Changes in Physicochemical Characteristics, Peel Color, and Juice Attributes of ‘Moro’ Blood Orange Fruit Treated with Glycine Betaine and Methyl Salicylate during Cold Quarantine Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Treatments and Storage Conditions
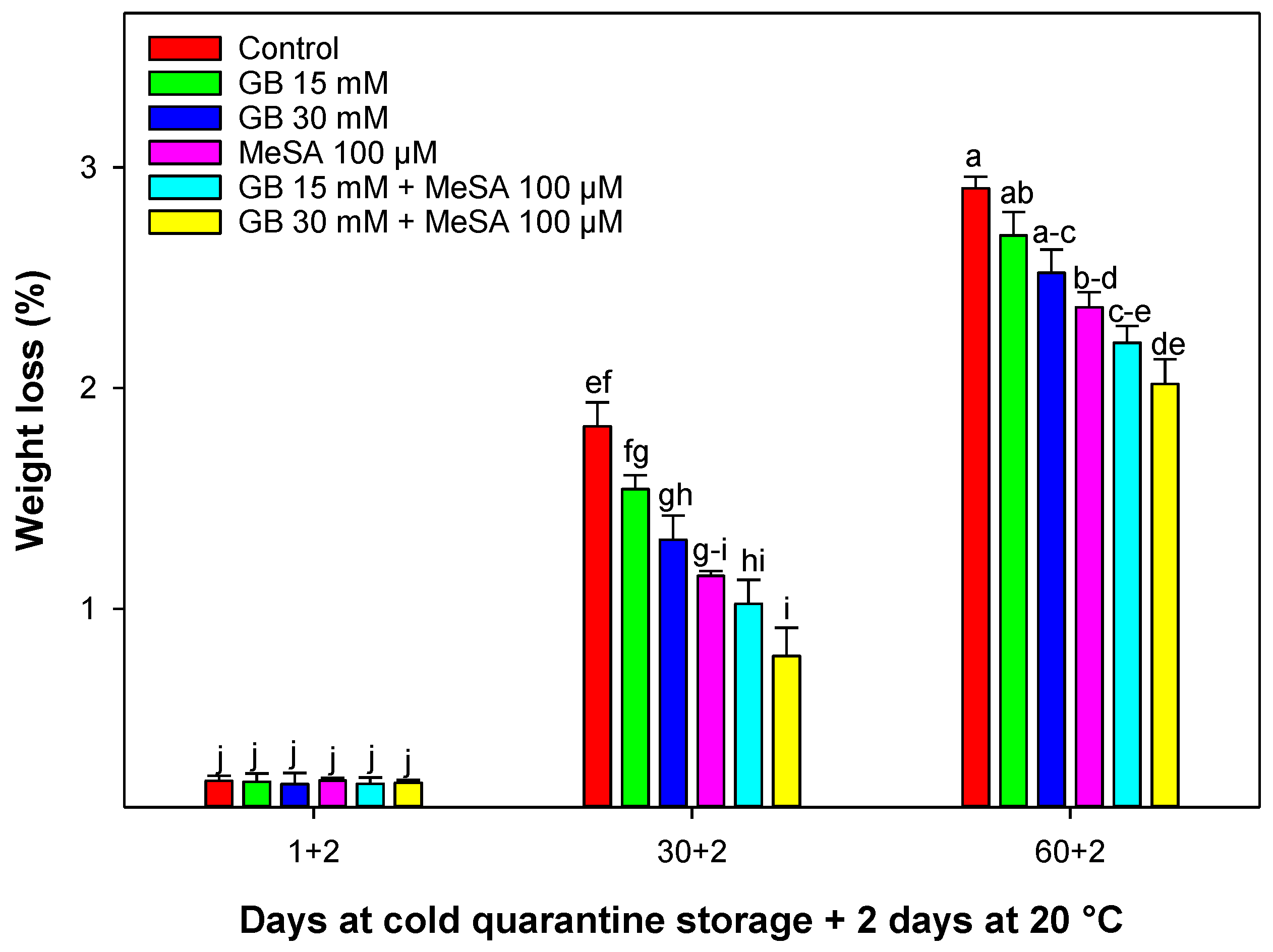
2.2. Weight Loss
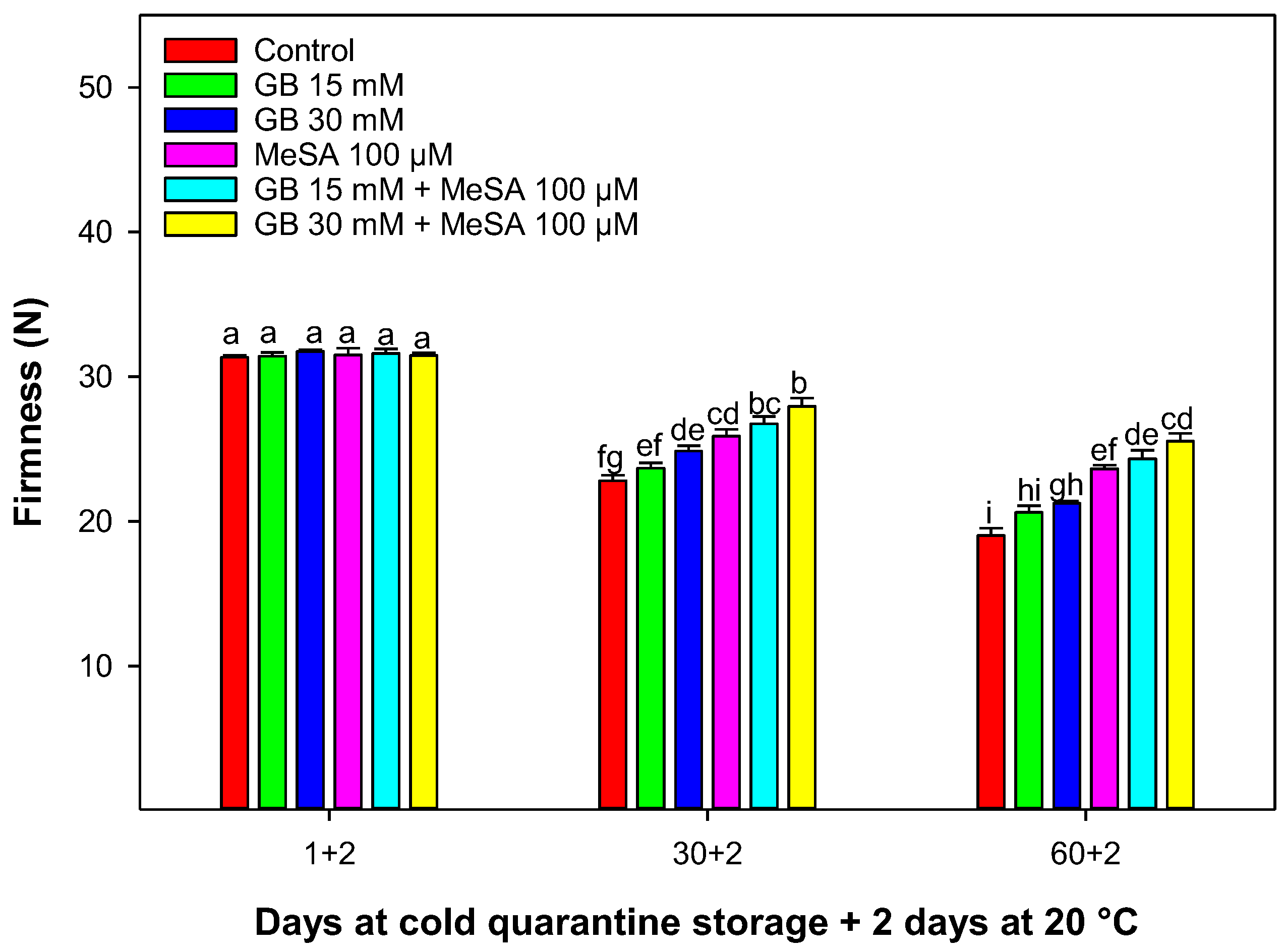
2.3. Firmness
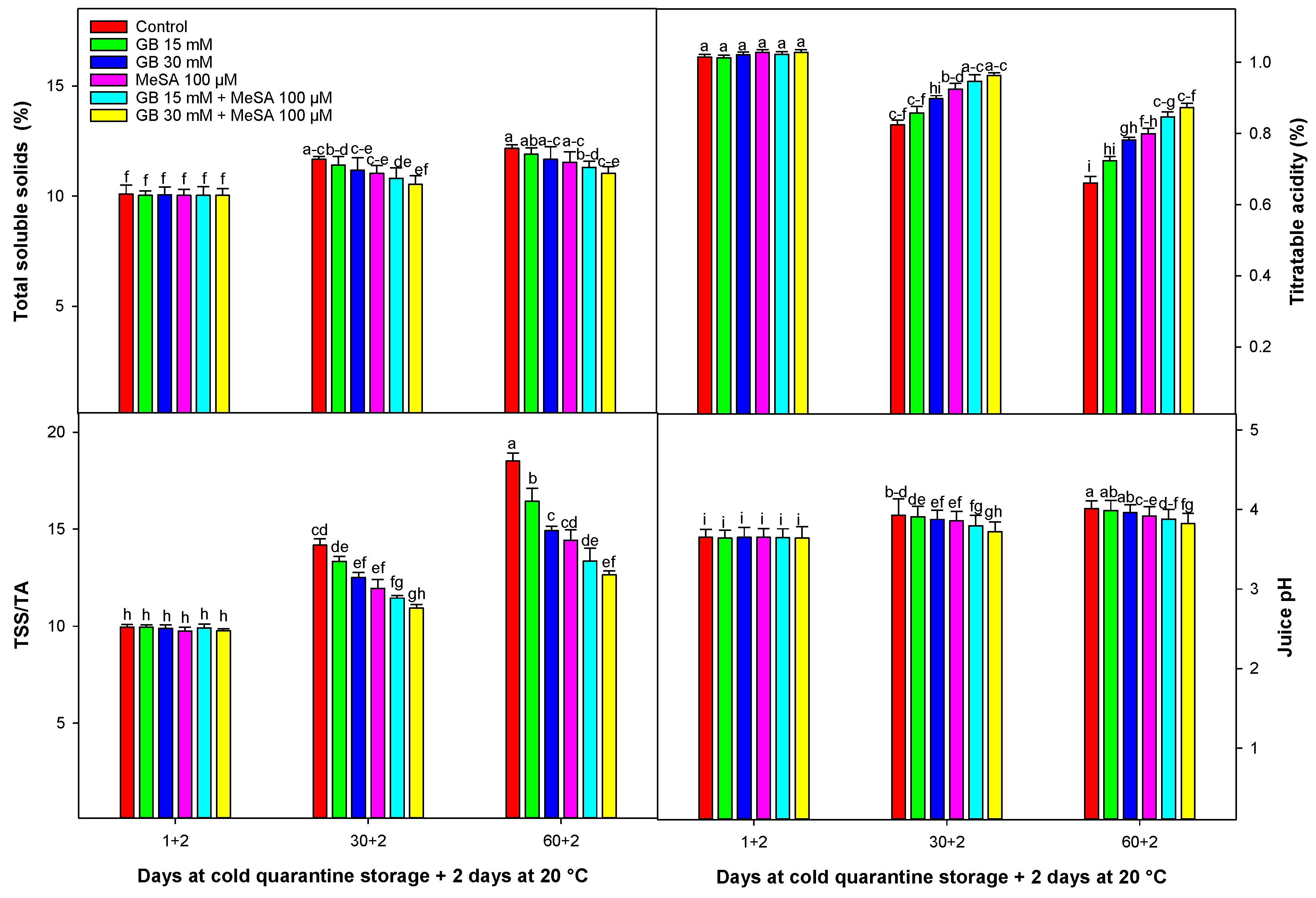
2.4. Chemical Attributes of Juice
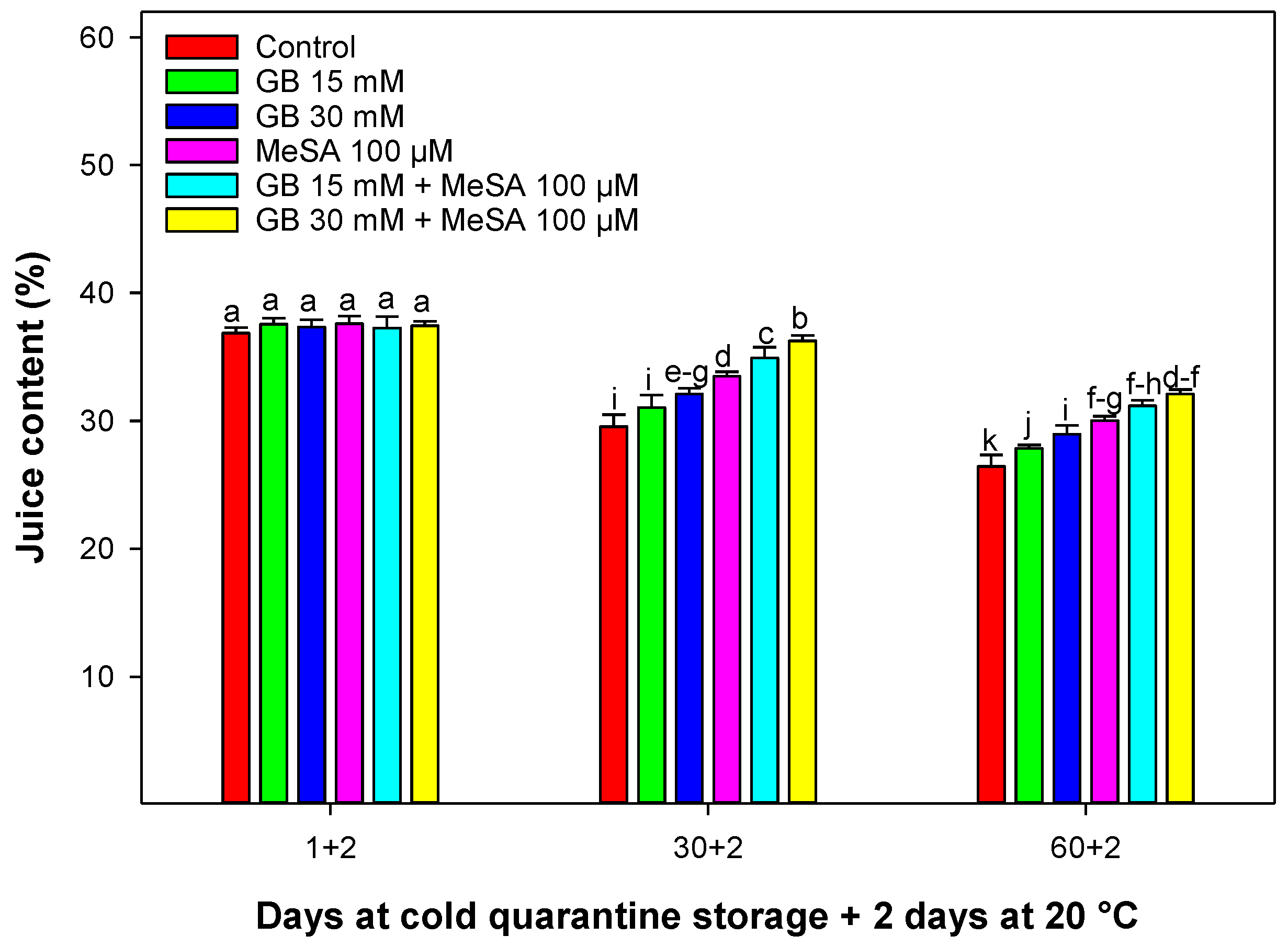
2.5. Juice Content
2.6. Peel Color
2.7. Statistical Analysis
3. Results
3.1. Weight Loss
3.2. Firmness
3.3. Chemical Attributes of Juice
3.4. Juice Content
3.5. Peel Color
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habibi, F.; García-Pastor, M.E.; Puente-Moreno, J.; Garrido-Auñón, F.; Serrano, M.; Valero, D. Anthocyanin in blood oranges: A review on postharvest approaches for its enhancement and preservation. Crit. Rev. Food Sci. Nutr. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carmona, L.; Zacarías, L.; Rodrigo, M.J. Stimulation of coloration and carotenoid biosynthesis during postharvest storage of ‘Navelina’ orange fruit at 12 C. Postharvest Biol. Technol. 2012, 74, 108–117. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Zapata, P.J.; Castillo, S.; Serrano, M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘Early Lory’ sweet cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Zuo, J.; Gao, L.; Fan, L. Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biol. Technol. 2016, 112, 114–120. [Google Scholar] [CrossRef]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous glycine betaine treatment enhances chilling tolerance of peach fruit during cold storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Wang, L.; Bokhary, S.U.F.; Xie, B.; Hu, S.; Jin, P.; Zheng, Y. Biochemical and molecular effects of glycine betaine treatment on membrane fatty acid metabolism in cold stored peaches. Postharvest Biol. Technol. 2019, 154, 58–69. [Google Scholar] [CrossRef]
- Wang, L.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y.; Wang, L.; Zheng, Y.; Jin, P. Amino acid metabolomic analysis involved in flavor quality and cold tolerance in peach fruit treated with exogenous glycine betaine. Food Res. Int. 2022, 157, 111204. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, P.; Huang, Y.; Shan, T.; Wang, L.; Li, Y.; Zheng, Y. Effect of hot water combined with glycine betaine alleviates chilling injury in cold-stored loquat fruit. Postharvest Biol. Technol. 2016, 118, 141–147. [Google Scholar] [CrossRef]
- Razavi, F.; Mahmoudi, R.; Rabiei, V.; Aghdam, M.S.; Soleimani, A. Glycine betaine treatment attenuates chilling injury and maintains nutritional quality of hawthorn fruit during storage at low temperature. Sci. Hortic. 2018, 233, 188–194. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, X.; Sun, H.; Zhou, Q.; Wei, B.; Cheng, S.; Ji, S.