The Natural Alternatives: The Impact of Plant Extracts on Snowbush (Breynia disticha Forst.) Cuttings’ Morpho-Physiological and Biochemical Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cutting Preparation
2.2. Planting and Caring for Cuttings
2.3. Experimental Design and Data Recording
2.4. Statistical Analysis
3. Results and Discussions
3.1. Effect of the Rooting Treatments on Growth Parameters of Snowbush Cuttings
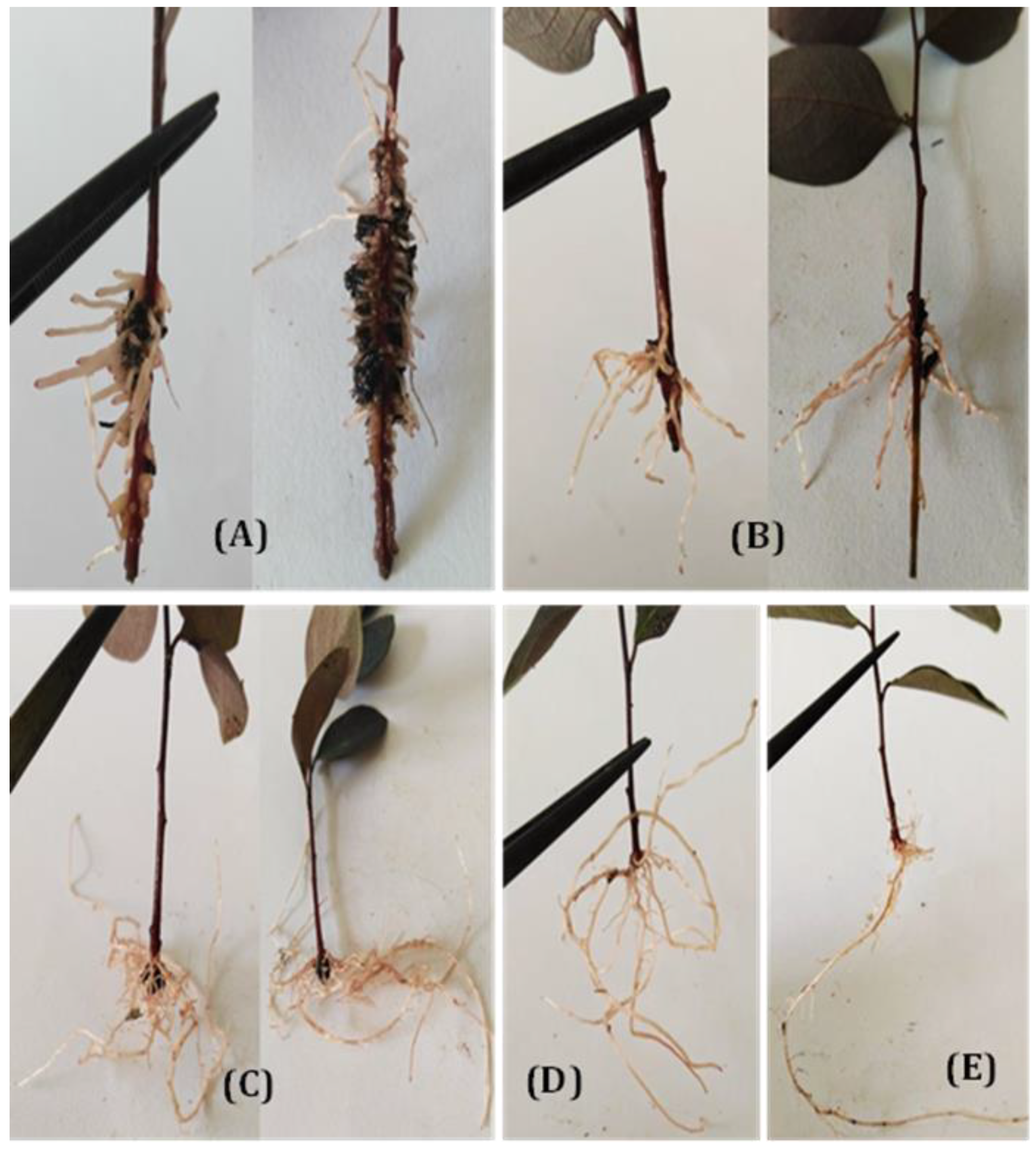
3.1.1. Rooting Behavior (Rooting Percentage, Root Number, and Root Length)
3.1.2. Shoot Length
3.1.3. Root Fresh and Dry Weight
3.2. Effect of the Rooting Treatments on Chemical Analysis of Snowbush Cuttings
3.2.1. Total Carbohydrates and Total Soluble Phenols
3.2.2. Nitrogen, Phosphorus, and Potassium
3.2.3. Plant Endogenous Hormones (IAA, GA3, and ABA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schum, A.; Preil, W. Induced Mutations in Ornamental Plants, Somaclonal Variation and Induced Mutations in Crop Improvement; Springer: Berlin/Heidelberg, Germany, 1998; pp. 333–366. [Google Scholar]
- Adam, K.L. Lavender Production, Products, Markets, and Entertainment Farms; A Publication of ATTRA; National Sustainable Agriculture Information Service: Butte, MT, USA, 2006. [Google Scholar]
- Potter, D.J. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Arjana, I.G.M.; Situmeang, Y.P.; Suaria, I.N.; Mudra, N.K.S. Effect of plant material and variety for production and quality Chrysanthemum. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 407–409. [Google Scholar] [CrossRef]
- Nakhooda, M.; Jain, S.M. A Review of Eucalyptus propagation and Conservation. Propag. Ornam. Plants 2016, 16, 101–119. [Google Scholar]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative propagation of cannabis by stem cuttings: Effects of leaf number, cutting position, rooting hormone, and leaf tip removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Agbo, C.; Obi, I. Variability in Propagation Potentials of Stem Cuttings of Different Physiological Ages of Gongronema latifolia Benth. World J. Agric. Sci. 2007, 3, 576–581. [Google Scholar]
- Hassanein, A.M. Factors influencing plant propagation efficiency via stem cuttings. J. Hortic. Sci. Ornamental Plants 2013, 5, 171–176. [Google Scholar]
- Weaver, R.J. Plant Growth Substances in Agriculture; W.H. Freeman and Co.: San Francisco, CA, USA, 1972. [Google Scholar]
- Cutler, H.G.; Schneider, B.A. Plant Growth Regulator Handbook; Plant Growth Regulator Society of America: La Grange, GA, USA, 1990. [Google Scholar]
- Dunsin, O.; Ajiboye, G.; Adeyemo, T. Effect of alternative hormones on the root ability of Parkia biglobosa. J. Agric. For. Soc. Sci. 2016, 12, 113–11812. [Google Scholar]
- Turetskaya, R.; Polikarpova, F. Plant Propagation Using Plant Growth Regulators; Publ. Science: Moscow, Russia, 1968. (In Russian) [Google Scholar]
- Chee, P.P. Stimulation of adventitious rooting of Taxus species by thiamine. Plant Cell Rep. 1995, 14, 753–757. [Google Scholar] [CrossRef]
- Hamouda, A.M.A.; Hendi, D.M.G.; Abu-El-Leel, O.F.A. Improving basil growth, yield and oil production by Aloe vera extract and active dry yeast. Egypt J. Hortic. 2012, 39, 45–71. [Google Scholar]
