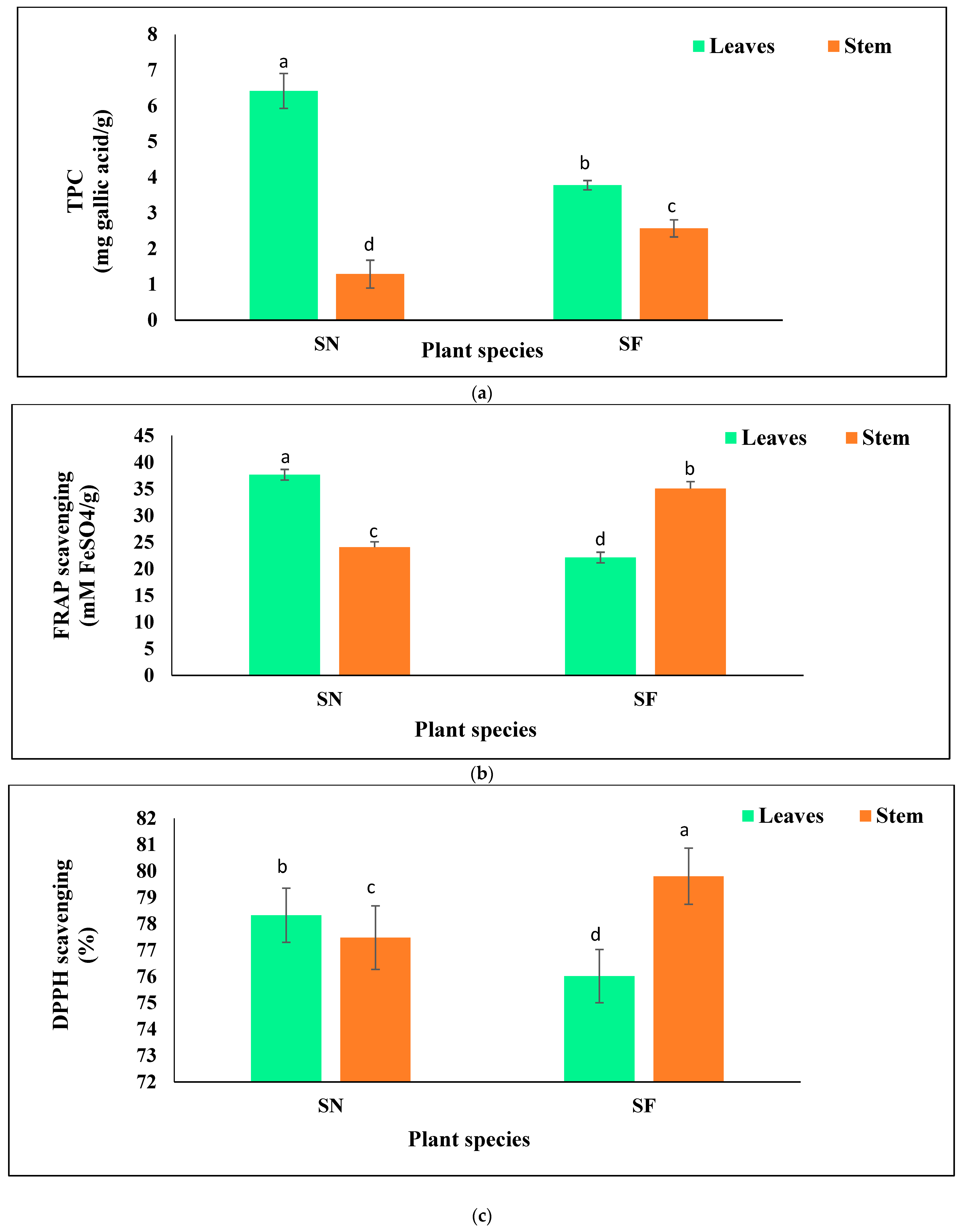
3.4. Estimation of TPC and Antioxidant Activity
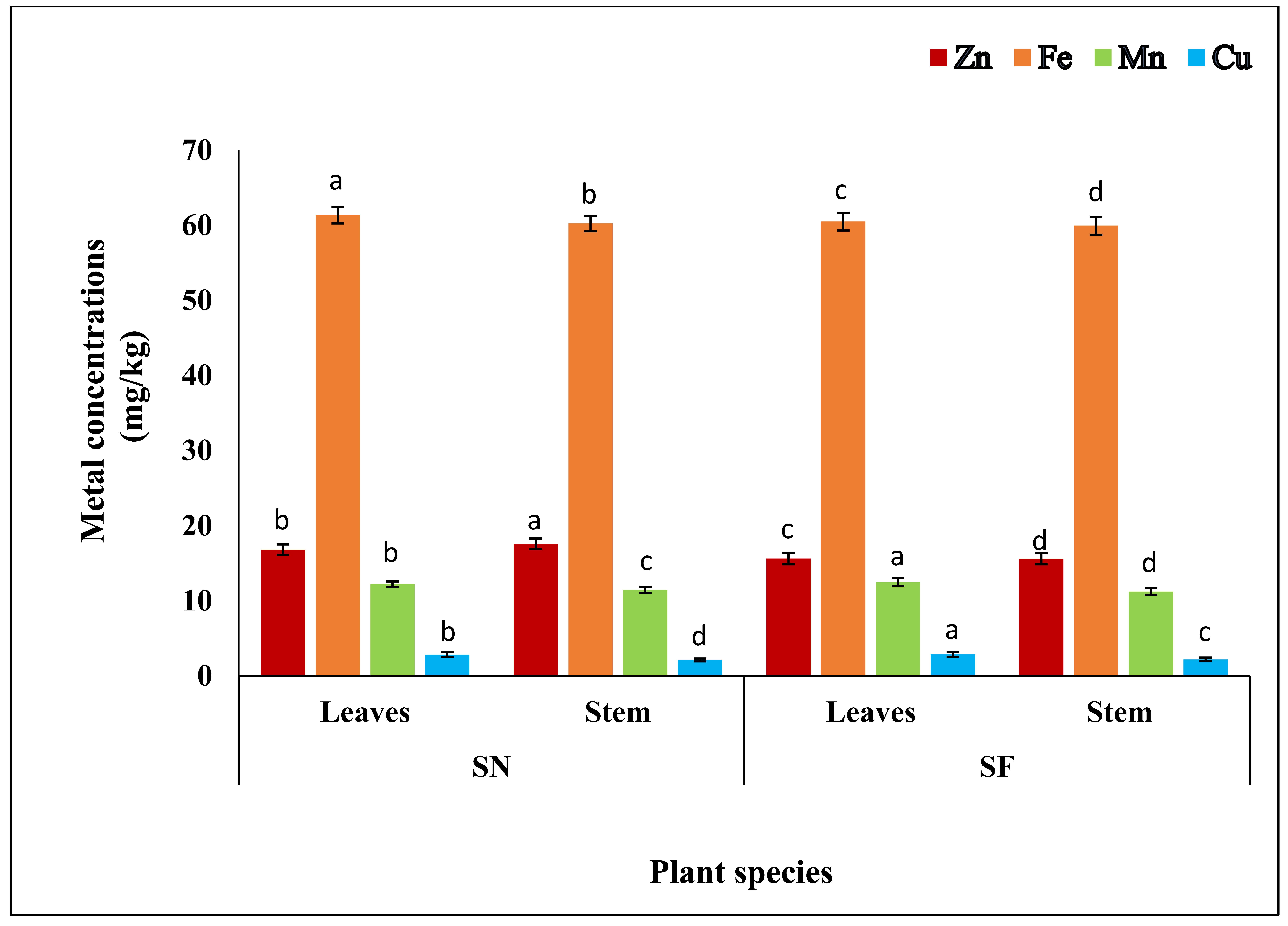
3.5. Estimation of Metal Concentrations in Leaves and Stem Parts of Plants
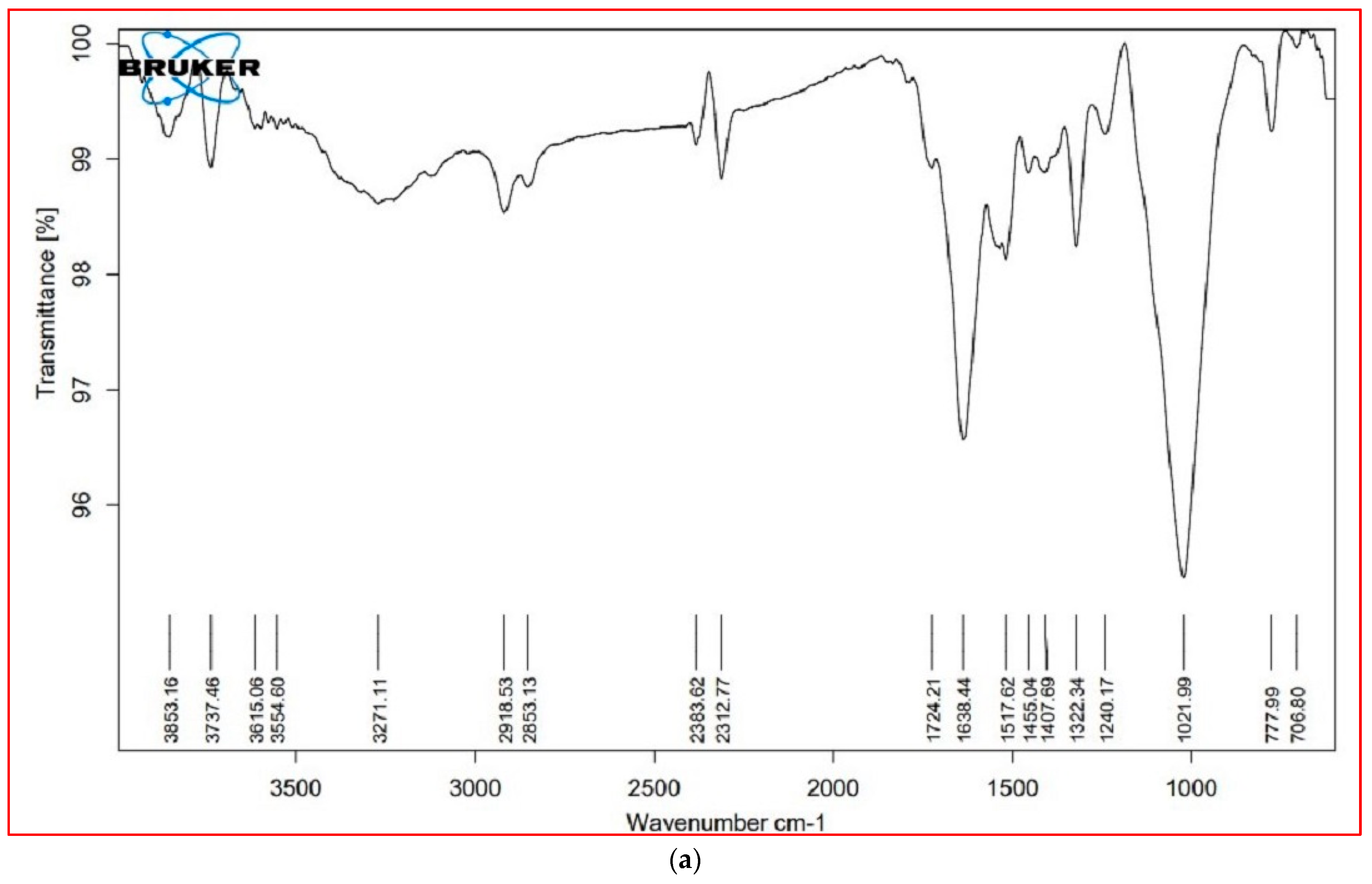
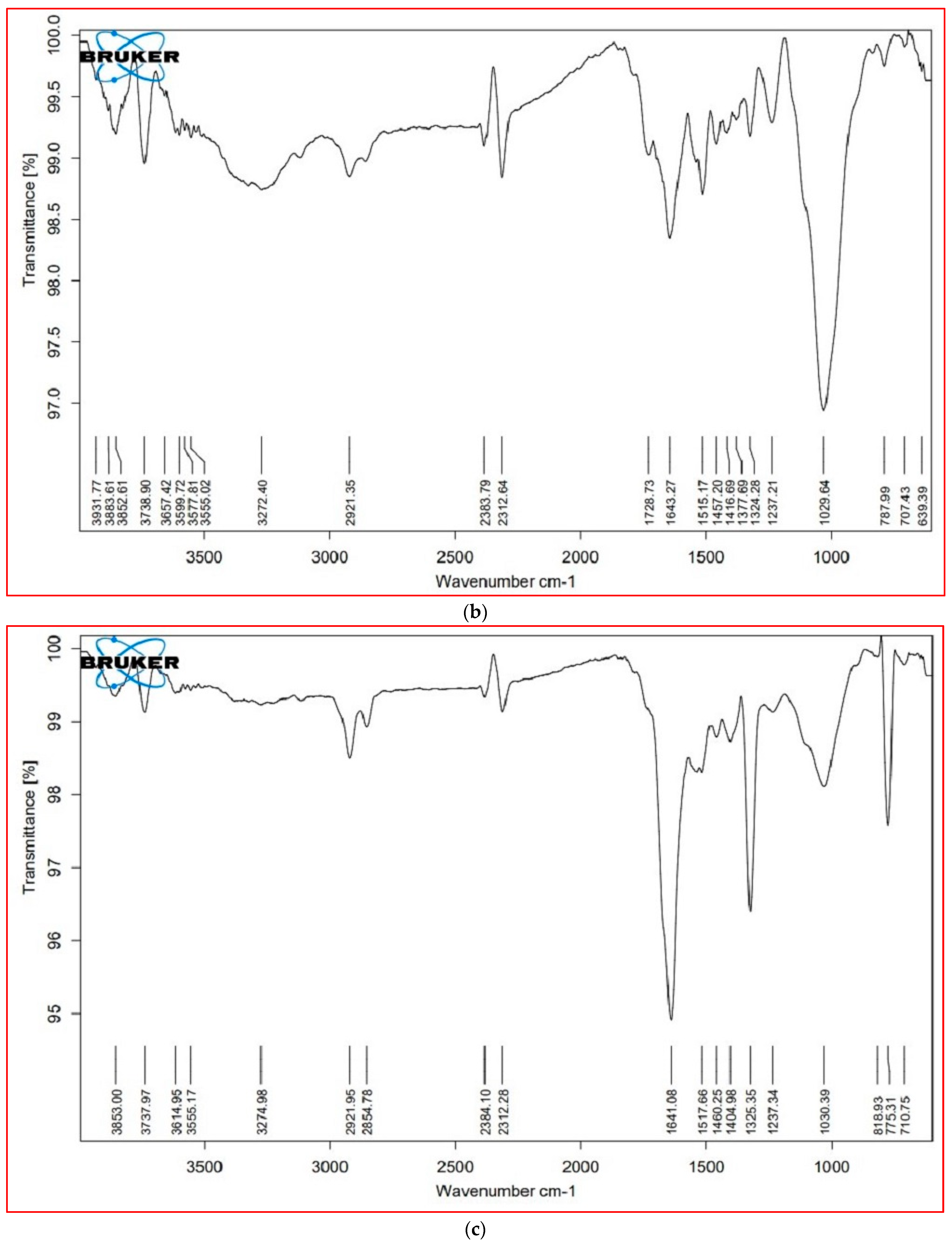
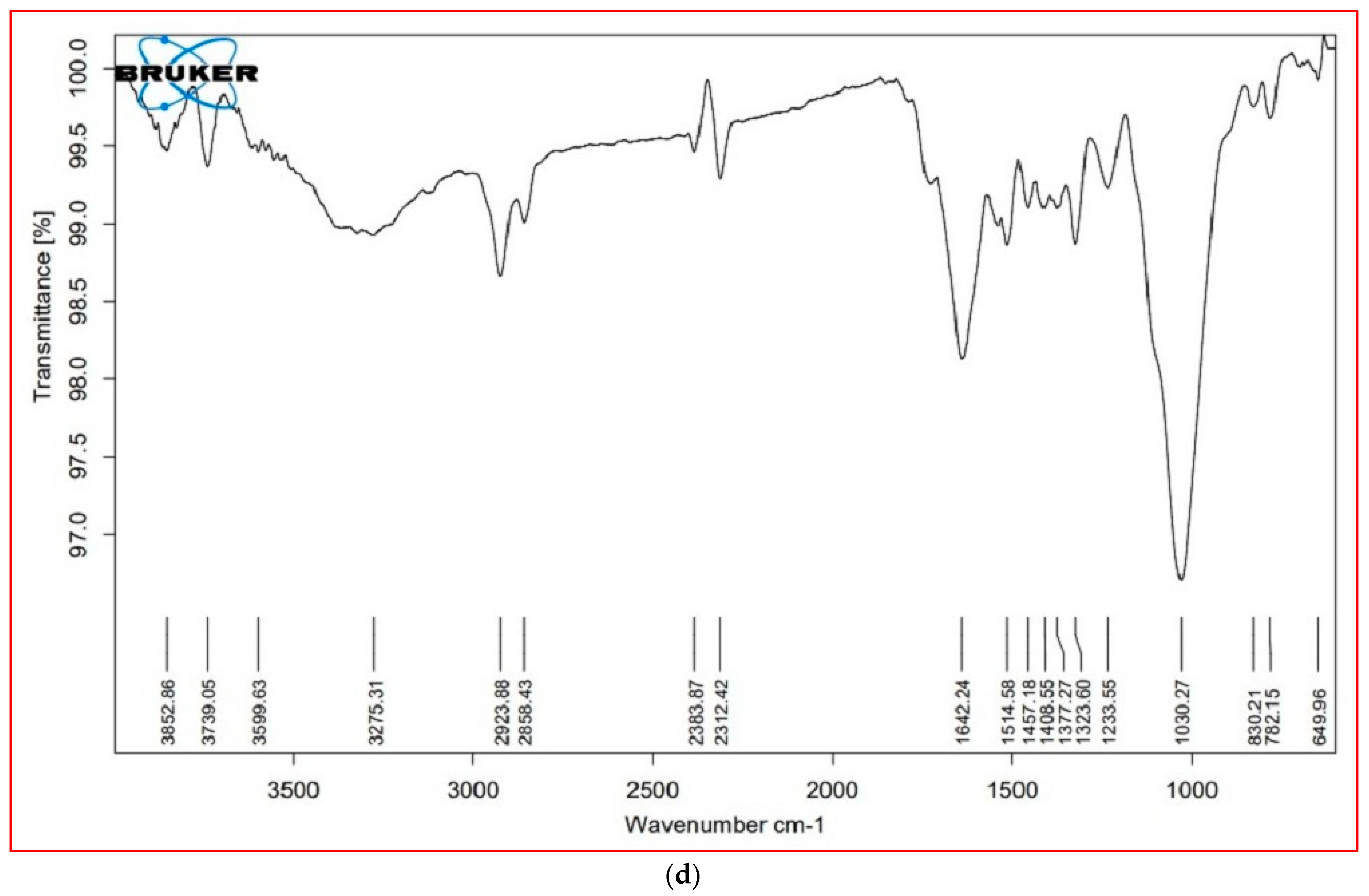
3.6. FTIR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Map of Salt-affected Soils (GSASmap). 2021. Available online: https://www.fao.org/global-soil-partnership/gsasmap/en/ (accessed on 16 January 2022).
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques, 1st ed.; Zaman, M., Ed.; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Dagar, J.C. Salinity research in India: An overview. Bull. Natl. Inst. Ecol. 2005, 15, 69–80. [Google Scholar]
- Mandal, A.K.; Reddy, G.O.; Ravisankar, T.; Yadav, R.K. Computerized database of salt-affected soils for coastal region of India. J. Soil Salin. Water Qual. 2018, 10, 1–13. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Mishra, A.; Tanna, B. Halophytes: Potential resources for salt stress tolerance genes and promoters. Front. Plant Sci. 2017, 8, 829. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A. Ectopic expression of C4 photosynthetic pathway genes improves carbon assimilation and alleviate stress tolerance for future climate change. Physiol. Mol. Biol. Plants. 2020, 26, 195–209. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Srivastava, A.K.; Pandey, G.K.; Suprasanna, P. Halophytes in biosaline agriculture: Mechanism, utilization, and value addition. Land Degrad. Dev. 2018, 29, 1081–1095. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Morphological and anatomical adaptations of halophytes: A review. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture, 1st ed.; Grigore, M.N., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 1079–1221. [Google Scholar]
- Al Hassan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unravelling salt tolerance mechanisms in halophytes: A comparative study on four Mediterranean Limonium species with different geographic distribution patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Podar, D.; Macalik, K.; Réti, K.O.; Martonos, I.; Török, E.; Carpa, R.; Székely, G. Morphological, physiological and biochemical aspects of salt tolerance of halophyte Petrosimonia triandra grown in natural habitat. Physiol. Mol. Biol. Plants. 2019, 25, 1335–1347. [Google Scholar] [CrossRef]
- Joshi, A.; Kanthaliya, B.; Rajput, V.; Minkina, T.; Arora, J. Assessment of phytoremediation capacity of three halophytes: Suaeda monoica, Tamarix indica and Cressa critica. Biologia Futr. 2020, 71, 301–312. [Google Scholar] [CrossRef]
- Joshi, A.; Kanthaliya, B.; Arora, J. Halophytic Plant Existence in Indian Salt Flats: Biodiversity, Biology, and Uses. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar]
- Joshi, A.; Kanthaliya, B.; Arora, J. Halophytes: The Nonconventional Crops as Source of Biofuel Production. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture, 1st ed.; Grigore, M.N., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Joshi, A.; Kanthaliya, B.; Arora, J. Halophytes of Thar Desert: Potential source of nutrition and feedstuff. Int. J. Bioass. 2018, 8, 5674–5683. [Google Scholar]
- Sharma, V.; Joshi, A.; Ramawat, K.G.; Arora, J. Bioethanol production from halophytes of Thar Desert: A “green gold”. In Environment at Crossroads: Challenges, Dynamics and Solutions; Basu, S.K., Zandi, P., Chalaras, S.K., Eds.; Haghshenass Publishing: Guilan Prov, Iran, 2017; pp. 219–235. [Google Scholar]