-j. Glycine betaine treatment alleviates loss of aroma-related esters in cold-stored ‘Nanguo’ pears by regulating the lipoxygenase pathway. Food Chem. 2020, 316, 126335. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Sun, Y.; Ge, W.; Wei, B.; Cheng, S.; Ji, S. Exogenous glycine betaine treatment alleviates low temperature-induced pericarp browning of ‘Nanguo’ pears by regulating antioxidant enzymes and proline metabolism. Food Chem. 2020, 306, 125626. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, M.; He, H.; Jiang, Y.; Yang, J.; Ji, S. Glycine betaine alleviated peel browning in cold-stored ‘Nanguo’ pears during shelf life by regulating phenylpropanoid and soluble sugar metabolisms. Sci. Hortic. 2020, 262, 109100. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Razavi, F.; Rabiei, V.; Gohari, G.; Palou, L. Application of glycine betaine coated chitosan nanoparticles alleviate chilling injury and maintain quality of plum (Prunus domestica L.) fruit. Int. J. Biol. Macromol. 2022, 207, 965–977. [Google Scholar] [CrossRef]
- Chen, L.-L.; Shan, W.; Cai, D.-L.; Chen, J.-Y.; Lu, W.-J.; Su, X.-G.; Kuang, J.-F. Postharvest application of glycine betaine ameliorates chilling injury in cold-stored banana fruit by enhancing antioxidant system. Sci. Hortic. 2021, 287, 110264. