The Effects of Enhanced Ultraviolet-B Radiation on Leaf Photosynthesis and Submicroscopic Structures in Mangifera indica L. cv. ‘Tainong No 1’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design and Treatment Method
2.3. Sampling for Biochemical Assays
2.4. Experimental Methods and Techniques
2.4.1. Determination of Leaf Physiological and Biochemical Parameters
2.4.2. Observation of Leaf Anatomy and Chloroplast Ultrastructure
2.4.3. Quantitative Real–Time PCR (qRT–PCR)
2.5. Statistical Analysis
3. Results and Analysis
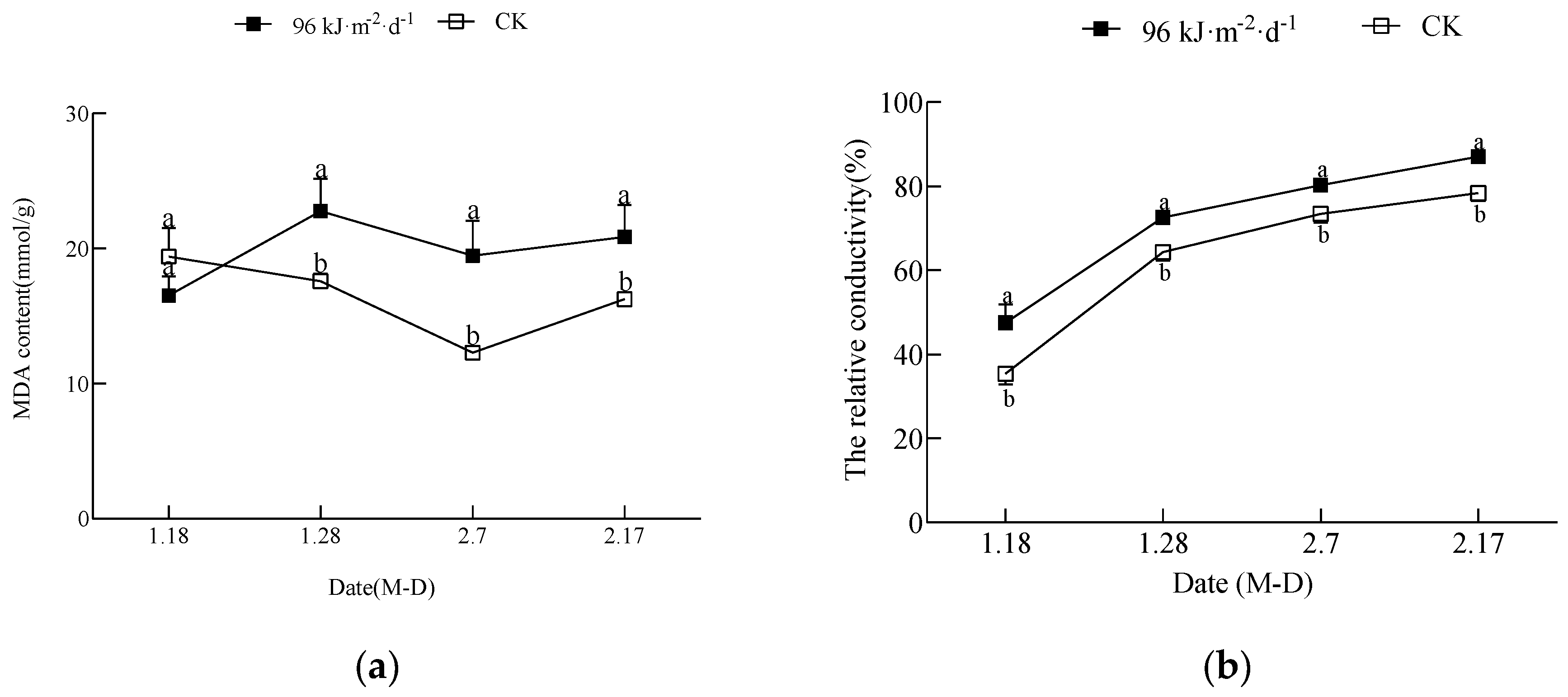
3.1. Effect of Enhanced UV-B Radiation on Leaves
3.2. Effect of Enhanced UV-B Radiation on the Photosynthetic Properties of Leaves
3.3. Enhanced UV-B Radiation Treatment Effect on the Photosynthetic Pigment Content of Leaves
3.4. Effect of Enhanced UV-B Radiation on the Stomata and Epidermal Submicrostructure of Leaves
3.5. Effect of Enhanced UV-B Radiation on the Submicrostructure of Mesophyll Cells in Leaves
3.6. Effect of Enhanced UV-B Radiation on the Submicrostructure of Mesophyll Cells in Leaves
3.7. Effect of Enhanced UV-B Radiation on the Gene Expression of Rubisco Major and Minor Subunits in Leaves
4. Discussion
4.1. The Submicrostructure of the Choloplast is Damaged and the Expression of the rbcL Gene Is Inhibited by High−Dose Enhanced UV-B Radiation, Leading to Nonstomatal Limitation of Photosynthesis in Mango Leaves
4.2. Stomatal Morphology is Destroyed and Density Is Reduced by High−Dose Enhanced UV-B Radiation, Leading to Stomatal Limitation of Photosynthesis in Mango Leaves
4.3. Passive Defense Mechanism Induced by High−Dose Enhanced UV-B Radiation in Mango Leaves
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.B.; Guan, D.X.; Yuan, F.H.; Zhang, X.J. Research advances on the biological effects of elevated ultraviolet-B radiation on terrestrial plants. J. For. Res. 2009, 20, 383–390. [Google Scholar] [CrossRef]
- Madronich, S.; McKenzie, R.L.; Caldwell, M.; Bjorn, L.O. Changes in ultraviolet radiation reaching the Earth’s surface. Ambio 1995, 24, 143–152. [Google Scholar]
- Markham, K.R.; Tanner, G.J.; Caasi-Lit, M.; Whitecross, M.I.; Nayudu, M.; Mitchell, K.A. Possible protective role for 3′,4′-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry 1998, 49, 1913–1919. [Google Scholar] [CrossRef]
- Wright, L.A.; Murphy, T.M. Short-Wave Ultraviolet Light Closes Leaf Stomata. Am. J. Bot. 1982, 69, 1196. [Google Scholar] [CrossRef]
- Wu, X.C.; Lin, W.X.; Guo, Y.C.; Ke, Y.Q.; Liang, Y.Y.; Chen, Y.F. Effect of enhancing ultraviolet-b radiation on antioxidant systems in rice seedling leaves. Fujian J. Agric. Sci. 2001, 16, 51–55. [Google Scholar]
