Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Morphological Traits
2.3. Fragrance Analysis and Volatile Compounds Investigation
2.3.1. Sensory Analysis
2.3.2. GC-MS Analysis
2.4. Chemical Characterization of Rose Petal Methanol Extracts
2.4.1. Preparation of Methanol Extracts
2.4.2. Determination of Total Phenolic Content
2.4.3. Determination of Total Flavonoid Content
2.4.4. Determination of Total Monomeric Anthocyanin Content
2.4.5. Determination of Vitamin C Content
2.4.6. Quantitative Analysis of Selected Phenolic Compounds
2.5. Antioxidant Potential
2.5.1. DPPH Assay
2.5.2. FRAP Assay
2.6. Neuroprotective Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphological Traits
3.2. Fragrance Analysis and Volatile Compounds Investigation
3.2.1. Sensory Evaluation
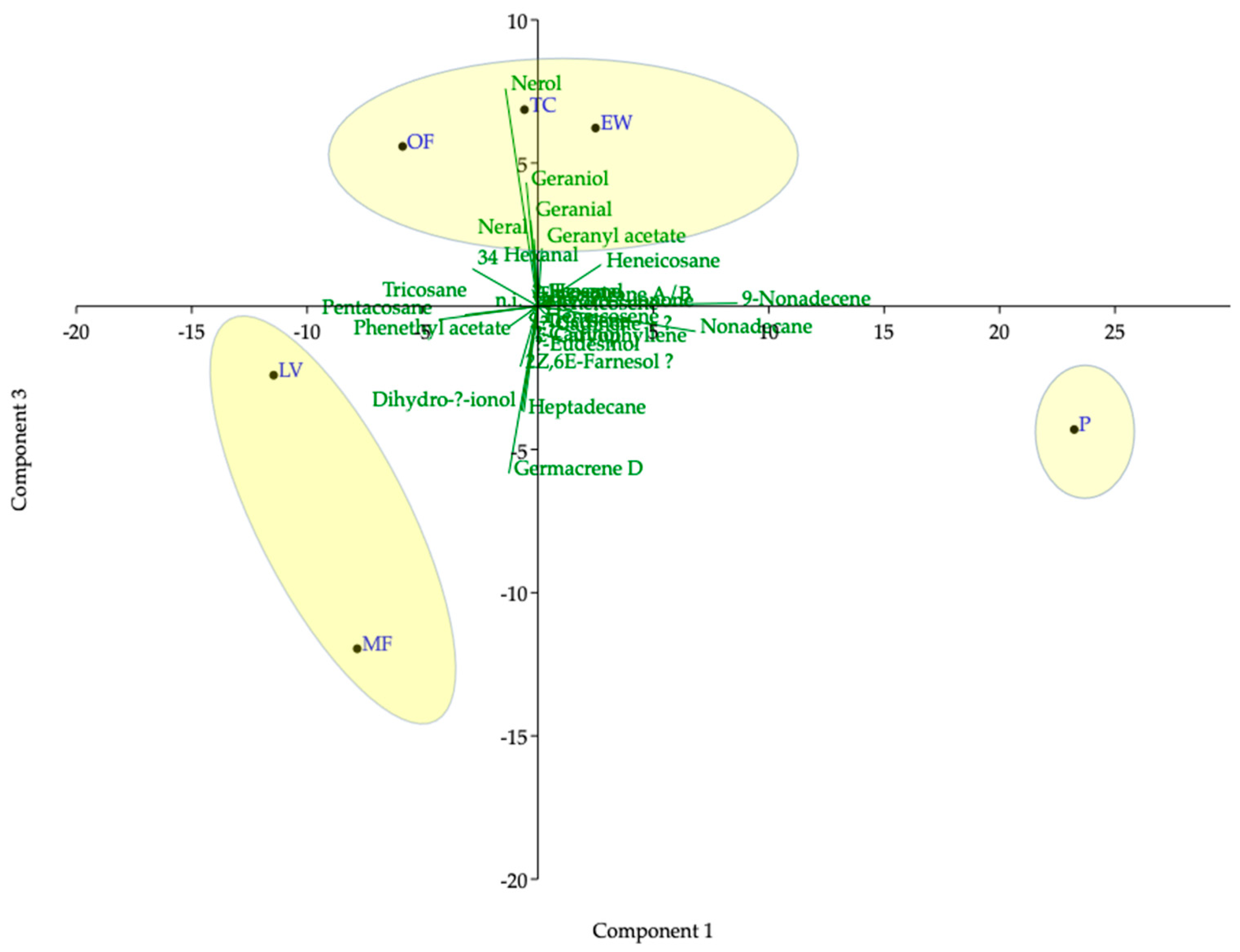
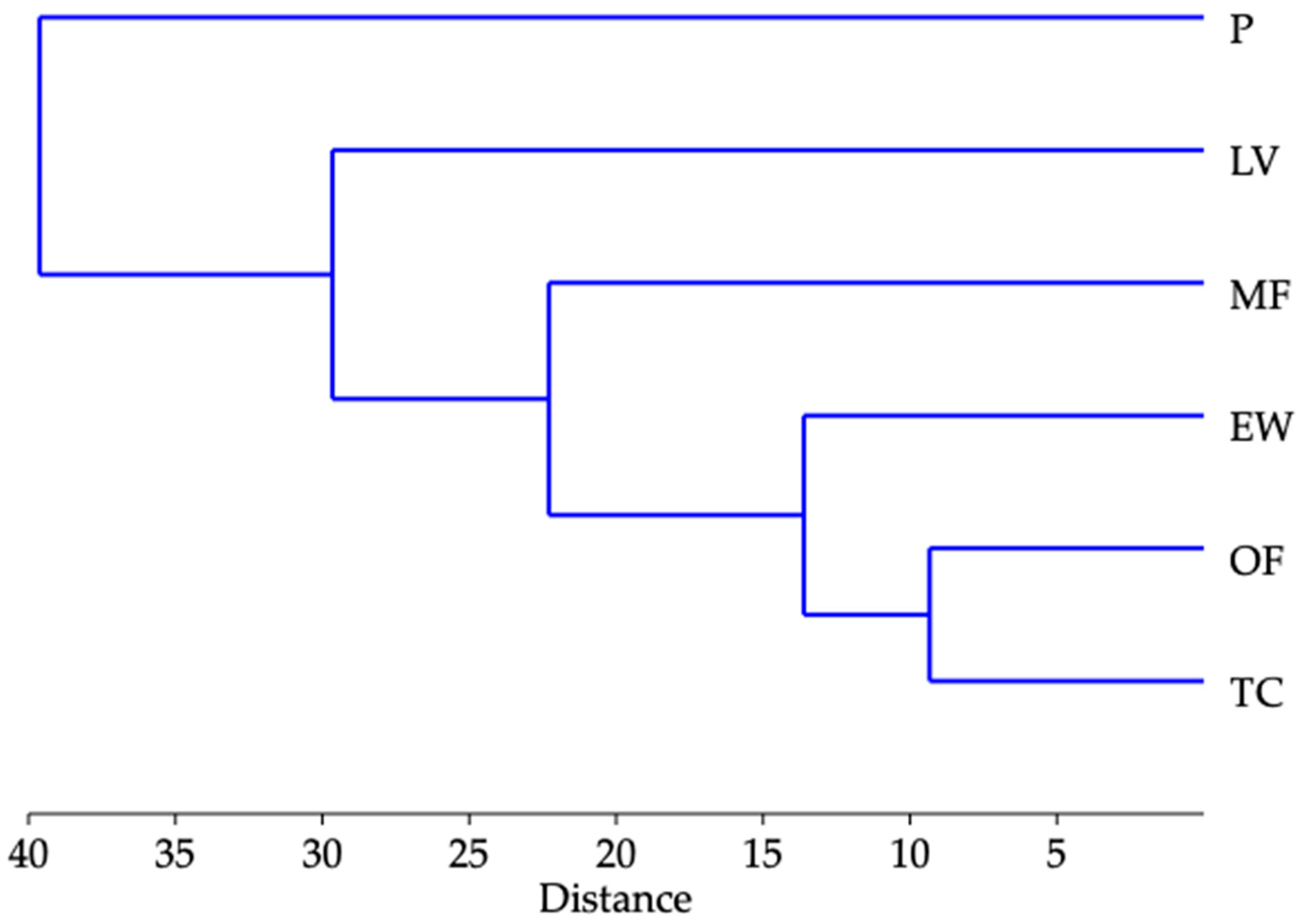
3.2.2. Chemical Profile of Essential Oils
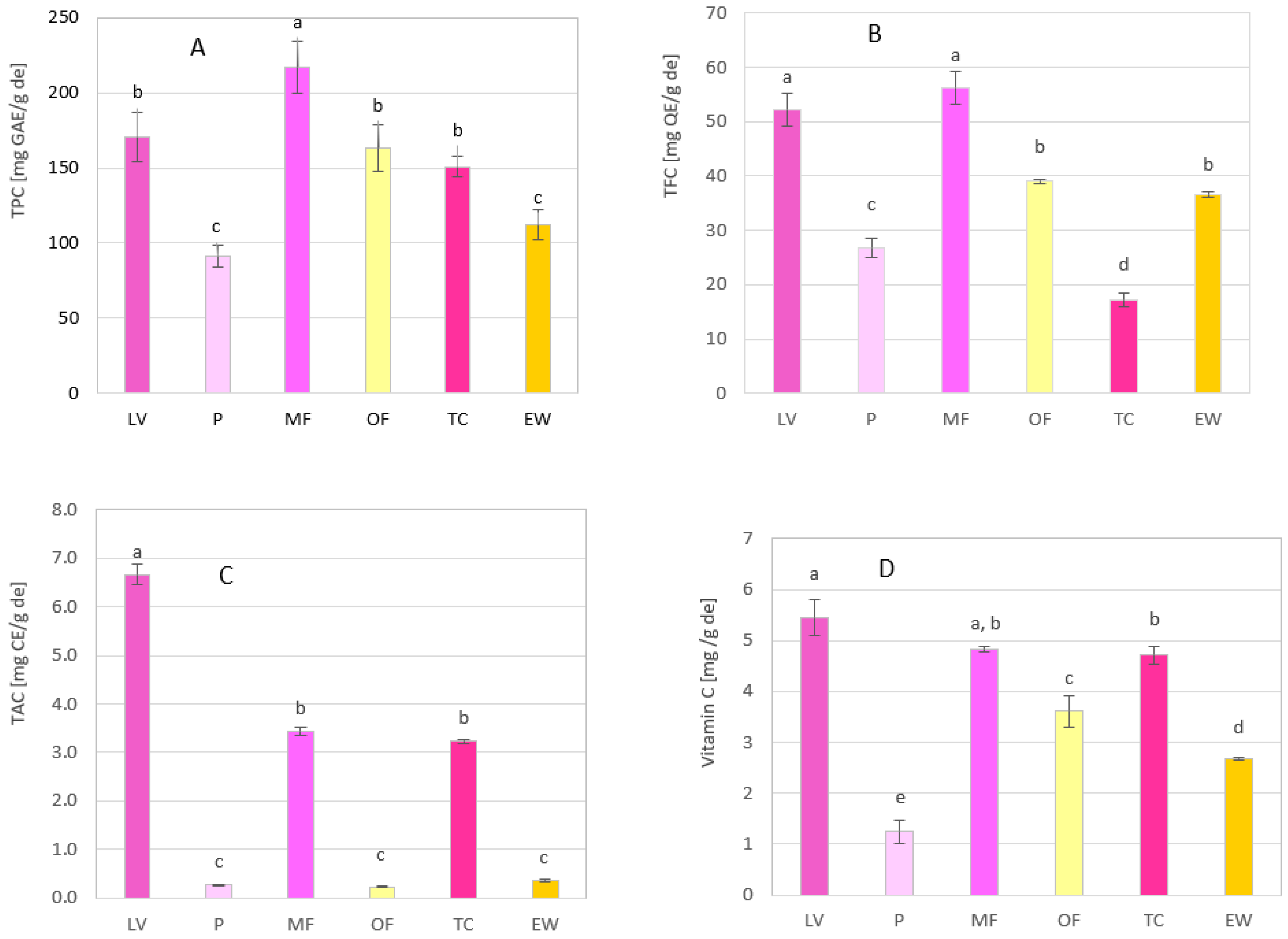
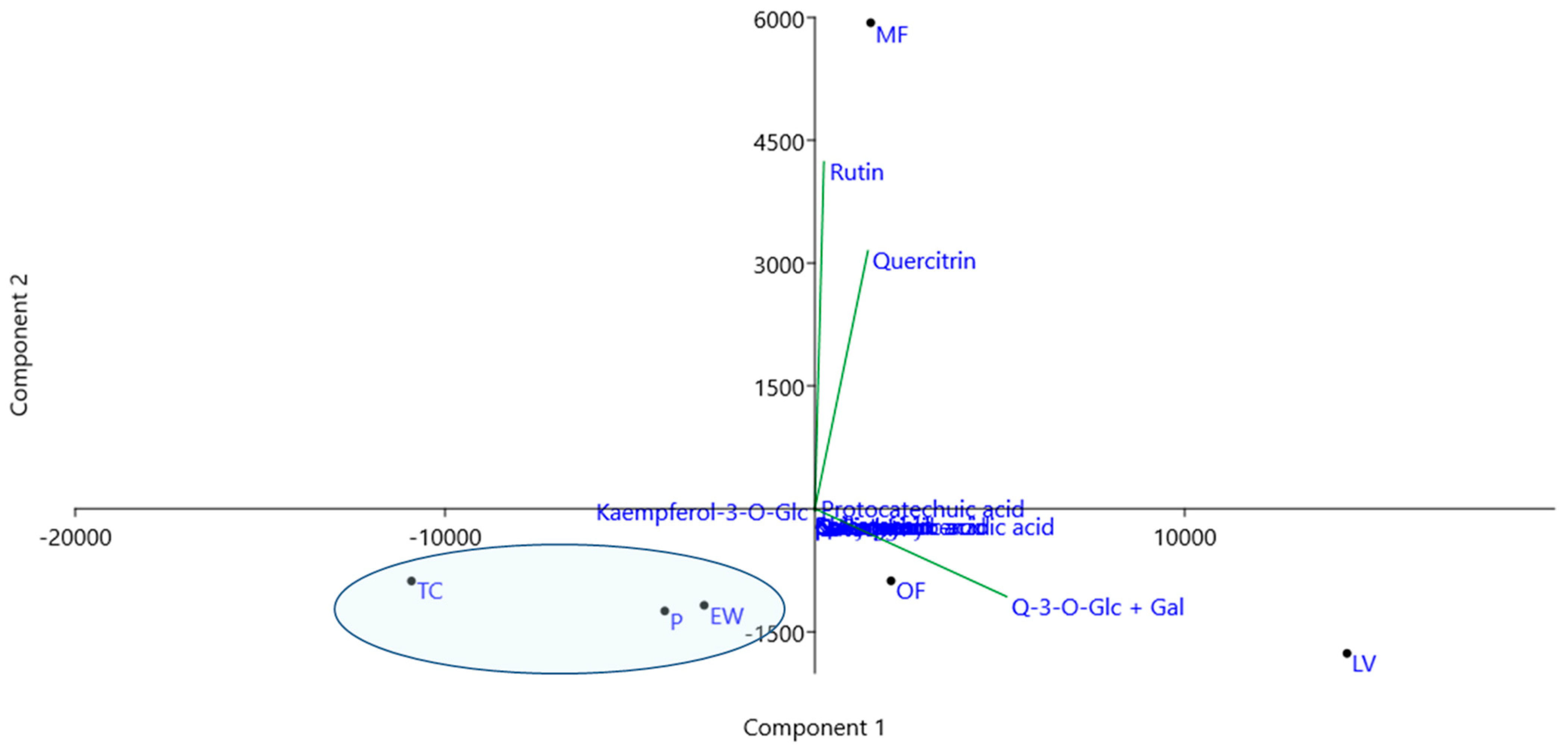
3.3. Chemical Profile of Methanol Extracts of Rose Petals
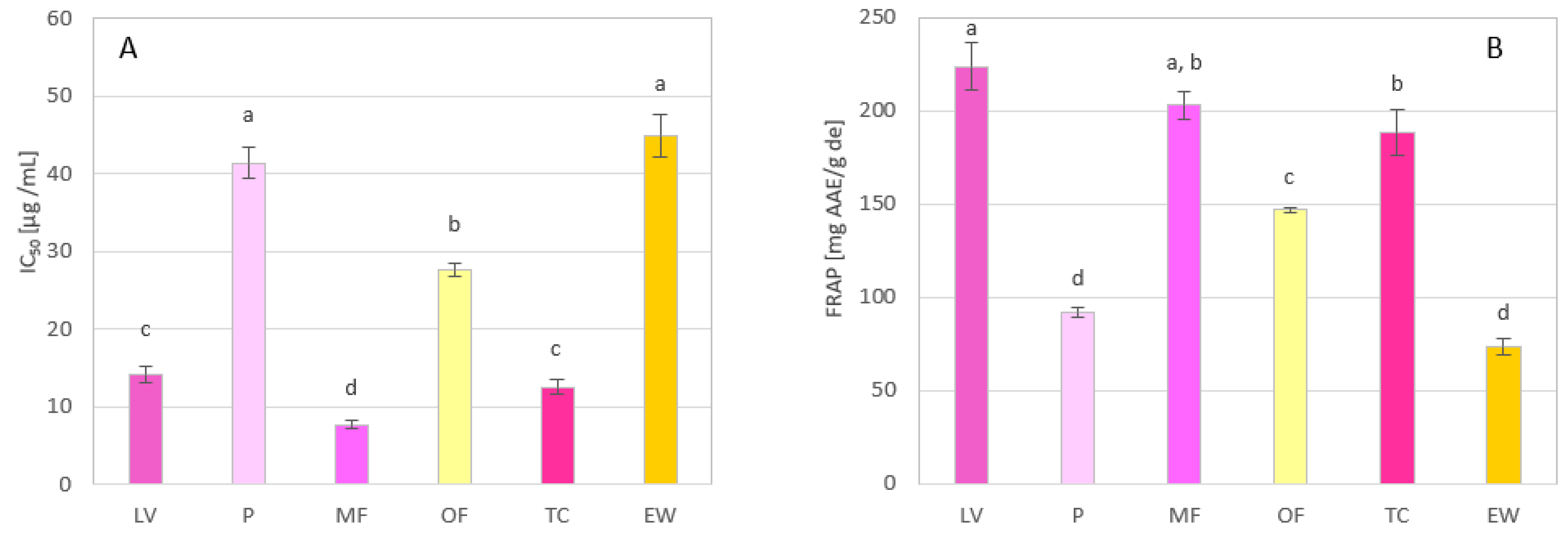
3.4. Antioxidant Activity of Methanol Extracts of Rose Petals
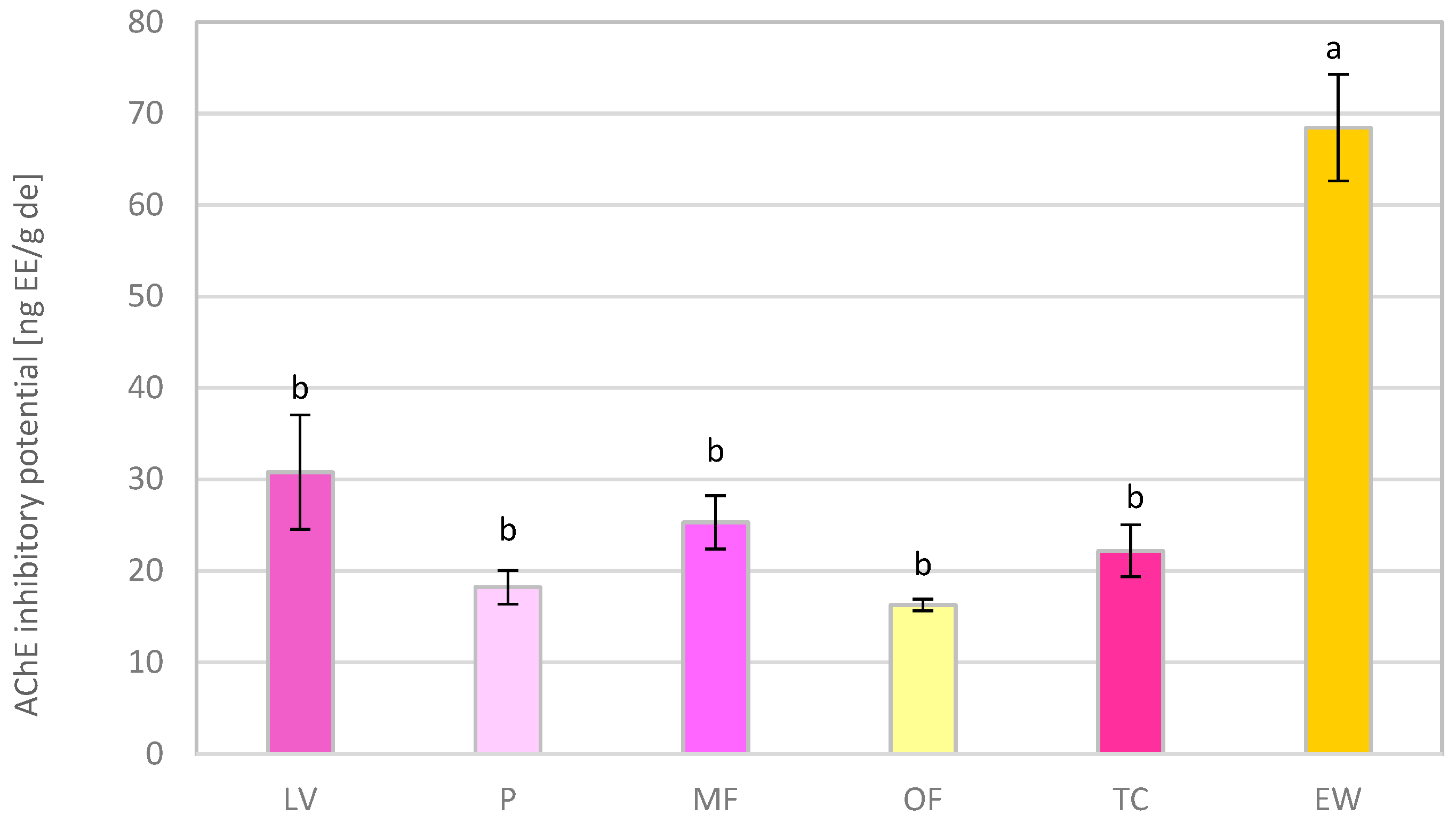
3.5. Neuroprotective Activity of Methanol Extracts of Rose Petals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khosh-Khui, M.; Teixeira da Silva, J. In vitro culture of the Rosa species. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues Vol. II, 1st ed.; Teixeira da Silva, J., Ed.; Global Science Books: London, UK, 2006; pp. 514–526. [Google Scholar]
- Moein, M.; Zarshenas, M.M.; Delnavaz, S. Chemical composition analysis of rose water samples from Iran. Pharm. Biol. 2014, 52, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh, Z.; Khosh-Khui, M. Factors affecting tissue culture of Damask rose (Rosa damascena Mill.). Sci. Hortic. 2005, 105, 475–482. [Google Scholar] [CrossRef]