- Surjushe, A.; Vasani, R.; Saple, D. Aloe vera, A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Lanka, S. A review on Aloe vera-the wonder medicinal plant. J. Drug Deliv. Ther. 2018, 8, 94–99. [Google Scholar] [CrossRef]
- Sahu, K.P.; Giri, D.D.; Singh, R.; Pandey, P.; Gupta, S.; Shrivastava, A.K.; Kumar, A.; Pandey, D.K. Effect of Aloe vera on some annual plants. Sci. Res. Pharmacol. Pharm. 2013, 4, 599–610. [Google Scholar]
- Phiri, C.; Mbewe, D.N. Influence of Moringa oleifera by extract on germination and seedling survival of three common legumes. Int. J. Agric. Biol. 2010, 12, 315–317. [Google Scholar]
- Mishra, G.; Singh, P.; Verma, R.; Kumar, S.; Srivastav, S.; Jha, K.K.; Khosa, R.L. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant, An overview. Der Pharm. Lett. 2011, 3, 141–164. [Google Scholar]
- Foidl, N.; Makkar, H.P.S.; Becker, K. The potential of Moringa Oleifera for agricultural and industrial uses. In The Miracle Tree: The Multiple Attributes of Moringa; Lowell, J., Fuglie, C.T.A., Eds.; Church World Service: Wageningen, The Netherlands, 2001; pp. 45–76. [Google Scholar]
- Jacob, S.J.P.; Shenbagaraman, S. Evolution of antioxidant and antimicrobial activities of the selected green leafy vegetables. Int. J. PharmTech Res. 2011, 3, 148–152. [Google Scholar]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 470–478. [Google Scholar] [CrossRef]
- Al-Amad, I.; Qrunfleh, M. Effect of Babylon Weeping Willow (Salix babylonica L.) Extracts on Rooting of Stem Cuttings of Olive (Olea europaea L.) “Nabali”, 1130th ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2016; pp. 391–396. [Google Scholar]
- El-Shazly, A.; El-Sayed, A.; Fikrey, E. Bioactive secondary metabolites from Salix tetrasperma Roxb. Z. Naturforschung. C 2012, 67, 353–359. [Google Scholar] [CrossRef]
- El-Sayed, M.; El-Hashash, M.; Mohamed, R.; Abdel-Lateef, E. Phytochemical Investigation and in vitro antioxidant activity of different leaf extracts of Salix mucronate. Thunb. J. Appl. Pharm. Sci. 2015, 5, 80–85. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y. Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. Int. J. Food Sci. Technol. 2010, 45, 1837–1842. [Google Scholar] [CrossRef]
- Abid, S.; Touqeer, S. Antimicrobial and antioxidant activity of Breynia disticha and Vernonia elaegnifolia. J. Appl. Pharm. 2015, 7, 178–182. [Google Scholar] [CrossRef]
- Onyegbule, F.A.; Ilouno, I.O.; Ikeh, C.; Umeokoli, B.O.; Eze, P.M. Evaluation of phytochemical constituents, analgesic, anti-inflammatory, antimicrobial and antioxidant activities of extracts of Breynia nivosa leaves. Planta Med. 2014, 80, LP18. [Google Scholar] [CrossRef]
- Amadi, E.S.; Oveka, C.A.; Onveagba, R.A.; Ugbogu, O.C.; Okoli, I. Antimicrobial screening of Breynia nivosus and ageratum conzynoides against dental caries organisms. J. Biol. Sci. 2007, 7, 354–358. [Google Scholar] [CrossRef]
- Jude, E.O.; Koofreh, D.; Azare, B.A. Antimalarial activities of Breynia nivosa. J. Herb. Drugs 2015, 5, 168–172. [Google Scholar]
- Vic, F. Propagation of Breynia disticha “roseo-picta” by hardwood cuttings. Int. Plant Propagator’s Soc. 1987, 37, 130–131. [Google Scholar]
- Pregl, F. Quantitative Organic Microanalysis, 4th ed.; I. Chudrial: London, UK, 1945. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentica Hall Inc.: Engleweed Cliffs, NJ, USA, 1967. [Google Scholar]