- Bais, A.F.; Bernhard, G.; McKenzie, R.L.; Aucamp, P.J.; Young, P.J.; Ilyas, M.; Deushi, M. Ozone–climate interactions and effects on solar ultraviolet radiation. Photochem. Photobiol. Sci. 2019, 18, 602–640. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.P.; Li, X.; Li, Z.R.; Ling, C.T.; He, Y.M.; Li, Y. Research progress on the characteristics and mechanisms of transgenerational plasticity of plants in response to enhanced UV-B radiation. Plant Physiol. J. 2022, 58, 797–805. (In Chinese) [Google Scholar]
- Taalas, P.; Kaurola, J.; Kylling, A.; Shindell, D.; Sausen, R.; Dameris, M.; Steil, B. The impact of greenhouse gases and halogenated species on future solar UV radiation doses. Geophys. Res. Lett. 2000, 27, 1127–1130. [Google Scholar] [CrossRef] [Green Version]
- De Bakker, N.V.J.; Van Bodegom, P.M.; Van De Poll, W.H.; Boelen, P.; Nat, E.; Rozema, J.; Aerts, R. Is UV-B radiation affecting charophycean algae in shallow freshwater systems? New Phytol. 2005, 166, 957–966. [Google Scholar] [CrossRef]
- Correia, C.M.; Torres-Pereira, M.S.; Torres-Pereira, J. Growth, photosynthesis and UV-B absorbing compounds of Portuguese Barbela wheat exposed to ultraviolet-B radiation. Environ. Pollut. Ser. A 1999, 104, 383–388. [Google Scholar] [CrossRef]
- Flores-Moya, A.; Hanelt, D.; Figueroa, F.L.; Altamirano, M.; Viñegla, B.; Salles, S. Involvement of solar UV-B radiation in recovery of inhibited photosynthesis in the brown alga Dictyota dichotoma (Hudson) Lamouroux. J. Photochem. Photobiol. B 1999, 49, 129–135. [Google Scholar] [CrossRef]
- Hu, Z.; Li, H.; Chen, S.; Yang, Y. Chlorophyll content and photosystem II efficiency in soybean exposed to supplemental ultraviolet-B radiation. Photosynthetica 2013, 51, 151–157. [Google Scholar] [CrossRef]
- Heinze, M.; Hanschen, F.S.; Wiesner-Reinhold, M.; Baldermann, S.; Gräfe, J.; Schreiner, M.; Neugart, S. Effects of Developmental Stages and Reduced UVB and Low UV Conditions on Plant Secondary Metabolite Profiles in Pak Choi (Brassica rapa subsp. chinensis). J. Agric. Food Chem. 2018, 66, 1678–1692. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Teramura, A.H.; Tevini, M. The changing solar ultraviolet climate and the ecological consequences for higher plants. Trends Ecol. Evol. 1989, 4, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.W.; Zhang, X.S.; Liu, L.K.; Guo, Y.; Fan, Y.L.; Yang, X.Y.; Li, Y.X. Comparing intraspecific responses of 12 winter wheat cultivars to different doses of ultraviolet-B radiation. J. Photochem. Photobiol. B 2013, 119, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Z.C. Differential response of leaf gas exchange to enhanced ultraviolet-B (UV-B) radiation in three species of herbaceous climbing plants. Acta Ecol. Sin. 2009, 29, 124–129. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Conf. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, X.Q.; Zhao, C.Z.; Cheng, X.Y. Effects of enhanced UV-B radiation on growth and photosynthetic responses of four species of seedling in subalpine forests of the eastern Tibet plateau. Environ. Exp. Bot. 2011, 74, 151–156. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U.; Lbtz, C.; Wiencke, C. Ultrastructure and photosynthesis in the supralittoral green macroalga Prasiola crispa from Sp itsbergen (Norway) under UV exposure. Phycologia 2006, 45, 168–172. [Google Scholar] [CrossRef]
- Robert, L.H.; Lester, P. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar]
- Deshmukh, P.S.; Sairam, R.K.; Shukla, D.S. Measurement of ionleakage as a screening technique for drought resistance in wheat genotypes. Indian J. Plant Physiol. 1991, 34, 89–91. [Google Scholar]
- Wang, H.; Guo, Y.J.; Zhu, J.J.; Yue, K.; Zhou, K.B. Characteristics of Mango Leaf Photosynthetic Inhibition by Enhanced UV-B Radiation. Horticulturae 2021, 7, 557. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, Q. Influence of lanthanum on chloroplast ultrastructure of soybean leaves under ultraviolet-B stress. J. Rare Earths. 2009, 27, 304–307. [Google Scholar] [CrossRef]
- Wu, X.C.; Lin, W.X.; Huang, Z.L. Influence of enhanced ultraviolet-B radiation on photosynthetic physiologies andultrastructure of leaves in two different resistivity rice cultivars. J. Plant Ecol. 2007, 27, 554–564. (In Chinese) [Google Scholar]
- Wang, J.; Zhang, J.; Yang, J.X.; Wang, S.M.; Tian, L.P. Effects of Enhanced UV-B Radiation on the Leaves Microstructure of Cotton. Xinjiang Agric. Sci. 2010, 47, 1619–1626. (In Chinese) [Google Scholar]
- Wu, N.B.; Ma, H.Q.; Hu, L.T.; Hong, H.; Sun, J.C.; Zhang, Y.H.; Dai, D.L. Effect of enhanced UV-B radiation on photosynthetic structure and photosynthetic characteristics of Mentha piperita. China J. Chin. Mater. Med. 2009, 34, 2995–2998. [Google Scholar] [PubMed]
- Yang, Y.J.; Guo, S.P.; Yang, S.L.; Zhang, Y.H.; Liu, H.G.; Meng, F.X.; Duan, Y.J.; Yang, Z.X.; Yang, X.Q.; Yuan, J.M.; et al. Effects of enhanced UV-B radiation on photosynthetic physiology and ultrastructure of leaves in mango (Mangifera indica L.). J. Fruit Sci. 2021, 38, 1524–1539. (In Chinese) [Google Scholar]
- Liu, P.; Zhou, K.B.; Ding, S.; Cai, H.Z. Antioxidative Response of Adult Mango(Mangifera indica L.) Leaves to Enhanced UV-radiation. J. Mount. Agric. Biotech. 2010, 29, 397–402. (In Chinese) [Google Scholar]
- He, J.M.; She, X.P.; Liu, C.; Zhao, W.M. Stomatal and nonstomatal limitations of photosynthesis in mung bean leaves under the combination of enhanced UV-B radiation and NaCl stress. J. Plant Physiol. Mol. Biol. 2004, 30, 53–58. [Google Scholar]
- Pandey, N.; Pandey-Rai, S. Modulations of physiological responses and possible involvement of defense-related secondary metabolites in acclimation of Artemisia annua L. against short-term UV-B radiation. Planta 2014, 240, 611–627. [Google Scholar] [CrossRef]