- Haque, M.I.; Siddiqui, S.A.; Jha, B.; Rathore, M.S. Interactive Effects of Abiotic Stress and Elevated CO2 on Physio-Chemical and Photosynthetic Responses in Suaeda Species. J. Plant Growth Regul. 2021, 41, 2930–2948. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Wen, W.; Yang, G. The antioxidant system in Suaeda salsa under salt stress. Plant Signal. Behav. 2020, 15, 1771939. [Google Scholar] [CrossRef]
- Joshi, A.; Kanthaliya, B.; Arora, J. Evaluation of growth and antioxidant activity in Suaeda monoica and Suaeda nudiflora callus cultures under sequential exposure to saline conditions. Curr. Biotechnol. 2019, 8, 42–52. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Lata, C.; Kumar, S. Eco-Physiological responses of Aeluropus lagopoides (grass halophyte) and Suaeda nudiflora (non-grass halophyte) under individual and interactive sodic and salt stress. S. Afr. J. Bot. 2016, 105, 36–44. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, T.; Gulzar, S.; Aziz, I.; Gul, B.; Khan, M.A. Salt tolerance of a cash crop halophyte Suaeda fruticosa: Biochemical responses to salt and exogenous chemical treatments. Acta Physiol. Plant. 2012, 34, 2331–2340. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef]
- Ghanem, A.E.M.F.; Mohamed, E.; Kasem, A.M.; El-Ghamery, A.A. Differential salt tolerance strategies in three halophytes from the same ecological habitat: Augmentation of antioxidant enzymes and compounds. Plants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Ltaeif, H.B.; Sakhraoui, A.; González-Orenga, S.; LandaFaz, A.; Boscaiu, M.; Vicente, O.; Rouz, S. Responses to salinity in four Plantago species from Tunisia. Plants 2021, 10, 1392. [Google Scholar] [CrossRef]
- Sinha, R. The Sambhar Lake: The Largest Saline Lake in Northwestern India. In Landscapes and Landforms of India: World Geomorphological Landscapes; Kale, V., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 239–244. [Google Scholar]
- Cherekar, M.N.; Pathak, A.P. Chemical assessment of Sambhar Soda Lake, a Ramsar site in India. J. Water Chem. Technol. 2016, 38, 244–247. [Google Scholar] [CrossRef]
- USDA Handbook. Diagnosis and Improvement of Saline and Alkali Soils; Richards, L.A., Ed.; Oxford & IBH Publ. Co. Pvt. Ltd.: New Delhi, India, 1960; p. 60. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939); US Department of Agriculture: Washington, DC, USA, 1954.