- Black, C.A. “Methods of Soil Analysis”. Part 1. Physical and Mineralogical; ASA Madison: Madison, WI, USA, 1965. [Google Scholar]
- Fales, F.W. The assimilation and degradation of carbohydrates by yeast cells. J. Biol. Chem. 1951, 193, 113–124. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 13th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1970; pp. 376–384. [Google Scholar]
- Daniel, H.D.; George, C.M. Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. J. Am. Soc. Hortic. Sci. 1972, 97, 651–654. [Google Scholar]
- Chen, Y.P.; Yang, W.Y. Determination of GA3, IAA, ABA and ZT in dormant buds of Allium ovalifolium by HPLC. J. Sichuan Agric. Univ. 2005, 23, 498–500. [Google Scholar]
- Costst. Software Program for the Design and Analysis of Agronomic Research Experiments, Chort. Software, Costat 3-30; Costat: Berkeley, CA, USA, 1986.
- Singh, B.; Rawat, J.M.S. Effects of cuttings types and hormonal concentration on vegetative propagation of Zanthoxylum armatum in Garhwal Himalaya India. J. For. Res. 2017, 28, 419–423. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Youssef, A.S.M.; Hegazy, N.E. Effect of IBA, rooting media and planting date on vegetative propagation of button wood tree (Conocarpus erectus L.). Ann. Agric. Sci. Moshtohor 2014, 52, 123–132. [Google Scholar]
- Massoud, H.Y.A.; Abd El-Baset, M.M.; Ghozzy, A.A. Effect of some natural products as an alternative chemical growth regulator on rooting response‚ growth and chemical composition of rosemary cutting. J. Plant Prod. Mansoura Univ. 2017, 8, 797–803. [Google Scholar] [CrossRef]
- Haissig, B.E. Influence of auxins and auxin synergists on adventitious root primordium initiation and development. N. Z. J. For. Sci. 1974, 4, 311–323. [Google Scholar]
- Webster, P.L.; Van Hof, J. DNA synthesis and mitosis in meristems, requirements for RNA and protein synthesis. Am. J. Bot. 1970, 57, 130–139. [Google Scholar] [CrossRef]
- Tripathi, A.N.; Pandey, G.; Shukla, P.K. Effect of auxins on rooting behavior of Buphorbia pulcherrima willd. Progress. Hortic. 2003, 35, 111–113. [Google Scholar]
- Bhagya, H.P.; Lalithya, K.A.; Bharathi, K. Influence of growth hormones and nodal cutting on rooting of Vitex negundo L. Indian J. Agric. Res. 2014, 48, 81–88. [Google Scholar] [CrossRef]
- Dawa, S.; Rather, Z.A.; Tundup, P.; Tamchos, T. Effect of growth regulators and growth media on rooting of semi hardwood cuttings of rose root stocks. Int. J. Curr. Microbiol. App. Sci. 2014, 6, 1042–1051. [Google Scholar]
- Abd El Hameed, N.S. Effect of Indole butyric acid (IBA), cutting type and planting date on cuttings rooting of Myrtus communis. Middle East J. Agric. Res. 2018, 7, 1135–1145. [Google Scholar]
- Hartmann, H.T.; Kester, D.E. Plant Propagation, Principles and Practices, 2nd ed.; Printice-Hall. Inc.: Engle Wood Cliffs, NJ, USA, 1968; pp. 66–304. [Google Scholar]
- Pierick, R.L.M. In Vitro Culture of Higher Plants; Kluwer-Acad. Publ.: Dadrecht, The Netherlands, 1986; p. 69. [Google Scholar]
- Hussein, M.M.M. Studies on the rooting and consequent plant growth on the stem cuttings of Thunbergia grandiflora (Roxb.ex Rottl.) Roxb.2- Effect of indole-3-butyric acid. World J. Agric. Sci. 2008, 4, 811–817. [Google Scholar]
- Davies, F.T., Jr.; Lazarte, J.E.; Joiner, J.N. Initiation and development of root in juvenile and mature leaf bud cuttings of Ficus pumila L. Am. J. Bot. 1982, 69, 804–811. [Google Scholar] [CrossRef]