- Baydar, H.; Baydar, N.G. The effects of harvest date, fermentation duration and Tween 20 treatment on essential oil content and composition of industrial oil rose (Rosa damascena Mill.). Ind. Crop. Prod. 2005, 21, 251–255. [Google Scholar] [CrossRef]
- Vinokur, Y.; Rodov, V.; Reznick, N.; Goldman, G.; Horev, B.; Umiel, N.; Friedman, H. Rose petal tea as an antioxidant rich beverage: Cultivar effects. J. Food Sci. Educ. 2006, 71, 42–47. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants–A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Vukosavljev, M.; Stranjanac, I.; van Dongen, B.W.P.; Voorrips, R.E.; Miric, M.; Bozanic Tanjga, B.; Arens, P.; Smulders, M.J.M. A novel source of food–garden rose petals. Acta Hortic. 2023, 1362, 165–171. [Google Scholar] [CrossRef]
- Mawarni, E.; Ginting, C.N.; Chiuman, L.; Girsang, E.; Handayani, R.A.S.; Widowati, W. Antioxidant and elastase inhibitor potential of petals and receptacle of rose rlower (Rosa damascena). Pharm. Sci. Res. (PSR) 2020, 7, 105–113. [Google Scholar]
- Hegde, A.S.; Gupta, S.; Sharma, S.; Srivatsan, V.; Kumari, P. Edible rose flowers: A doorway to gastronomic and nutraceutical research. Food Res. Int. 2022, 162, 111977. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Khamechian, T.; Fallah, M.H.; Kermani, M.M. Chemical composition and antimicrobial activity of Rosa damascena Mill essential oil. J. Biol. Act. Prod. Nat. 2011, 1, 19–26. [Google Scholar]
- Yousefi, B.; Jaimand, K. Chemical variation in the essential oil of Iranian Rosa damascena landraces under semi-arid and cool conditions. Int J Hortic Sci Technol 2018, 5, 81–92. [Google Scholar]
- Yaghoobi, M.; Farimani, M.M.; Sadeghi, Z.; Asghari, S.; Rezadoost, H. Chemical analysis of Iranian Rosa damascena essential oil, concrete, and absolute oil under different bio-climatic conditions. Ind. Crop. Prod. 2022, 187, 115266. [Google Scholar] [CrossRef]