- Jordan, B.R.; Chow, W.S.; Strid, Å.; Anderson, J.M. Reduction incabandpsbA RNA transcripts in response to supplementary ultraviolet-B radiation. FEBS Lett. 1991, 284, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.; Srivastava, G.; Prasad, S.M.; Abraham, G. Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Pestic. Biochem. Physiol. 2008, 92, 30–37. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Keiller, D.R.; Holmes, M.G. Effects of long-term exposure to elevated UV-B radiation on the photosynthetic performance of five broad-leaved tree species. Photosynth. Res. 2001, 67, 229–240. [Google Scholar] [CrossRef]
- Surabhi, G.; Reddy, K.R.; Singh, S.K. Photosynthesis, fluorescence, shoot biomass and seed weight responses of three cowpea (Vigna unguiculata (L.) Walp.) cultivars with contrasting sensitivity to UV-B radiation. Environ. Exp. Bot. 2009, 66, 160–171. [Google Scholar] [CrossRef]
- Bornman, J.F.; Teramura, A.H. Effects of Ultraviolet-B Radiation on Terrestrial Plants. In Environmental UV Photobiology; Plenum Press: New York, NY, USA, 1993; pp. 427–471. [Google Scholar]
- Musil, C.F. Differential effects of elevated ultraviolet-B radiation on the photochemical and reproductive performances of dicotyledonous and monocotyledonous arid-environment ephemerals. Plant Cell Environ. 1995, 18, 844–854. [Google Scholar] [CrossRef]
- Moody, S.A.; Coop, D.J.; Paul, N.D. Plants and UV-B: Effects of elevated UV-B radiation and elevated CO2 on heathland communities. Soc. Exp. Biol. Semin. Ser. 1997, 64, 283–304. [Google Scholar]
- Nogués, S.; Allen, D.J.; Morison, J.I.L.; Baker, N.R. Characterization of Stomatal Closure Caused by Ultraviolet-B Radiation. Plant Physiol. 1999, 121, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Teramura, A.H.; Sullivan, J.H. Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth. Res. 1994, 39, 463–473. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K. Responses of the Morphological and Anatomical Structure of the Plant Leaf to Environmental Change. Chih Wu Hsueh T’ung Pao. 2005, 22, 18–127. (In Chinese) [Google Scholar]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Mohammed, A.R. Effects of ultraviolet-B radiation on cotton (Gossypium hirsutum L.) morphology and anatomy. Ann.Bot. 2003, 91, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Y.; An, L.Z.; Tan, L.L.; Hou, Z.D.; Wang, X.L. Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 taxa in vitro. Environ. Exp. Bot. 2000, 43, 45–53. [Google Scholar] [CrossRef]
- Kofidis, G.; Bosabalidis, A.M.; Moustakas, M. Contemporary seasonal and altitudinal variations of leaf structural features in oregano (Origanum vulgare L.). Ann. Bot. 2003, 92, 635–645. [Google Scholar] [CrossRef] [PubMed]