- Mato, M.C.; Vieitez, A.M. Changes in auxin protectors and IAA oxidase during the rooting of chestnut shoots in vitro. Physiol. Plant. 1986, 66, 491–494. [Google Scholar] [CrossRef]
- Mirihagalla, M.K.P.N.; Fernando, K.M.C. Effect of Aloe vera Gel for Inducing Rooting of Stem Cuttings and Air layering of Plants. J. Dry Zone Agric. 2020, 6, 13–26. [Google Scholar]
- Wise, K.; Gill, H.; Selby-Pham, J. Willow bark extract and the biostimulant complex Root Nectar® increase propagation efficiency in chrysanthemum and lavender cuttings. Sci. Hortic. 2020, 263, 109108. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chakraborty, B.; Nandy, S. Aloe vera plant, review with significant pharmacological activities. Mintage J. Pharm. Med. Sci. 2013, 2, 21–24. [Google Scholar]
- El Sherif, F. Aloe vera leaf extract as a potential growth enhancer for populus trees grown under in vitro conditions. Am. J. Plant Biol. 2017, 2, 101–105. [Google Scholar]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U. Molecular physiology of adventitious root formation in Petunia hybrida cuttings, involvement of wound response and primary metabolism. New Phytol. 2019, 181, 613–625. [Google Scholar] [CrossRef]
- Callahan, D.L.; Kolev, S.D.; Richard, A.; Salt, D.E.; Baker, A.J. Relationships of nicotianamine and other amino acids with nickel, zinc and iron in Thlaspi hyperaccumulators. New Phytol. 2007, 176, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Davis, R.H.; Maro, N.P. Aloe vera and gibberellin. Anti-inflammatory activity in diabetes. J. Am. Podiatr. Med. Assoc. 1989, 79, 24–26. [Google Scholar] [CrossRef]
- Santos, K.M.; Fisher, P.R.; Argo, W.R. Stem versus foliar uptake during propagation of Petunia × hybrida vegetative cuttings. HortScience 2009, 44, 1974–1977. [Google Scholar] [CrossRef]
- Kling, G.; Meyer, M., Jr. Effects of phenolic compounds and indoleacetic acid on adventitious root initiation in cuttings of Phaseolus aureus, Acer saccharinum, and Acer griseum. HortScience 1983, 18, 352–354. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment, a review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Basu, R.; Bose, T.; Roy, B.; Mukhopadhyay, A. Auxin synergists in rooting of cuttings. Physiol. Plant. 1969, 22, 649–652. [Google Scholar] [CrossRef]
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- Lee, A.; Cho, K.; Jang, S.; Rakwal, R.; Iwahashi, H.; Agrawal, G.K.; Shim, J.; Han, O. Inverse correlation between jasmonic acid and salicylic acid during early wound response in rice. Biochem. Biophys. Res. Commun. 2004, 318, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; Ramón Guerrero, J.; Angel Cano, E.; Acosta, M.; Sánchez-Bravo, J. Origin and basipetal transport of the IAA responsible for rooting of carnation cuttings. Physiol. Plant. 2002, 114, 303–312. [Google Scholar] [CrossRef]
- Fuglie, L.J. Natural Nutrition for the tropics. In The Miracle Tree, Moringa oleifers; Xlibris Corporation: Bloomington, IN, USA, 2000. [Google Scholar]
- Yasmeen, A. Exploring the Potential of Moringa (Moringa oleifera) Leaf Extract as Natural Plant Growth Enhancer. Ph.D. Thesis, University of Agriculture, Faisalabad, Pakistan, 2011. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Tilahun, A.; Manahlie, B.; Abebe, G.; Negash, G. Effect of cutting position and indole butyric acid (auxin) concentration on rooting response of Araucaria heterophylla. Afr. J. Biotech. 2019, 18, 86–91. [Google Scholar]