- Merwin, H.D.; Peech, M. Exchangeability of soil potassium in the sand, silt, and clay fractions as influenced by the nature of the complementary exchangeable cation 1. Soil Sci. Soc. Am. J. 1951, 15, 125–128. [Google Scholar] [CrossRef]
- Prakash, L.; Prathapasenan, G. Efect of NaCl salinity and putrescine on shoot growth, tissue ion concentration and yield of rice (Oryzasativa L. var. GR-3). J. Agron. Crop Sci. 1988, 160, 325–334. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem. J. 2003, 74, 289–297. [Google Scholar] [CrossRef]
- Bates, C.J.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Farkas, G.L.; Kiraly, Z. Role of phenolic compound in the physiology of plant diseases and disease resistance. Phytopathol Zeitsch. 1962, 44, 105–150. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharma. Bulletin. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Petelka, J.; Abraham, J.; Bockreis, A.; Deikumah, J.P.; Zerbe, S. Soil heavy metal (loid) pollution and phytoremediation potential of native plants on a former gold mine in Ghana. Water Air Soil Pollu. 2019, 230, 267. [Google Scholar] [CrossRef]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. In Climate Change and Agriculture; Intech Open: London, UK, 2019; p. 13. [Google Scholar] [CrossRef]
- Kar, A. The Thar or the Great Indian Sand Desert. In Landscapes and Landforms of India; Kale, V.S., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 79–90. [Google Scholar]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Ghazaryan, K.A.; Movsesyan, H.S.; Minkina, T.M.; Sushkova, S.N.; Rajput, V.D. The identification of phytoextraction potential of Melilotus officinalis and Amaranthus retroflexus growing on copper-and molybdenum-polluted soils. Environ. Geochem. Health. 2021, 43, 1327–1335. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, P.; Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Mangalassery, S.; Dayal, D.; Kumar, A.; Bhatt, K.; Nakar, R.; Kumar, A.; Misra, A.K. Pattern of salt accumulation and its impact on salinity tolerance in two halophyte grasses in extreme saline desert in India. Indian J. Exp. Biol. 2017, 55, 542–548. [Google Scholar]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants. 2014, 6, plu049. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahman, M.A.; Miah, M.G.; Saha, S.R.; Karim, M.A.; Mostofa, M.G. Mechanistic insight into salt tolerance of Acacia auriculiformis: The importance of ion selectivity, osmoprotection, tissue tolerance, and Na + exclusion. Front. Plant Sci. 2017, 8, 155. [Google Scholar] [CrossRef]
- Ahmed, H.A.I.; Shabala, L.; Shabala, S. Understanding the mechanistic basis of adaptation of perennial Sarcocornia quinqueflora species to soil salinity. Physiol. Plant. 2021, 172, 1997–2010. [Google Scholar] [CrossRef]
- Kumar, A.; Mann, A.; Kumar, A.; Kumar, N.; Meena, B.L. Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Int. J. Phytoremediation 2021, 23, 1041–1051. [Google Scholar] [CrossRef]
- Chaudhary, D.R. Ion accumulation pattern of halophytes. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; Hasanuzzaman, M., Shabala, S., Fujita, M., Eds.; CAB International Publishing: Wallingford, UK, 2019; pp. 137–151. [Google Scholar]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.P. Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Ahmadi, F.; Mohammadkhani, N.; Servati, M. Halophytes play important role in phytoremediation of salt-affected soils in the bed of Urmia Lake, Iran. Sci. Rep. 2022, 12, 12223. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, K.C.; Venkatesan, K.; Balakrishnan, V.; Chellappan, K.P.; Balasubramanian, T. Restoration of saline land by halophytes for Indian soils. Soil Biol. Biochem. 2007, 39, 2661–2664. [Google Scholar] [CrossRef]
- Devi, S.; Nandwal, A.S.; Angrish, R.; Arya, S.S.; Kumar, N.; Sharma, S.K. Phytoremediation potential of some halophytic species for soil salinity. Int. J. Phytoremediation 2016, 18, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Domènech, L.L.; Tifrea, A.; Grigore, M.N.; Boscaiu, M.; Vicente, O. Proline and glycine betaine accumulation in two succulent halophytes under natural and experimental conditions. Plant Biosyst. 2016, 150, 904–915. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Parida, A.K.; Panda, A.; Rangani, J. Metabolomics-guided elucidation of abiotic stress tolerance mechanisms in plants. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 89–131. [Google Scholar]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Marco, P.; Carvajal, M.; del Carmen Martinez-Ballesta, M. Efficient leaf solute partioning in Salicornia fruticosa allows growth under salinity. Environ. Exper. Bot. 2019, 157, 177–186. [Google Scholar] [CrossRef]