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Chen, C.; Zhang, S.; Zhao, X.; Wu, C.; Kou, X.; Xue, Z. Glycine betaine inhibits postharvest softening and quality decline of winter jujube fruit by regulating energy and antioxidant metabolism. Food Chem. 2023, 410, 135445. [Google Scholar] [CrossRef] [PubMed]
- Molaei, S.; Rabiei, V.; Soleimani, A.; Razavi, F. Exogenous application of glycine betaine increases the chilling tolerance of pomegranate fruits cv. Malase Saveh during cold storage. J. Food Process. Preserv. 2021, 45, e15315. [Google Scholar] [CrossRef]
- Huang, S.; Lim, S.Y.; Lau, H.; Ni, W.; Li, S.F.Y. Effect of glycinebetaine on metabolite profiles of cold-stored strawberry revealed by 1H NMR-based metabolomics. Food Chem. 2022, 393, 133452. [Google Scholar] [CrossRef]
- Habibi, F.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatment with glycine betaine enhances chilling tolerance of blood orange fruit by increasing antioxidant defence systems and osmoregulation during cold storage. Sci. Hortic. 2022, 305, 111352. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Sarkhosh, A.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with methyl salicylate and glycine betaine synergistically enhanced chilling tolerance and maintained bioactive compounds of blood orange fruit subjected to cold quarantine storage. LWT 2023, 185, 115141. [Google Scholar] [CrossRef]
- Habibi, F.; Guillén, F.; Serrano, M.; Valero, D. Physicochemical changes, peel colour, and juice attributes of blood orange cultivars stored at different temperatures. Horticulturae 2021, 7, 320. [Google Scholar] [CrossRef]