- Abdel-Rahman, S.S.A.; Abdul-Hafeez, E.Y.; Asmaa, M.M.S. Improving rooting and growth of Conocarpus Erectus stem cuttings using Indole-3-Butyric Acid 22 (IBA) and some biostimulants. Sci. J. Flowers Ornam. Plants 2020, 7, 109–129. [Google Scholar] [CrossRef]
- Izadi, M.; Shahsavar, A.R.; Mirsoleimani, A. Relation between leaf and stem biochemical constituents and rooting ability of olive cuttings. Int. J. Hort. Sci. Technol. 2016, 3, 231–242. [Google Scholar]
- Pacholczak, A.; Nowakowska, K.; Mika, N.; Borkowska, M. The effects of the biostimulator Goteo on the rooting of ninebark stem cuttings. Folia Hort. 2011, 28, 109–116. [Google Scholar] [CrossRef][Green Version]
- Fabbri, A.; Bartolini, G.; Lombardi, M.; Kailis, S. Olive Propagation Manual; CSIRO Publishing: Melbourne, Australia, 2004; 133p. [Google Scholar]
- Yoo, Y.K.; Kim, K.S. Seasonal variation in rooting ability, plant hormones, carbohydrate, nitrogen, starch and soluble sugar contents in cuttings of white Forsythia (Abeliophyllum distichum Nakai). J. Kor. Soc. Hort. Sci. 1996, 37, 554–560. [Google Scholar]
- Davies, P.J. The Plant Hormones, Their Nature, Occurrence and Functions. In Plant Hormones, Biosynthesis, Signal Transduction, Action; Davies, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1–15. [Google Scholar]
- Arslonov, M.N. Physiological changes occurring during root formation in lemon cuttings. Uzabekskii Biol. Zhurnal 1979, 5, 24–26. [Google Scholar]
- Cannon, M.J.; Myszka, D.G.; Bagnate, D.J.; Alpers, D.H.; West, F.G.; Grissom, C.B. Equilibrium and kinetic analyses of the interactions between vitamin B12 binding proteins and colalamins by surface plasman resonance. Anal. Biochem. 2002, 305, 1–9. [Google Scholar] [CrossRef]
- Aslmoshtaghi, E.; Shahsavar, A.R. Endogenous soluble sugars, starch contents and phenolic compounds in easy- and difficult-to-root olive cuttings. J. Biol. Environ. Sci. 2010, 4, 83–86. [Google Scholar]
- Trobec, M.; Stampar, F.; Veberic, R.; Osterc, G. Fluctuations of different endogenous phenolic compounds and cinnamic acid in the first days of the rooting process of cherry rootstock “Gisela 5” leafy cuttings. J. Plant Physiol. 2005, 162, 589–597. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indole acetic acid in Malus “Jork 9”. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef]
- Scagel, C.F.; Linderman, R.G. Influence of ectomycorrhizal fungi inoculation on growth and root IAA concentrations of transplanted conifers. Tree Physiol. 1998, 18, 739–747. [Google Scholar] [CrossRef]
- Shin, H.K.; Chun, C.K.; Choi, S.T. Seasonal changes of rooting ability in herbaceous cuttings of Gypsophilla paniculata L. cv. Bristol Fairy. J. Kor. Soc. Hort. Sci. 1988, 29, 319–327. [Google Scholar]
- Sagee, A.; Raviv, M.; Medina, S.; Becker, D.; Cosse, A. Involvement of rooting factors and free IAA in the root ability of Citrus species stem cuttings. Sci. Hort. 1992, 51, 187–195. [Google Scholar] [CrossRef]
- Fouda, R.A.; Abdel-Kader, H.H.; El-Hindi, K.H.; Massoud, H.Y.; Ibrahim, F.R. Effect of wounding, auxin type and concentration on rooting of lemon verbena (Aloysia triphylla (l’her.) britton) plant. J. Plant Prod. Mansoura Univ. 2012, 3, 2927–2943. [Google Scholar] [CrossRef]