- Mali, B.S.; Chitale, R.D. Comparision of accumulation of organic and inorganic osmolyte in Trianthema portulacastrum L. growing in saline and non-saline habitats. Eco. Env. Cons. 2020, 26, 155–158. [Google Scholar]
- Akcin, A.; Yalcin, E. Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata Pall. and Suaeda prostrata Pall. subsp. prostrata (Amaranthaceae). Braz. J. Bot. 2016, 39, 101–106. [Google Scholar] [CrossRef]
- Youssef, A.M. Salt tolerance mechanisms in some halophytes from Saudi Arabia and Egypt. Res. J. Agricul. Biol. Sci. 2009, 5, 191–206. [Google Scholar]
- Ibraheem, F.; Al-Zahrani, A.; Mosa, A. Physiological adaptation of three wild halophytic Suaeda species: Salt tolerance strategies and metal accumulation capacity. Plants 2022, 11, 537. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Mandzhieva, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic content changes in plants under salt stress. In Ecophysiology and responses of plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Reginato, M.; Cenzano, A.M.; Arslan, I.; Furlán, A.; Varela, C.; Cavallin, V.; Luna, V. Na2SO4 and NaCl salts differentially modulate the antioxidant systems in the highly stress tolerant halophyte Prosopis strombulifera. Plant Physiol. Biochem. 2021, 167, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kanthaliya, B.; Meena, S.; Rajput, V.D.; Minkina, T.; Arora, J. Proteomic and Genomic Approaches to Study Plant Physiological Responses under Heavy Metal Stress. In Heavy Metal Toxicity in Plants: Physiological and Molecular Adaptations; Aftab, T., Hakeem, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 231–247. [Google Scholar]
- Sánchez-Gavilán, I.; Rufo, L.; Rodriguez, N.; de la Fuente, V. On the elemental composition of the Mediterranean euhalophyte Salicornia patula Duval-Jouve (Chenopodiaceae) from saline habitats in Spain (Huelva, Toledo and Zamora). Environ. Sci. Pollu. Res. 2021, 28, 2719–2727. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Semenkov, I.; Klink, G.; Tarigholizadeh, S.; Sushkova, S. Phylogenetic analysis of hyperaccumulator plant species for heavy metals and polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2021, 43, 1629–1654. [Google Scholar] [CrossRef]
- Caparrós, P.G.; Ozturk, M.; Gul, A.; Batool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Altay, V.; Toderich, K.N. Halophytes have potential as heavy metal phytoremediators: A comprehensive review. Environ. Exper. Bot. 2022, 193, 104666. [Google Scholar] [CrossRef]
- Alam, M.R.; Islam, R.; Tran, T.K.A.; Le Van, D.; Rahman, M.M.; Griffin, A.S.; Yu, R.M.K.; MacFarlane, G.R. Global patterns of accumulation and partitioning of metals in halophytic saltmarsh taxa: A phylogenetic comparative approach. J. Hazard. Mater. 2021, 414, 125515. [Google Scholar] [CrossRef]
- Mousavi Kouhi, S.M.; Moudi, M. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted saline–sodic soil. Environ. Sci. Pollu. Res. 2020, 27, 10027–10038. [Google Scholar] [CrossRef] [PubMed]
- Afifi, A.A.; Youssef, R.A.; Hussein, M.M. Fourier transform infrared spectometry study on early stage of salt stress in Jujube plant. Life Sci. J. 2013, 10, 1973–1981. [Google Scholar]
- Akyuz, S.; Akyuz, T.; Celik, O.; Atak, C. FTIR spectroscopy of protein isolates of salt-tolerant soybean mutants. J. Appl. Spectroscopy. 2018, 84, 1019–1023. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Kumar, J.; Nikam, T.D.; Suprasanna, P. FT-IR profiling reveals differential response of roots and leaves to salt stress in a halophyte Sesuvium portulacastrum (L.) L. Biotechnol. Rep. 2019, 23, e00352. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Parameters | Values |
|---|---|---|
| 1 | Soil pH | 9.89 ± 0.6 |
| 2 | ECe (dS/m−1) | 65 ± 0.7 |
| 3 | Organic carbon (%) | 0.19 ± 0.04 |
| 4 | P2O5 (kg/ha) | 8.49 ± 0.32 |
| 5 | K2O (kg/ha) | 84.13 ± 0.79 |
| 6 | Na+ (mg/100 g dry soil) | 1485 ± 16.11 |
| 7 | K+ (mg/100 g dry soil) | 41.23 ± 2.4 |
| 8 | Ca2+ (mg/100 g dry soil) | 19.51 ± 1.8 |
| 9 | Cl− (mg/100 g dry soil) | 1.02 ± 0.74 |
| 10 | Iron (mg/kg) | 4185 ± 70.4 |
| 11 | Zinc (mg/kg) | 38.64 ± 1.85 |
| 12 | Manganese (mg/kg) | 131.9 ± 4.6 |
| 13 | Copper (mg/kg) | 6.47 ± 0.97 |
| Species | Plant Parts | Na+ | K+ | Ca2+ | Cl− | Na+/K+ Ratio |
|---|---|---|---|---|---|---|
| S. nudiflora | Leaves | 45.56 ± 1.34 b | 12.65 ± 1.05 b | 11.72 ± 1.12 b | 11.68 ± 1.21 b | 3.60 |
| Stem | 21.32 ± 1.08 d | 7.63 ± 0.98 d | 6.89 ± 0.65 d | 5.56 ± 0.99 d | 2.79 | |
| S. fruticosa | Leaves | 71.01 ± 1.71 a | 19.54 ± 1.45 a | 11.54 ± 0.91 a | 14.02 ± 1.01 a | 3.63 |