- Moein, M.; Karami, F.; Tavallali, H.; Ghasemi, Y. Composition of the essential oil of Rosa damascena Mill. from south of Iran. Iran. J. Pharm. Sci. 2010, 6, 59–62. [Google Scholar]
- Shohayeb, M.; Abdel-Hameed, E.S.S.; Bazaid, S.A.; Maghrabi, I. Antibacterial and antifungal activity of Rosa damascena MILL essential oil, different extracts of rose petals. Glob. J. Pharmacol 2014, 8, 1–7. [Google Scholar]
- Mikanagi, Y.; Yokoi, M.; Ueda, Y.; Saito, N. Flower flavonol and anthocyanin distribution in subgenus Rosa. Biochem. Syst. Ecol. 1995, 23, 183–200. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Xing, J.; Sun, M.; Zhan, Z.Q.; Corke, H. Phenolic antioxidants (hydrolyzable tannins, flavonols, and anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF MS from Rosa chinensis flowers. J. Agric. Food Chem. 2005, 53, 9940–9948. [Google Scholar] [CrossRef]
- Schmitzer, V.; Stampar, F. Changes in anthocyanin and selected phenolics in “KORcrisett” rose flowers due to substrate pH and foliar application of sucrose. Acta Hortic. 2010, 870, 89–96. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Changes in the phenolic concentration during flower development of rose ‘KORcrisett’. J. Am. Soc. Hortic. Sci. 2009, 134, 491–496. [Google Scholar] [CrossRef]
- Yon, J.M.; Kim, Y.B.; Park, D. The ethanol fraction of white rose petal extract abrogates excitotoxicity-induced neuronal damage in vivo and in vitro through inhibition of oxidative stress and proinflammation. Nutrients 2018, 10, 1375. [Google Scholar] [CrossRef]
- Vanderjagt, T.J.; Ghattas, R.; VanderJagt, D.J.; Crossey, M.; Glew, R.H. Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico. Life Sci. 2002, 70, 1035–1040. [Google Scholar] [CrossRef]
- UPOV. Guidelines for the Conduct of Tests Distinctness, Uniformity and Stability-Rosa L.; International Union for The Protection of New Varieties of Plants: Geneva, Switzerland, 2010. [Google Scholar]
- EDQM (Council of Europe). European Pharmacopoeia, 3rd ed; Directorate for the Quality of Medicines, Council of Europe: Strasbourg, France, 1996; pp. 121–122. [Google Scholar]