- Ibironke, O.A. Root initiation of Bougainvillea from cuttings using different rooting hormones. Adv. Plants Agric. Res. 2019, 9, 121–125. [Google Scholar] [CrossRef]
- Dada, C.A.; Kayode, J.; Arowosegbe, S. Effects of rooting hormones on the juvenile stem cuttings of Annona muricata Linn. (Annonaceae). World News Nat. Sci. 2019, 23, 336–342. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 7th ed.; Freeman: New York, NY, USA, 2005; 686p. [Google Scholar]
- Jiang, K.; Feldman, L.J. Regulation of root apical meristem development. Annu. Rev. Cell Dev. Biol. 2005, 21, 485–509. [Google Scholar] [CrossRef] [PubMed]
- Ponce, G.; Barlow, P.W.; Feldman, L.J.; Cassab, G.I. Auxin and ethylene interactions control mitotic activity of the quiescent center, root cap size and pattern of cap cell differentiation in maize. Plant Cell Environ. 2005, 28, 719–732. [Google Scholar] [CrossRef]
- Aloni, R. The induction of vascular tissue by auxin. In Plant Hormones: Biosynthesis, Signal Transduction, Action! Davies, P.J., Ed.; Kluwer: Dordrecht, The Netherlands, 2004; pp. 471–492. [Google Scholar]
- Petridou, M.K.; Porlingis, I. Pre-sowing application of gibberellic acid on seeds used for the mung bean bioassay, promotes root formation in cuttings. Sci. Hortic. 1997, 70, 203–210. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann, and Kester’s Plant Propagation: Principles and Practices, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2014; pp. 395–399. [Google Scholar]
- Coleman, W.K.; Greyson, R.I. Promotion of root initiation by gibberellic acid in leaf discs of tomato (Lycopersicon esculentum) cultured in vitro. New Phytol. 1977, 78, 47–54. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting actions of rhizobacteria. Adv. Bot. Res. 2009, 51, 283–320. [Google Scholar]
- Liang, Y.T.; Long, Z.R. Principles and Techniques of Vegetative Propagation of Plants; Chinese Forestry Press: Beijing, China, 1993. [Google Scholar]
- Zhang, X.; Li, L.Y.; Yang, X. The change of endogenous hormone content in the loniceramacranthoides hands-mazz cutting rooting process. J. Chin. Med. Mater. 2012, 35, 521–525. [Google Scholar]
- Yamaguchi, M.; Sharp, R.E. Complexity and coordination of root growth at low water potentials: Recent advances from transcriptomic and proteomic analyses. Plant Cell Environ. 2010, 33, 590–603. [Google Scholar] [CrossRef]

| Treatments | Rooting % | Root Number/Cutting | Root Length (cm) | Shoot Length (cm) | Rooting % | Root Number/Cutting | Root Length (cm) | Shoot Length (cm) |
|---|---|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | |||||||
| Control | 0.0 g | 0.0 h | 0.0 h | 0.0 i | 0.0 g | 0.0 g | 0.0 j | 0.0 h |
| NAA at 2000 ppm | 87.5 ± 7.2 a | 21.69 ± 1.10 a | 0.82 ± 0.03 g | 11.17 ± 0.14 e | 93.8 ± 6.25 a | 22.08 ± 0.79 a | 0.85 ± 0.04 i | 10.93 ± 0.11 ef |
| IBA at 2000 ppm | 81.2 ± 6.2 ab | 8.83 ± 0.39 b | 2.13 ± 0.02 f | 11.34 ± 0.13 e | 87.5 ± 7.21 a | 8.66 ± 0.13 b | 2.03 ± 0.09 h | 11.27 ± 0.14 de |
| Honey 75% | 37.5 ± 7.2 ef | 2.63 ± 0.23 f | 3.21 ± 0.10 e | 10.39 ± 0.16 g | 31.2 ± 6.25 ef | 2.75 ± 0.14 e | 3.26 ± 0.08 g | 10.62 ± 0.20 f |