| Gene Name | Primers |
|---|---|
| rbcL | F: 5′−ACGCCGGTACAGTAGTAGGT−3′ |
| R: 5′−GAATCCCCAGTCCAACCACG−3′ | |
| rbcS | F: 5′−TCAGGCCTCCATTGTTGACC−3′ |
| R: 5′−ACCTGCATGCATTGGACTCT−3′ | |
| Actin | F: 5′−ATCTGCTGGAAGGTGCTGAG−3′ |
| R: 5′−CCAAGCAGCATGAAGATCAA−3′ |
| Date | Treatment | Chl a (mg·g−1) | Chl b (mg·g−1) | Chl (a + b) (mg·g−1) | Chl a/b | Caro (mg·g−1) |
|---|---|---|---|---|---|---|
| 1.18 | CK | 1.16 ± 0.26 a | 0.24 ± 0.07 a | 1.89 ± 0.25 a | 3.24 ± 0.09 a | 0.32 ± 0.02 a |
| T | 0.95 ± 0.10 a | 0.27 ± 0.01 a | 1.21 ± 0.10 b | 3.14 ± 0.08 a | 0.23 ± 0.04 b | |
| 1.28 | CK | 1.38 ± 0.05 a | 0.41 ± 0.01 a | 1.82 ± 0.04 a | 2.96 ± 0.03 a | 0.30 ± 0.02 a |
| T | 1.25 ± 0.11 a | 0.412 ± 0.05 a | 1.67 ± 0.10 a | 2.88 ± 0.03 a | 0.24 ± 0.02 b | |
| 2.7 | CK | 1.30 ± 0.09 a | 0.41 ± 0.06 a | 1.72 ± 0.14 a | 3.07 ± 0.04a | 0.28 ± 0.02 a |
| T | 0.87 ± 0.10 b | 0.24 ± 0.06 b | 1.11 ± 0.15 b | 2.85 ± 0.04 b | 0.22 ± 0.01 b | |
| 2.17 | CK | 1.35 ± 0.04 a | 0.42 ± 0.02 a | 1.70 ± 0.10 a | 2.88 ± 0.02 a | 0.33 ± 0.01 a |
| T | 1.12 ± 0.07 b | 0.28 ± 0.02 b | 1.38 ± 0.09 b | 2.45 ± 0.17 b | 0.27 ± 0.01 b |
| Date | Treatment | Stomatal Density/Number·cm−2 | Upper Epidermis Thickness/μm | Lower Epidermis Thickness/μm | Palisade Tissue Thickness/μm | Spongy Tissue Thickness/μm | Palisade Spongy Ratios | Leaf Blade Thickness/μm |
|---|---|---|---|---|---|---|---|---|
| 2.7 | CK | 694.8 ± 8.1 a | 12.0 ± 1.0 a | 13.0 ± 0.8 a | 74.9 ± 1.8 b | 100.3 ± 2.3 b | 1.0 ± 0.05 a | 227.1 ± 6.2 b |
| T | 605.4 ± 8.1 b | 13.8 ± 1.5 a | 11.3 ± 0.8 a | 103.8 ± 2.5 a | 140.7 ± 2.9 a | 0.7 ± 0.01 b | 289.2 ± 5.9 a | |
| 2.17 | CK | 697.0 ± 4.1 a | 9.7 ± 1.6 b | 10.1 ± 1.2 a | 100.6 ± 2.4 b | 110.1 ± 1.6 b | 0.7 ± 0.01 a | 228.4 ± 2.0 b |
| T | 662.8 ± 1.6 b | 15.6 ± 1.0 a | 7.9 ± 0.8 a | 119.1 ± 8.2 a | 162.5 ± 2.7 a | 0.5 ± 0.07 b | 263.5 ± 8.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Peng, J.; Qian, M.; Shui, X.; Du, J.; Liu, F.; Zhou, K. The Effects of Enhanced Ultraviolet-B Radiation on Leaf Photosynthesis and Submicroscopic Structures in Mangifera indica L. cv. ‘Tainong No 1’. Horticulturae 2023, 9, 83. https://doi.org/10.3390/horticulturae9010083
Chen T, Peng J, Qian M, Shui X, Du J, Liu F, Zhou K. The Effects of Enhanced Ultraviolet-B Radiation on Leaf Photosynthesis and Submicroscopic Structures in Mangifera indica L. cv. ‘Tainong No 1’. Horticulturae. 2023; 9(1):83. https://doi.org/10.3390/horticulturae9010083
Chicago/Turabian StyleChen, Tiantian, Junjie Peng, Minjie Qian, Xian Shui, Jingjia Du, Feng Liu, and Kaibing Zhou. 2023. "The Effects of Enhanced Ultraviolet-B Radiation on Leaf Photosynthesis and Submicroscopic Structures in Mangifera indica L. cv. ‘Tainong No 1’" Horticulturae 9, no. 1: 83. https://doi.org/10.3390/horticulturae9010083