- Lesjak, M.; Simin, N.; Orcic, D.; Franciskovic, M.; Knezevic, P.; Beara, I.; Aleksic, V.; Svircev, E.; Buzas, K.; Mimica-Dukic, N. Binary and tertiary mixtures of Satureja hortensis and Origanum vulgare essential oils as potent antimicrobial agents against Helicobacter pylori. Phytother. Res. 2015, 30, 476–484. [Google Scholar] [CrossRef]
- McLafferty, F.W. Wiley Registry of Mass Spectral Data 7th edition with NIST 2005 Spectral Data; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Business Media: Carol Stream, IL, USA, 2012. [Google Scholar]
- Lesjak, M.M.; Beara, I.N.; Orčić, D.Z.; Anačkov, G.T.; Balog, K.J.; Francišković, M.M.; Mimica-Dukić, N.M. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and anti-inflammatory agents. Food Chem. 2011, 124, 850–856. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Food Analysis Laboratory Manual, 2nd ed.; Springer: New York, NY, USA, 2010; pp. 55–60. [Google Scholar]
- Simin, N.; Orcic, D.; Cetojevic-Simin, D.; Mimica-Dukic, N.; Anackov, G.; Beara, I.; Mitic-Culafic, D.; Bozin, B. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of small yellow onion (Allium flavum L. subsp. flavum, Alliaceae). LWT Food Sci. Technol. 2013, 54, 139–146. [Google Scholar] [CrossRef]
- Pintać, D.; Četojević-Simin, D.; Berežni, B.; Orčić, D.; Mimica-Dukić, N.; Lesjak, M. Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties. Food Chem. 2019, 286, 686–695. [Google Scholar] [CrossRef]
- Khaleghi, A.; Khadivi, A. Morphological characterization of Damask rose (Rosa × damascena Herrm.) germplasm to select superior accessions. Genet. Resour. Crop Evol. 2020, 67, 1981–1997. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 307–1899. [Google Scholar]
- Chemical Entities of Biological Interest (ChEBI). Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=88528 (accessed on 20 August 2023).