| Honey 100% | 43.8 ± 6.2 e | 1.25 ± 0.14 g | 4.18 ± 0.03 c | 10.82 ± 0.17 f | 43.8 ± 6.25 de | 1.50 ± 0.20 f | 4.25 ± 0.14 de | 10.69 ± 0.24 f |
| Aloe extract 75% | 75.0 ± 0.0 abc | 4.33 ± 0.13 de | 5.04 ± 0.12 b | 12.85 ± 0.12 c | 68.8 ± 6.25 bc | 4.41 ± 0.15 d | 5.46 ± 0.07 b | 12.80 ± 0.16 b |
| Aloe extract 100% | 75.0 ± 0.0 abc | 5.98 ± 0.48 c | 6.04 ± 0.02 a | 13.24 ± 0.02 b | 81.2 ± 6.25 ab | 6.14 ± 0.21 c | 6.35 ± 0.05 a | 13.25 ± 0.12 b |
| Willow extract 75% | 68.8 ± 6.2 bc | 3.42 ± 0.20 def | 4.26 ± 0.10 c | 12.69 ± 0.06 c | 62.5 ± 7.21 c | 3.54 ± 0.28 de | 4.11 ± 0.15 e | 11.93 ± 0.04 c |
| Willow extract 100% | 75.0 ± 0.0 abc | 4.58 ± 0.14 d | 4.90 ± 0.03 b | 11.99 ± 0.06 d | 68.8 ± 6.25 bc | 4.29 ± 0.17 d | 4.62 ± 0.09 c | 11.64 ± 0.10 cd |
| Moringa extract 75% | 50.0 ± 0.0 de | 3.13 ± 0.12 ef | 3.10 ± 0.08 e | 13.35 ± 0.09 ab | 50.0 ± 0.0 cd | 3.33 ± 0.23 e | 3.24 ± 0.08 g | 13.82 ± 0.26 a |
| Moringa extract 100% | 62.5 ± 7.2 cd | 4.17 ± 0.09 ed | 3.72 ± 0.09 d | 13.59 ± 0.06 a | 56.2 ± 6.25 cd | 4.21 ± 0.12 d | 3.77 ± 0.04 f | 13.84 ± 0.14 a |
| Cinnamon | 25.0 ± 0.0 f | 1.38 ± 0.23 g | 4.08 ± 0.14 c | 9.79 ± 0.08 h | 18.8 ± 6.25 f | 1.25 ± 0.14 f | 4.48 ± 0.08 cd | 9.25 ± 0.26 g |
| Treatments | Root Fresh Weight (mg) | Root Dry Weight (mg) | Root Fresh Weight (mg) | Root Dry Weight (mg) |
|---|---|---|---|---|
| 1st Season | 2nd Season | |||
| Control | 0.0 i | 0.0 h | 0.0 j | 0.0 h |
| NAA at 2000 ppm | 65 ± 6 h | 6 g | 95 ± 3 i | 9 g |
| IBA at 2000 ppm | 360 ± 7 c | 31 bc | 352 ± 1 d | 30 d |
| Honey 75% | 162 ± 3 f | 19 e | 180 ± 7 g | 21 e |
| Honey 100% | 109 ± 5 g | 102 f | 133 ± 102 h | 14 f |
| Aloe extract 75% | 456 ± 8 b | 37 a | 463 ± 14 b | 37 b |
| Aloe extract 100% | 492 ± 6 a | 39 a | 487 ± 7 a | 40 a |
| Willow extract 75% | 380 ± 10 c | 30 c | 352 ± 12 d | 31 cd |
| Willow extract 100% | 446 ± 15 b | 34 b | 422 ± 9 c | 34 bc |
| Moringa extract 75% | 286 ± 4 e | 19 e | 256 ± 6 f | 17 f |
| Moringa extract 100% | 311 ± 9 d | 27 d | 289 ± 4 e | 23 e |
| Cinnamon | 112 ± 7 g | 14 f | 133 ± 6 h | 15 f |
| Treatments | Total Carbohydrates (%) | Total Soluble Phenols (mg/g DW) | Total Carbohydrates (%) | Total Soluble Phenols (mg/g DW) |
|---|---|---|---|---|
| 1st Season | 2nd Season | |||
| NAA at 2000 ppm | 20.63 ± 0.16 hi | 3.64 ± 0.02 cd | 20.43 ± 0.29 g | 3.71 ± 0.16 c |
| IBA at 2000 ppm | 25.43 ± 0.47 c | 4.08 ± 0.04 a | 25.25 ± 0.074 c | 4.16 ± 0.05 a |
| Honey 75% | 21.30 ± 0.30 gh | 2.54 ± 0.06 g | 21.23 ± 0.30 fg | 2.52 ± 0.04 e |
| Honey 100% | 22.06 ± 0.11 fg | 2.78 ± 0.09 f | 22.11 ± 0.04 ef | 2.58 ± 0.03 e |
| Aloe extract 75% | 27.86 ± 0.46 b | 3.95 ± 0.03 ab | 27.67 ± 0.29 b | 3.94 ± 0.05 b |
| Aloe extract 100% | 29.79 ± 0.09 a | 4.04 ± 0.06 a | 31.61 ± 0.52 a | 4.14 ± 0.03 a |
| Willow extract 75% | 23.60 ± 0.31 d | 3.73 ± 0.10 bcd | 23.58 ± 0.29 d | 3.85 ± 0.03 bc |
| Willow extract 100% | 25.85 ± 0.42 c | 3.84 ± 0.07 abc | 26.22 ± 0.39 c | 3.96 ± 0.02 b |
| Moringa extract 75% | 22.35 ± 0.07 ef | 3.38 ± 0.07 e | 21.24 ± 0.09 fg | 3.49 ± 0.02 d |
| Moringa extract 100% | 23.15 ± 0.44 de | 3.55 ± 0.10 de | 22.38 ± 0.60 e | 3.69 ± 0.02 c |
| Cinnamon | 19.88 ± 0.12 i | 2.46 ± 0.14 g | 18.29 ± 0.31 h | 2.15 ± 0.02 f |
| Treatments | N (g/kg) | P(g/kg) | K (g/kg) | N (g/kg) | P (g/kg) | K (g/kg) |
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | |||||