- Sdiri, S.; Navarro, P.; Monterde, A.; Benabda, J.; Salvador, A. Effect of postharvest degreening followed by a cold-quarantine treatment on vitamin C, phenolic compounds and antioxidant activity of early-season citrus fruit. Postharvest Biol. Technol. 2012, 65, 13–21. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Cao, J.; Kang, C.; Chen, Y.; Karim, N.; Wang, Y.; Sun, C. Physiochemical changes in Citrus reticulata cv. Shatangju fruit during vesicle collapse. Postharvest Biol. Technol. 2020, 165, 111180. [Google Scholar] [CrossRef]
- Chen, C. Pigments in Citrus Fruit: Mutants, Compounds, Genes, and Beyond. In The Citrus Genome. Compendium of Plant Genomes; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 195–209. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Valero, D. Susceptibility of blood orange cultivars to chilling injury based on antioxidant system and physiological and biochemical responses at different storage temperatures. Foods. 2020, 9, 1609. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, A.A.; Huysamer, M.; Barry, G.H. Extended low-temperature shipping adversely affects rind colour of ‘Palmer Navel’ sweet orange [Citrus sinensis (L.) Osb.] due to carotenoid degradation but can partially be mitigated by optimising post-shipping holding temperature. Postharvest Biol. Technol. 