- Fu, Y.; Zhang, Y.; Zeng, S.; Luo, L.; Xi, H.; Li, P.; Wang, D.; Liao, T.; Chen, J.; Sun, S.; et al. The effect of long-chain alkanes on flavour release and olfactory characteristics of rose essential oil. Flavour Fragr. J. 2021, 37, 72–80. [Google Scholar] [CrossRef]
- Rusdi, N.; Goh, H.H.; Baharum, S. GC-MS/Olfactometric characterisation and aroma extraction dilution analysis of aroma active compounds in Polygonum minus essential oil. Plant Omics 2016, 9, 289–291. [Google Scholar] [CrossRef]
- Li, Z.; Howell, K.; Fang, Z.; Zhang, P. Sesquiterpenes in grapes and wines: Occurrence, biosynthesis, functionality, and influence of winemaking processes. Compr. Rev. Food Sci. Food Saf. 2019, 19, 247–281. [Google Scholar] [CrossRef]
- Adams, T.B.; Hallagan, J.B.; Putnam, J.M.; Gierke, T.L.; Doull, J.; Munro, I.C.; Newberne, P.; Portoghese, P.S.; Smith, R.L.; Wagner, B.M.; et al. The FEMA GRAS assessment of alicyclic substances used as flavour ingredients. Food Chem. Toxicol. 1996, 34, 763–828. [Google Scholar] [CrossRef]
- Beara, I.; Torović, L.j.; Pintać, D.; Majkić, T.; Orčić, D.; Mimica-Dukić, N.; Lesjak, M. Polyphenolic profile, antioxidant and neuroprotective potency of grape juices and wines from Fruška Gora region (Serbia). Int. J. Food Prop. 2017, 20, S2552–S2568. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Šibul, F.S.; Orčić, D.Z.; Svirčev, E.; Mimica-Dukić, N.M. Optimization of extraction conditions for secondary biomolecules from various plant species. Chem. Ind. 2016, 70, 473–483. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Shamsher, S.B. Antioxidant activity and ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of Rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol. 2009, 47, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shin, Y. Antioxidant compounds and activities of edible roses (Rosa hybrida spp.) from different cultivars grown in Korea. Appl. Biol. Chem. 2017, 60, 129–136. [Google Scholar] [CrossRef]
- Lutz, M.; Hernández, J.; Henríquez, C. Phenolic content and antioxidant capacity in fresh and dry fruits and vegetables grown in Chile. CyTA J. Food 2015, 13, 541–547. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Choi, J.K.; Lee, Y.B.; Lee, K.H.; Im, H.C.; Kim, Y.B.; Choi, E.K.; Joo, S.S.; Jang, S.K.; Han, N.S.; Kim, C.H. Extraction conditions for phenolic compounds with antioxidant activities from white rose petals. J. Appl. Biol. Chem. 2015, 58, 117–124. [Google Scholar] [CrossRef]
- Kumari, P.; Raju, D.V.S.; Prasad, K.V.; Saha, S.; Panwar, S.; Paul, S.; Banyal, N.; Bains, A.; Chawla, P.; Fogarasi, M.; et al. Characterization of anthocyanins and their antioxidant activities in Indian rose varieties (Rosa × hybrida) using HPLC. Antioxidants 2022, 11, 2032. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and cardiovascular disease: An update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Magana, A.A.; Reed, R.L.; Koluda, R.; Miranda, C.L.; Maier, C.S.; Stevens, J.F. Vitamin C activates the folate-mediated one-carbon cycle in C2C12 myoblasts. Antioxidants 2020, 9, 217. [Google Scholar] [CrossRef]
- Food and Nutrition Board Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000; pp. 95–185. [Google Scholar]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; van Buren, L.; Wagner, E.; Wiseman, S.; van de Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and Vitamin C composition. Free. Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Conti Filho, C.E.; Loss, L.B.; Marcolongo-Pereira, C.; Rossoni Junior, J.V.; Barcelos, R.M.; Chiarelli-Neto, O.; da Silva, B.S.; Passamani Ambrosio, R.; Castro, F.C.A.Q.; Teixeira, S.F.; et al. Advances in Alzheimer’s disease’s pharmacological treatment. Front. Pharmacol. 2023, 14, 1101452. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Ara, L.; Hajimehdipoor, H.; Kolivand, H.; Mohammadi Motamed, S. Acetylcholinesterase inhibitory effects of some plants from Rosaceae. Res. J. Pharmacogn. 2015, 2, 33–37. [Google Scholar]
- Jazayeri, S.B.; Amanlou, A.; Ghanadian, N.; Pasalar, P.; Amanlou, M. A preliminary investigation of anticholinesterase activity of some Iranian medicinal plants commonly used in traditional medicine. DARU J. Pharm. Sci. 2014, 22, 17. [Google Scholar] [CrossRef]