| NAA at 2000 ppm | 11.65 ± 0.17 d | 2.13 ± 0.14 d | 9.32 ± 0.25 de | 10.95 ± 0.32 b | 2.03 ± 0.04 e | 10.85 ± 0.15 de |
| IBA at 2000 ppm | 14.40 ± 0.20 a | 2.88 ± 0.06 b | 14.25 ± 0.85 a | 14.38 ± 0.22 a | 2.98 ± 0.07 b | 14.00 ± 0.40 ab |
| Honey 75% | 9.73 ± 0.54 e | 2.55 ± 0.06 c | 8.25 ± 0.62 ef | 8.60 ± 0.35 c | 2.48 ± 0.08 cd | 10.25 ± 0.62 ef |
| Honey 100% | 1.48 ± 0.30 e | 2.75 ± 0.05 b | 11.50 ± 0.28 bc | 10.23 ± 0.19 b | 2.75 ± 0.05 bc | 13.00 ± 0.40 bc |
| Aloe extract 75% | 12.50 ± 0.21 c | 3.18 ± 0.04 a | 13.75 ± 0.47 a | 13.95 ± 0.43 a | 3.18 ± 0.04 ab | 15.25 ± 0.25 a |
| Aloe extract 100% | 13.55 ± 0.37 b | 3.35 ± 0.02 a | 14.75 ± 0.47 a | 14.37 ± 0.35 a | 3.48 ± 0.06 a | 15.25 ± 0.25 a |
| Willow extract 75% | 1.48 ± 0.34 e | 2.85 ± 0.02 b | 9.25 ± 0.62 de | 10.15 ± 0.27 b | 3.00 ± 0.04 b | 11.25 ± 0.47 de |
| Willow extract 100% | 11.50 ± 0.25 d | 2.95 ± 0.02 b | 11.75 ± 0.10 b | 10.65 ± 0.23 b | 2.95 ± 0.06 b | 13.25 ± 0.47 bc |
| Moringa extract 75% | 6.53 0.24±g | 2.00 ± 0.04 de | 10.25 ± 0.32 cd | 6.93 ± 0.17 d | 2.10 ± 0.04 de | 12.00 ± 0.40 cd |
| Moringa extract 100% | 8.65 ± 0.17 f | 2.75 ± 0.02 b | 12.38 ± 0.23 b | 8.85 ± 0.17 c | 2.75 ± 0.06 bc | 13.25 ± 0.47 bc |
| Cinnamon | 5.73 ± 0.20 g | 1.90 ± 0.07 e | 7.28 ± 0.26 f | 4.95 ± 0.21 e | 1.52 ± 0.44 f | 9.38 ± 0.83 f |
| Treatments | IAA (μg/g f.w.) | GA3(μg/g f.w.) | ABA(μg/g f.w.) |
|---|---|---|---|
| 2nd Season | |||
| IBA at 2000 ppm | 249.7 ± 6.6 a | 376.4 ± 14.3 a | 0.87 ± 0.07 d |
| Honey 100% | 62.9 ± 0.9 e | 92.4 ± 1.7 e | 2.62 ± 0.10 a |
| Aloe extract 100% | 203.0 ± 7.0 b | 283.5 ± 8.6 b | 1.04 ± 0.01 d |
| Willow extract 100% | 126.9 ± 5.4 c | 234.9 ± 7.7 c | 1.36 ± 0.09 c |
| Moringa extract 100% | 99.4 ± 1.1 d | 185.0 ± 2.6 d | 1.71 ± 0.10 b |
| Cinnamon | 53.1 ± 0.9 e | 89.5 ± 1.2 e | 2.77 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Banna, H.; Haroun, S.A.; Albishi, T.S.; Rashed, A.A.; Albadrani, M.; Abdelaal, K.; Alkhateeb, O.A.; Abdou, A.H. The Natural Alternatives: The Impact of Plant Extracts on Snowbush (Breynia disticha Forst.) Cuttings’ Morpho-Physiological and Biochemical Characteristics. Horticulturae 2023, 9, 1122. https://doi.org/10.3390/horticulturae9101122
El-Banna H, Haroun SA, Albishi TS, Rashed AA, Albadrani M, Abdelaal K, Alkhateeb OA, Abdou AH. The Natural Alternatives: The Impact of Plant Extracts on Snowbush (Breynia disticha Forst.) Cuttings’ Morpho-Physiological and Biochemical Characteristics. Horticulturae. 2023; 9(10):1122. https://doi.org/10.3390/horticulturae9101122
Chicago/Turabian StyleEl-Banna, Heba, Samia A. Haroun, Tasahil S. Albishi, Afaf Abdullah Rashed, Muayad Albadrani, Khaled Abdelaal, Omar Abdullah Alkhateeb, and Ahmed Hassan Abdou. 2023. "The Natural Alternatives: The Impact of Plant Extracts on Snowbush (Breynia disticha Forst.) Cuttings’ Morpho-Physiological and Biochemical Characteristics" Horticulturae 9, no. 10: 1122. https://doi.org/10.3390/horticulturae9101122
APA StyleEl-Banna, H., Haroun, S. A., Albishi, T. S., Rashed, A. A., Albadrani, M., Abdelaal, K., Alkhateeb, O. A., & Abdou, A. H. (2023). The Natural Alternatives: The Impact of Plant Extracts on Snowbush (Breynia disticha Forst.) Cuttings’ Morpho-Physiological and Biochemical Characteristics. Horticulturae, 9(10), 1122. https://doi.org/10.3390/horticulturae9101122