2009, 53, 109–116. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, F.; Sarkhosh, A.; Guillén, F.; Serrano, M.; Valero, D. Changes in Physicochemical Characteristics, Peel Color, and Juice Attributes of ‘Moro’ Blood Orange Fruit Treated with Glycine Betaine and Methyl Salicylate during Cold Quarantine Storage. Horticulturae 2023, 9, 1103. https://doi.org/10.3390/horticulturae9101103
Habibi F, Sarkhosh A, Guillén F, Serrano M, Valero D. Changes in Physicochemical Characteristics, Peel Color, and Juice Attributes of ‘Moro’ Blood Orange Fruit Treated with Glycine Betaine and Methyl Salicylate during Cold Quarantine Storage. Horticulturae. 2023; 9(10):1103. https://doi.org/10.3390/horticulturae9101103
Chicago/Turabian StyleHabibi, Fariborz, Ali Sarkhosh, Fabián Guillén, María Serrano, and Daniel Valero. 2023. "Changes in Physicochemical Characteristics, Peel Color, and Juice Attributes of ‘Moro’ Blood Orange Fruit Treated with Glycine Betaine and Methyl Salicylate during Cold Quarantine Storage" Horticulturae 9, no. 10: 1103. https://doi.org/10.3390/horticulturae9101103
APA StyleHabibi, F., Sarkhosh, A., Guillén, F., Serrano, M., & Valero, D. (2023). Changes in Physicochemical Characteristics, Peel Color, and Juice Attributes of ‘Moro’ Blood Orange Fruit Treated with Glycine Betaine and Methyl Salicylate during Cold Quarantine Storage. Horticulturae, 9(10), 1103. https://doi.org/10.3390/horticulturae9101103