- Tarbiat, S.; Türütoğlu, A.S.; Ekingen, M. Acetylcholinesterase inhibitory potential and antioxidant activities of five cultivars of Rosa damascena Mill. from Isparta, Turkey. Curr. Top. Nutraceutical Res. 2020, 18, 354–359. [Google Scholar] [CrossRef]

| Character | Abbr. | Scores |
|---|---|---|
| Plant and leaf | ||
| Growth type | GT | 1—miniature, 2—dwarf, 3—bed, 4—shrub, 5—climber and 6—ground cover |
| Growth habit | GH | 1—upright, 3—semi-upright, 5—intermediate, 7—moderately spreading and 9—strongly spreading |
| Intensity of green color (upper side) | IGC | 3—light, 5—medium and 7—dark |
| Leaf anthocyanin coloration | LAC | 1—absent and 9—present |
| Glossiness of upper side | GUS | 1—absent or very weak, 3—weak, 5—medium, 7—strong and 9—very strong |
| Flower | ||
| Flowering laterals | FL | 1—absent and 9—present |
| Flower type | TP | 1—single, 2—semi-double and 3—double |
| Color group | CG | 1—white or near white, 2—white blend, 3—green, 4—yellow, 5—yellow blend, 6—orange, 7—orange blend, 8—pink, 9—pink blend, 10—red, 11—red blend, 12—purple red, 13—purple, 14—violet blend, 15—brown blend and 16—multi colored |
| Color of center (only varieties with flower type double) | CC | 1—green, 2—yellow, 3—orange, 4—pink, 5—red and 6—purple |
| Shape | SH | 1—round, 2—irregularly rounded and 3—star-shaped |
| Profile of upper part | PUP | 1—flat, 2—flattened convex and 3—convex |
| Profile of lower part | PLP | 1—concave, 2—flat, 3—flattened convex and 4—convex |
| Fragrance (observed by smelling) | FG | 1—absent or weak, 2—medium weak, 3—medium, 4—medium strong and 5—strong |
| Cultivar/Trait | ‘Eveline Wild’ | ‘Olivera Frayla’ | ‘Lavender Vaza’ | ‘Pear’ | ‘Theo Clevers’ | ‘Marija Frayla’ |
|---|---|---|---|---|---|---|
| Plant | ||||||
| GT | Shrub | Shrub | Shrub | Shrub | Shrub | Shrub |
| GH | Upright | Semi-upright | Semi-upright | Semi-upright | Upright | Upright |
| Height (cm) | 89.9 ± 3.96 d | 86.8 ± 3.42 cd | 80.0 ± 4.12 c | 59.8 ± 3.96 b | 108 ± 5.71 e | 38.8 ± 7.59 a |
| Leaf | ||||||
| IGC | Light | Medium | Medium | Medium | Dark | Medium |
| LAC | Present | Present | Absent | Absent | Present | Absent |
| GUS | Very weak | Very weak | Weak | Very weak | Weak | Weak |
| Length (cm) | 3.32 ± 0.58 a | 4.96 ± 0.11 b | 4.24 ± 0.15 ab | 4.12 ± 0.56 ab | 6.32 ± 1.06 c | 5.18 ± 0.58 bc |
| Width (cm) | 2.14 ± 0.32 a | 3.10 ± 0.24 a | 2.52 ± 0.17 a | 3.02 ± 0.33 a | 4.82 ± 0.68 b | 4.30 ± 0.84 b |
| Cultivar/Trait | ‘Eveline Wild’ | ‘Olivera Frayla’ | ‘Lavender Vaza’ | ‘Pear’ | ‘Theo Clevers’ | ‘Marija Frayla’ |
|---|---|---|---|---|---|---|
| Flowering shoot | ||||||
| NFS * | 5.20 ± 1.30 bc | 0 a | 4.60 ± 0.89 b | 5.20 ± 0.84 bc | 0 a | 6.20 ± 0.84 c |
| FL | Absent | Present | Absent | Present | Present | Absent |
| NFL | 0 a | 3.00 ± 0.70 c | 0 a | 1.80 ± 0.83 b | 2.80 ± 0.44 c | 0 a |
| NF/L | 0 a | 7.61 ± 2.5 b | 0 a | 1.00 ± 0.33 a | 6.00 ± 2.65 b | 0 a |
| Flower | ||||||
| TP | Double | Double | Double | Double | Double | Double |
| CG | Orange blend | Yellow | White | Pale pink | Pink | Red purple |
| CC | Orange | Yellow | Pink | / | Pink | / |
| SH | Rounded | Rounded | Irregularly rounded | Rounded | Rounded | Rounded |
| PUP | Flat | Flat convex | Flat convex | Flat | Flat convex | Flat |
| PLP | Flat | Concave | Concave | Flattened convex | Concave | Flat |
| FG | 5 | 5 | 3 | 3 | 5 | 4 |
| NOP | 56.4 ± 2.96 b | 54.4 ± 7.16 b | 84.0 ± 12.2 c | 26.2 ± 2.58 a | 169 ± 15.9 e | 138 ± 3.84 d |
| DM (cm) | 6.22 ± 0.19 ab | 5.68 ± 0.52 a | 6.60 ± 0.53 ab | 6.80 ± 0.71 b | 6.67 ± 0.51 b | 9.54 ± 0.27 c |
| PL (cm) | 3.42 ± 0.13 a | 4.18 ± 0.39 b | 2.86 ± 0.47 a | 3.42 ± 0.24 a | 2.92 ± 0.19 a | 4.32 ± 0.23 b |
| PW (cm) | 3.34 ± 0.61 ab | 3.14 ± 0.32 a | 2.58 ± 0.41 a | 2.96 ± 0.59 a | 2.68 ± 0.22 a | 4.14 ± 0.51 b |
| Fragrance Panel | ‘Eveline Wild’ | ‘Olivera Frayla’ | ‘Lavender Vaza’ | ‘Pear’ | ‘Theo Clevers’ | ‘Marija Frayla’ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/W | M | S | A/W | M | S | A/W | M | S | A/W | M | S | A/W | M | S | A/W | M | S | |||
| Top Notes | Citrus | orange | 1 * | 1 | 3 | |||||||||||||||
| lemon | 3 | 4 | 3 | 2 | 6 | 6 | ||||||||||||||
| apple | 3 | 2 | 2 | 2 | 1 | 4 | ||||||||||||||
| Aromatic | mint | 2 | ||||||||||||||||||