| Stem | 23.84 ± 1.19 c | 8.55 ± 0.77 c | 7.90 ± 0.84 c | 7.11 ± 1.18 c | 2.78 |
| Species | Plant Part | Proline Content (µmoles/g) | Soluble Sugar Content (mg/g) | Soluble Protein Content (mg/g) |
|---|---|---|---|---|
| S. nudiflora | Leaves | 7.99 ± 0.20 d | 9.87 ± 0.36 a | 7.23 ± 0.91 a |
| Stem | 22.41 ± 0.30 a | 6.35 ± 0.38 b | 3.24 ± 0.13 c | |
| S. fruticosa | Leaves | 8.82 ± 0.14 c | 9.43 ± 0.24 d | 4.37 ± 0.41 b |
| Stem | 18.57 ± 0.90 b | 6.02 ± 0.26 d | 1.98 ± s0.12 d |
| Species | Plant Parts | Metals | |||
|---|---|---|---|---|---|
| Zn | Fe | Mn | Cu | ||
| S. nudiflora | Leaves | 0.435 ± 0.17 | 0.014 ± 0.01 | 0.092 ± 0.05 | 0.438 ± 0.21 |
| Stem | 0.455 ± 0.23 | 0.014 ± 0.01 | 0.086 ± 0.03 | 0.329 ± 0.14 | |
| S. fruticosa | Leaves | 0.404 ± 0.15 | 0.014 ± 0.01 | 0.094 ± 0.07 | 0.446 ± 0.20 |
| Stem | 0.403 ± 0.11 | 0.014 ± 0.01 | 0.085 ± 0.03 | 0.341 ± 0.16 |
| Cellular Metabolites | Wavenumber (cm−1) | Probable Functional Group | |||
|---|---|---|---|---|---|
| S. nudiflora | S. fruticosa | ||||
| Leaves | Stem | Leaves | Stem | ||
| Lipids (3000–2000 cm−1) | 3853.16 | 3825.61 | 3853.00 | 3852.86 | O–H stretch (alcohols, phenols) |
| ±0.94 | ±0.91 | ±1.02 | ±0.98 | ||
| 3737.46 | 3738.90 | 3737.97 | 3739.05 | O–H stretch (alcohols) | |
| ±0.72 | ±0.51 | ±0.64 | ±0.85 | ||
| * | 2921.35 | 2921.95 | 2923.88 | O–H stretch (alcohols), S, O–H stretch (carboxylic acids), =C–H (benzene, alkynes, alkenes) | |
| ±0.63 | ±0.41 | ±0.33 | |||
| 2853.13 | * | 2854.78 | * | C–H stretch (alkenes), H–C=O:C–H stretch (aldehydes) | |
| ±0.55 | ±0.61 | ||||
| 2383.62 | 2383.79 | 2384.10 | 2383.87 | P–H (phosphine) | |
| ±0.52 | ±0.49 | ±0.52 | ±0.37 | ||
| 2312.77 | 2312.64 | 2312.28 | 2312.42 | C=C stretch (alkynes) | |
| ±0.43 | ±0.64 | ±0.50 | ±0.73 | ||
| Proteins (1800–1500 cm−1) | 1724.21 | 1728.73 | * | * | C=O (esters, carboxylic acids, ketones, aldehydes), C=C (benzenes) |
| ±0.39 | ±0.42 | ||||
| 1638.44 | 1643.27 | 1641.08 | 1642.24 | N-H bend (nitro compounds, amides), C–C stretch (amides), C=O stretch (carboxylic acid, ketone), C=C (benzene, alkenes) | |
| ±0.13 | ±0.28 | ±0.21 | ±0.26 | ||
| 1517.62 | 1515.17 | 1517.66 | 1514.58 | N-H bend (nitro compounds), C–O stretch (amides), C=C (benzenes), C=O (ketones) | |
| ±0.18 | ±0.25 | ±0.10 | ±0.14 | ||
| Carbohydrates (1500– 1000 cm−1) | 1455.04 | 1457.20 | 1460.25 | 1457.18 | C–C stretch (aromatics), C–H bend (alkanes), N–O stretch (nitro compounds), C-O stretch (esters), CO–H bend (aldehydes), O–H bend (alcohols |
| ±0.16 | ±0.23 | ±0.24 | ±0.20 | ||
| * | 1377.69 | * | 1377.27 | N=O, CO–H band, O–H band | |
| ±0.57 | ±0.13 | ||||
| 1322.34 | 1322.34 | ±0.27 | 1323.60 | S(=O)2 stretch (sulfones), N=O stretch (nitro compounds), O–H bend (carboxylic acids, alcohols) | |
| ±0.27 | ±0.63 | ||||
| 1240.17 | 1237.21 | 1237.34 | 1233.55 | C–N stretch (amines), C–O stretch (esters), C–O stretch (ethers, alcohols), O–H band (carboxylic acids) | |
| ±0.31 | ±0.29 | ±0.25 | ±0.54 | ||
| 1021.99 | 1029.64 | 1030.39 | 1030.27 | S=O stretch (sulfoxides), C–N stretch (amines), C–O stretch (esters, ethers, alcohols), =C–H bend (benzene, alkenes) (cellulose) | |
| ±0.18 | ±0.23 | ±0.12 | ±0.20 | ||
| Cell wall components (1000– 600 cm−1) | * | * | 818.93 | 830.21 | C–N stretch (amines), =C–H bend (benzene, alkynes) (xyloglucan) |
| ±0.11 | ±0.16 | ||||
| 777.99 | 787.99 | 775.31 | 782.15 | C–N stretch (amines), =C–H bend (benzene), C–C stretch | |
| ±0.08 | ±0.15 | ±0.12 | ±0.16 | ||
| * | 639.39 | * | 649.96 | C–N stretch (amines), =C–H bend (Bbenzene), C–C stretch (chloride) | |
| ±0.23 | ±0.31 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, A.; Rajput, V.D.; Verma, K.K.; Minkina, T.; Ghazaryan, K.; Arora, J. Potential of Suaeda nudiflora and Suaeda fruticosa to Adapt to High Salinity Conditions. Horticulturae 2023, 9, 74. https://doi.org/10.3390/horticulturae9010074
Joshi A, Rajput VD, Verma KK, Minkina T, Ghazaryan K, Arora J. Potential of Suaeda nudiflora and Suaeda fruticosa to Adapt to High Salinity Conditions. Horticulturae. 2023; 9(1):74. https://doi.org/10.3390/horticulturae9010074
Chicago/Turabian StyleJoshi, Abhishek, Vishnu D. Rajput, Krishan K. Verma, Tatiana Minkina, Karen Ghazaryan, and Jaya Arora. 2023. "Potential of Suaeda nudiflora and Suaeda fruticosa to Adapt to High Salinity Conditions" Horticulturae 9, no. 1: 74. https://doi.org/10.3390/horticulturae9010074
APA StyleJoshi, A., Rajput, V. D., Verma, K. K., Minkina, T., Ghazaryan, K., & Arora, J. (2023). Potential of Suaeda nudiflora and Suaeda fruticosa to Adapt to High Salinity Conditions. Horticulturae, 9(1), 74. https://doi.org/10.3390/horticulturae9010074