| anise | ||||||||||||||||||||
| eucalyptus | ||||||||||||||||||||
| Heart Notes | Floral | flowery | 2 | 5 | 5 | 7 | 2 | 3 | 2 | 8 | 5 | |||||||||
| rose-like | 10 | 2 | 8 | 3 | 4 | 7 | 6 | 10 | ||||||||||||
| jasmine | ||||||||||||||||||||
| Green | forest | 3 | 2 | 3 | ||||||||||||||||
| herbaceous | 2 | |||||||||||||||||||
| grass | 3 | 3 | 1 | 4 | ||||||||||||||||
| Fruity | fruity | 3 | 6 | 4 | 6 | 5 | 7 | 5 | ||||||||||||
| sweet, honey | 6 | 4 | 8 | 4 | 6 | 3 | 6 | 7 | ||||||||||||
| peach | 2 | 5 | 1 | 2 | 4 | |||||||||||||||
| Spicy | spicy | 3 | ||||||||||||||||||
| peppery | 3 | |||||||||||||||||||
| cinnamon | ||||||||||||||||||||
| Base Notes | Woody, earthy | moss | ||||||||||||||||||
| woody | 1 | 2 | ||||||||||||||||||
| coniferous | ||||||||||||||||||||
| Balsamic | balsamic | 1 | ||||||||||||||||||
| musky | ||||||||||||||||||||
| vanilla | 3 | 2 | 4 | 2 | ||||||||||||||||
| % of Total Peak Area | |||||||
|---|---|---|---|---|---|---|---|
| AI | Compound | EW | LV | MF | OF | P | TC |
| 800 | Hexanal | 1.95 | 0.44 | 1.62 | 0.58 | 0.61 | 3.98 |
| 848 | 2-Hexenal | 1.14 | 1.17 | 0.93 | 1.93 | 0.69 | 1.75 |
| 1228 | Nerol | 11.19 | 1.76 | 3.57 | 9.39 | n.d. | 12.96 |
| 1240 | Neral | 3.15 | n.d. | n.d. | 2.76 | n.d. | 2.31 |
| 1254 | Geraniol | 4.46 | n.d. | n.d. | 6.52 | n.d. | 4.24 |
| 1255 | Phenethyl acetate | n.d. | 6.40 | n.d. | n.d. | n.d. | n.d. |
| 1265 | Orcinol dimethyl ether | n.d. | n.d. | 0.87 | n.d. | n.d. | 0.98 |
| 1270 | Geranial | 3.33 | n.d. | n.d. | 4.29 | n.d. | 2.90 |
| 1297 | Theaspirane A/B | n.d. | n.d. | n.d. | 1.92 | 0.12 | 0.11 |
| 1314 | Theaspirane A/B | n.d. | n.d. | n.d. | 0.92 | 0.11 | 0.09 |
| 1323 | n.i. | 0.83 | n.d. | n.d. | n.d. | n.d. | 0.16 |
| 1353 | n.i. | 0.66 | n.d. | n.d. | n.d. | n.d. | 0.35 |
| 1361 | n.i. | n.d. | n.d. | 0.84 | n.d. | n.d. | n.d. |
| 1365 | Neryl acetate | 0.29 | n.d. | n.d. | n.d. | n.d. | 0.15 |
| 1384 | Geranyl acetate | 4.46 | n.d. | n.d. | n.d. | n.d. | 2.34 |
| 1418 | E-Caryophyllene | n.d. | 0.69 | 1.49 | n.d. | 0.53 | 0.22 |
| 1438 | Dihydro-β-ionone | n.d. | n.d. | 0.23 | 1.50 | 0.09 | 0.14 |
| 1444 | Dihydro-α-ionol | n.d. | n.d. | 7.68 | 3.15 | 1.60 | 0.34 |
| 1480 | Germacrene D | 2.94 | 3.23 | 11.08 | n.d. | 1.85 | 0.59 |
| 1486 | E-β-Ionone | n.d. | n.d. | 0.70 | n.d. | n.d. | 0.08 |
| 1490 | n.i. | n.d. | n.d. | 0.48 | n.d. | n.d. | 0.08 |
| 1495 | n.i. | n.d. | n.d. | 0.69 | n.d. | 0.10 | 0.08 |
| 1499 | α-Muurolene + Pentadecane | n.d. | n.d. | 0.88 | n.d. | 0.20 | 0.08 |
| 1513 | n.i. | n.d. | n.d. | 0.45 | n.d. | n.d. | 0.08 |
| 1522 | δ-Cadinene + ? | 0.41 | n.d. | 1.99 | n.d. | 0.26 | 0.11 |
| 1631 | γ-Eudesmol | n.d. | n.d. | 0.74 | n.d. | n.d. | 0.08 |
| 1641 | τ-Cadinol | n.d. | n.d. | 1.57 | n.d. | 0.08 | 0.08 |
| 1645 | n.i. | 0.27 | n.d. | n.d. | n.d. | n.d. | 0.08 |
| 1653 | α-Eudesmol | n.d. | n.d. | 3.14 | n.d. | 0.05 | 0.10 |
| 1676 | Heptadecene | n.d. | n.d. | n.d. | n.d. | 0.99 | 0.07 |
| 1698 | Heptadecane | 0.34 | n.d. | 7.07 | n.d. | 0.81 | 0.91 |
| 1724 | 2Z,6E-Farnesol ? | n.d. | n.d. | 6.51 | n.d. | 0.40 | 4.96 |
| 1873 | 9-Nonadecene | 14.52 | 5.47 | 5.01 | 5.83 | 26.38 | 9.61 |
| 1900 | Nonadecane | 9.09 | 5.09 | 3.78 | 7.32 | 22.04 | 5.23 |
| 1918 | n.i. | n.d. | n.d. | n.d. | n.d. | 0.42 | 1.36 |
| 1971 | 9-Icosene | 0.29 | n.d. | n.d. | n.d. | 0.52 | 0.36 |
| 1998 | Icosane | 0.89 | 0.65 | 0.40 | 0.65 | 1.72 | 0.98 |
| 2070 | Heneicosene | 0.64 | 0.25 | 0.17 | 0.27 | 1.01 | 0.86 |
| 2087 | Heneicosene | 0.45 | 0.33 | 0.87 | 0.45 | 1.38 | 0.83 |
| 2092 | 10(?)-Heneicosene | 0.35 | 0.36 | n.d. | 0.41 | 0.90 | 0.54 |
| 2100 | Heneicosane | 12.82 | 17.77 | 9.71 | 14.20 | 21.61 | 18.09 |
| 2198 | Docosane | 0.27 | 0.29 | 0.29 | 0.37 | 0.31 | 0.59 |
| 2288 | 9-Tricosene | 1.23 | 2.74 | 2.75 | 2.83 | 1.96 | 2.28 |
| 2299 | Tricosane | 6.98 | 14.61 | 11.71 | 13.33 | 6.05 | 11.20 |
| 2397 | Tetracosane | 0.42 | 0.87 | 0.45 | 0.78 | 0.20 | 0.50 |
| 2489 | Pentacosene | 0.56 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2492 | n.i. | n.d. | 1.74 | 0.50 | 1.13 | 0.38 | 0.22 |
| 2499 | Pentacosane | 6.64 | 19.03 | 6.34 | 9.70 | 2.48 | 3.89 |
| 2597 | Hexacosane | 0.31 | 0.75 | n.d. | 0.30 | 0.08 | 0.13 |
| 2700 | Heptacosane | 8.79 | 16.22 | 2.26 | 6.61 | 2.36 | 1.87 |
| Total monoterpens (%) | 26.9 | 1.76 | 3.86 | 25.8 | 0.24 | 25.1 | |
| Total sesquiterpens (%) | 3.35 | 3.92 | 36.0 | 4.66 | 5.06 | 6.78 | |
| Total linear hydrocarbons (%) | 64.6 | 84.4 | 50.8 | 63.0 | 90.8 | 58.0 | |
| Total identified (%) | 97.9 | 98.1 | 93.8 | 96.0 | 97.2 | 96.3 | |
| TPC | TFC | TAC | Vitamin C | |||||
|---|---|---|---|---|---|---|---|---|
| Genotype | mg GAE/g de | mg GAE/g fw | mg QE/g de | mg QE/g fw | mg CE/g de | µg CE/g fw | mg/g de | µg/g fw |
| LV | 170 ± 16.4 | 13.2 ± 1.27 | 52.3 ± 3.00 | 4.00 ± 0.233 | 6.660 ± 0.216 | 517 ± 16.7 | 5.45 ± 0.354 | 424 ± 27.5 |
| P | 91.4 ± 7.30 | 8.19 ± 0.65 | 26.8 ± 1.70 | 2.40 ± 0.152 | 0.260 ± 0.022 | 23.3 ±1.97 | 1.24 ± 0.226 | 111 ±20.3 |
| MF | 217 ± 17.6 | 21.5 ± 1.75 | 56.3 ± 3.00 | 5.58 ± 0.297 | 3.435 ± 0.087 | 340 ± 8.62 | 4.83 ± 0.053 | 479 ± 5.28 |
| OF | 163 ± 15.2 | 13.5 ± 1.26 | 39.0 ± 0.33 | 3.24 ± 0.028 | 0.230 ± 0.001 | 18.8 ± 0.398 | 3.61 ± 0.307 | 299 ± 25.5 |
| TC | 151 ± 6.78 | 14.0 ± 0.63 | 17.3 ± 1.19 | 1.61 ± 0.111 | 3.234 ± 0.043 | 301 ±3.92 | 4.71 ± 0.183 | 438 ± 17.1 |
| EW | 112 ± 9.63 | 9.94 ± 0.85 | 36.7 ± 0.52 | 3.25 ± 0.046 | 0.355 ± 0.031 | 31.4 ±2.77 | 2.68 ± 0.028 | 238 ± 2.52 |
| Content [μg/g de] a | ||||||
|---|---|---|---|---|---|---|
| Genotype | LV | P | MF | OF | TC | EW |
| Quinic acid | 19,037 b ± 2.0 | 21,722 ± 2.2 | 26,963 ± 2.7 | 36,142 ± 3.6 | 36,833 ± 3.7 | 17,513 ± 1.7 |
| p-Hydroxybenzoic acid | <1.2 | <1.2 | <1.2 | <1.2 | 5.33 ± 0.32 | <1.2 |
| Protocatechuic acid | 44.8 ± 3.59 | 3.26 ± 0.26 | 33.0 ± 2.64 | 2.17 ± 0.17 | 11.1 ± 0.89 | 8.69 ± 0.69 |
| p-Coumaric acid | 1.15 ± 0.10 | 2.98 ± 0.27 | 0.17 ± 0.02 | 1.04 ± 0.09 | 0.33 ± 0.03 | 0.87 ± 0.08 |
| Gallic acid | 27.3 ± 2.46 | 24.3 ± 2.19 | 22.5 ± 2.03 | 23.1 ± 2.08 | 18.9 ± 1.70 | 30.6 ± 2.76 |
| Naringenin | 1.04 ± 0.07 | 0.49 ± 0.03 | 0.59 ± 0.04 | 2.64 ± 0.18 | 0.41 ± 0.03 | 1.87 ± 0.13 |
| Kaempferol | 9.88 ± 0.69 | 15.3 ± 1.07 | 9.60 ± 0.67 | 23.9 ± 1.67 | 18.1 ± 1.27 | 18.1 ± 1.27 |
| Catechin | 255 ± 0.03 | 155 ± 0.02 | 113 ± 0.01 | 75.7 ± 0.01 | 100 ± 0.01 | 55.5 ± 0.01 |
| Epicatechin | 8.07 ± 0.001 | 2.13 ± 0.00 | 11.3 ± 0.001 | 1.27 ± 0.00 | <1.2 | <1.2 |
| Quercetin | 58.8 ± 0.18 | 13.1 ± 0.04 | 23.8 ± 0.07 | 37.8 ± 0.11 | 6.89 ± 0.02 | 18.4 ± 0.06 |
| Chlorogenic acid | 7.34 ± 0.37 | 2.19 ± 0.11 | 1.11 ± 0.06 | 0.65 ± 0.03 | 2.18 ± 0.11 | 1.60 ± 0.08 |
| Kaempferol-3-O-Glc | 60.9 ± 2.44 | 147 ± 5.88 | 76.2 ± 3.05 | 96.2 ± 3.85 | 74.1 ± 2.96 | 193.6 ±7.74 |
| Quercitrin | 6537 ± 392 | 1843 ± 111 | 7584 ± 455 | 3974 ± 238 | 467 ± 28.0 | 1952 ± 117 |
| Quercetin-3-O-Glc + Gal | 25,326 ± 1520 | 7481 ± 449 | 11,403 ± 684 | 13,231 ± 794 | 758 ± 45.5 | 8545 ± 513 |
| Rutin | 749 ± 22.5 | 390 ± 11.7 | 6241 ± 187 | 723 ± 21.7 | 179 ± 5.37 | 665 ± 20.0 |
| Total phenolics (mg/g de) c | 33.09 | 10.08 | 25.52 | 18.19 | 1.642 | 11.49 |
| IC50 (DPPH) | FRAP | AChE-IP | |
|---|---|---|---|
| Genotype | μg/mL | mg AAE/g de | ng EE/g de |
| LV | 11.45 ± 1.15 | 227 ± 16.8 | 30.8 ± 6.24 |
| P | 37.82 ± 2.99 | 91.9 ± 2.94 | 18.2 ± 1.86 |
| MF | 9.24 ± 0.61 | 220 ± 18.1 | 25.3 ± 2.91 |
| OF | 27.07 ± 3.21 | 148 ± 1.47 | 16.3 ± 0.64 |
| TC | 15.78 ± 0.18 | 189 ± 18.0 | 22.2 ± 2.80 |
| EW | 42.51 ± 4.05 | 74.6 ± 7.25 | 68.5 ± 5.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simin, N.; Lesjak, M.; Živanović, N.; Božanić Tanjga, B.; Orčić, D.; Ljubojević, M. Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars. Horticulturae 2023, 9, 1082. https://doi.org/10.3390/horticulturae9101082
Simin N, Lesjak M, Živanović N, Božanić Tanjga B, Orčić D, Ljubojević M. Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars. Horticulturae. 2023; 9(10):1082. https://doi.org/10.3390/horticulturae9101082
Chicago/Turabian StyleSimin, Nataša, Marija Lesjak, Nemanja Živanović, Biljana Božanić Tanjga, Dejan Orčić, and Mirjana Ljubojević. 2023. "Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars" Horticulturae 9, no. 10: 1082. https://doi.org/10.3390/horticulturae9101082
APA StyleSimin, N., Lesjak, M., Živanović, N., Božanić Tanjga, B., Orčić, D., & Ljubojević, M. (2023). Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars. Horticulturae, 9(10), 1082. https://doi.org/10.3390/horticulturae9101082









