Efficacy and Potential Mechanism of Essential Oils of Three Labiatae Plants against the Pathogenic Fungi of Root Rot Disease in Atractylodes chinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oils and Chemicals
2.2. Pathogenic Fungi
2.3. Measurement of In Vitro Antifungal Activity of Three EOs
2.4. Measurement of In Vitro Antifungal Activity of Three EOs
2.5. GC-MS Determination of O. vulgare EO and T. mongolicus EO
2.6. Measurement of Fungal Inhibitory Activities of Main Components
2.7. Determination of MIC
2.8. Effect of O. vulgare EO on the Mycelial Morphology of F. oxysporum
2.8.1. Optical Microscope Observation
2.8.2. Scanning Electron Microscopy (SEM)
2.8.3. Transmission Electron Microscopy (TEM)
2.9. Effect on Leakage of Cell Contents of F. oxysporum
2.10. Measurement of Intracellular Reactive Oxygen Species (ROS) Production
2.11. Effects of Exogenous ROS Scavengers on the Activity of O. vulgare EO
2.12. Intracellular GSH Detection
3. Results
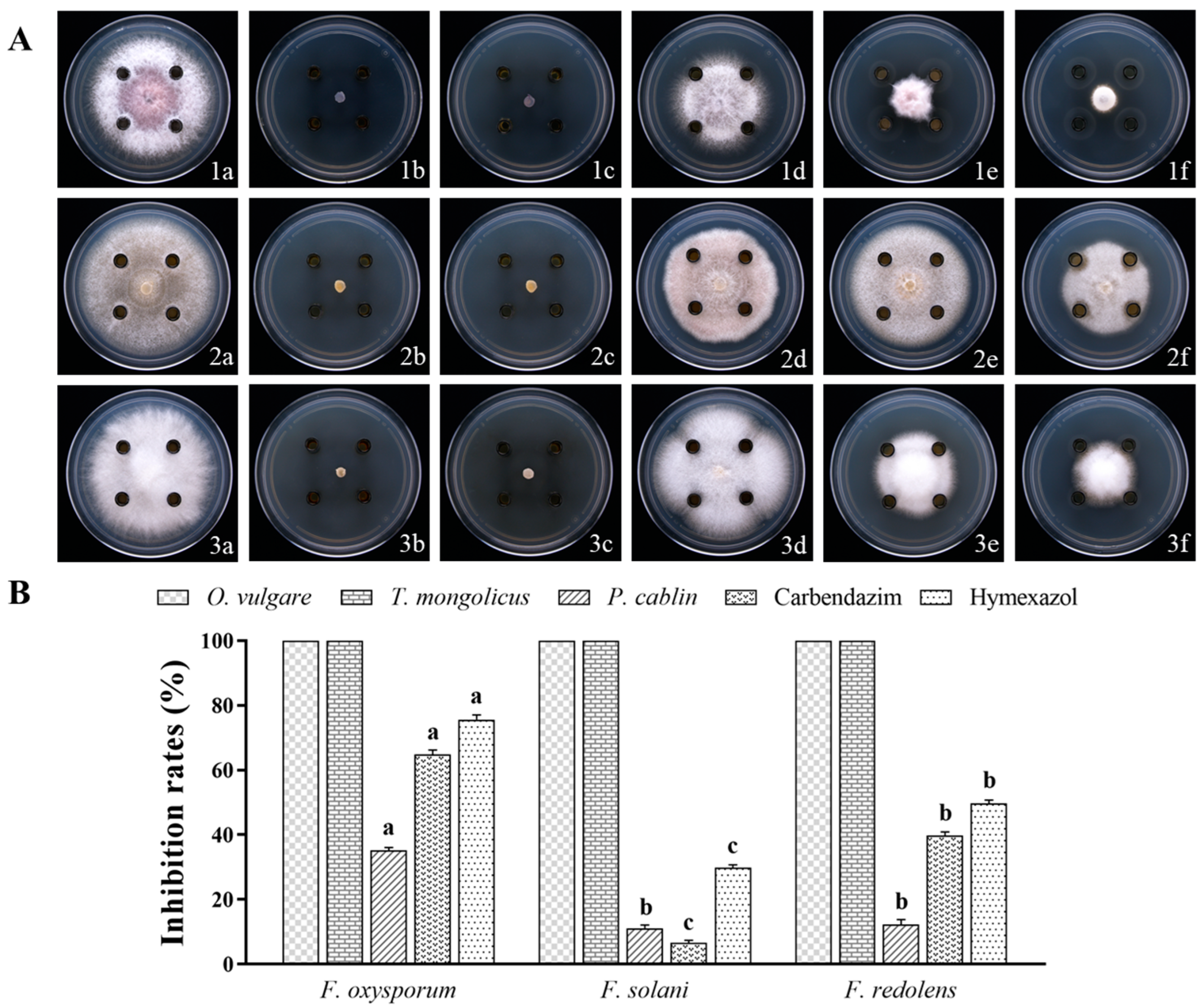
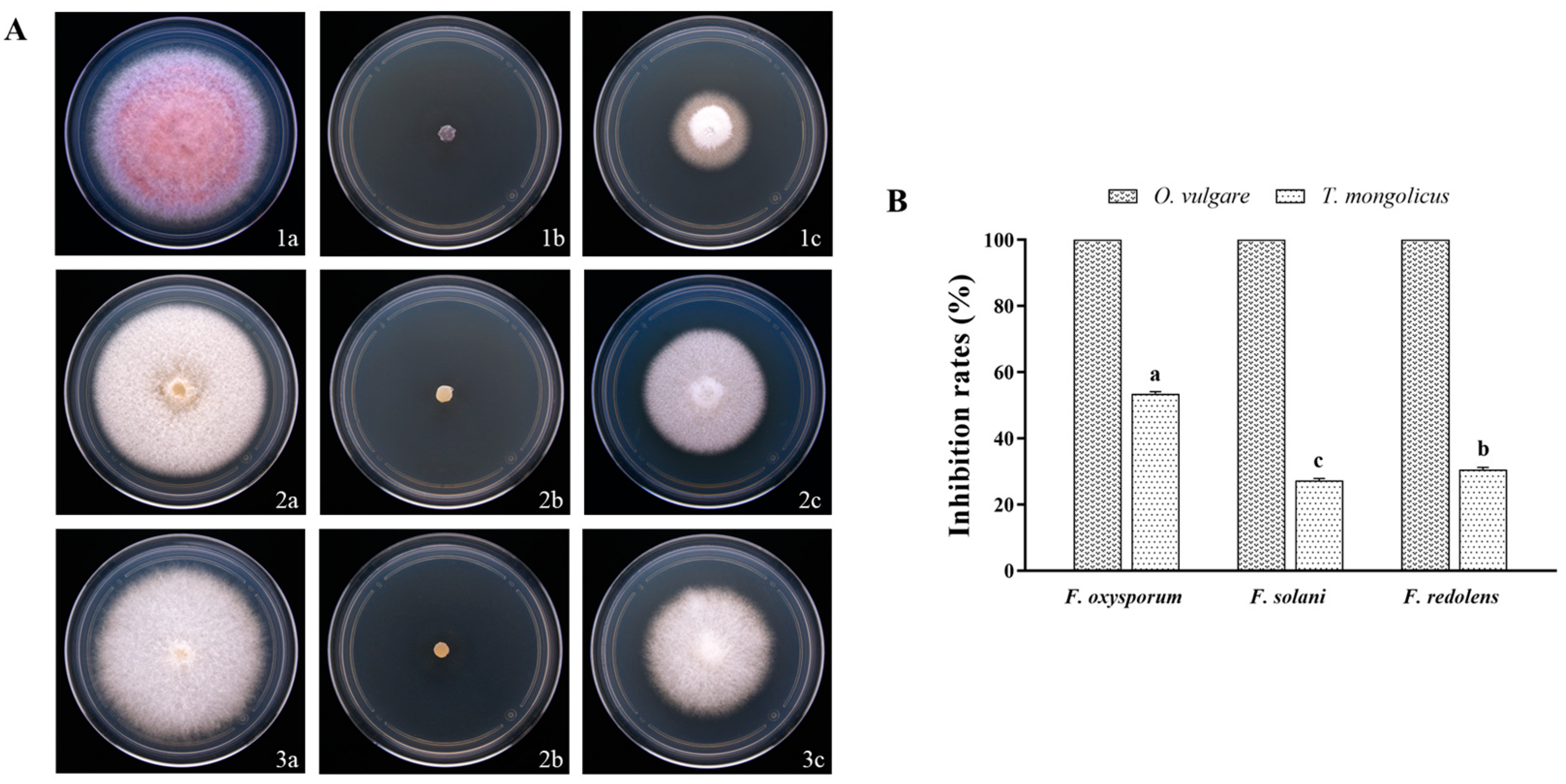
3.1. Inhibitory Effect of EOs on Pathogenic Fungi
3.2. Inhibitory Effects of EO Volatiles
3.3. Chemical Compositions
3.4. Antifungal Activities of Predominant Constituents
3.5. Determination of MIC
3.6. Effects of O. vulgare EO on Morphology and Ultrastructure of F. oxysporum
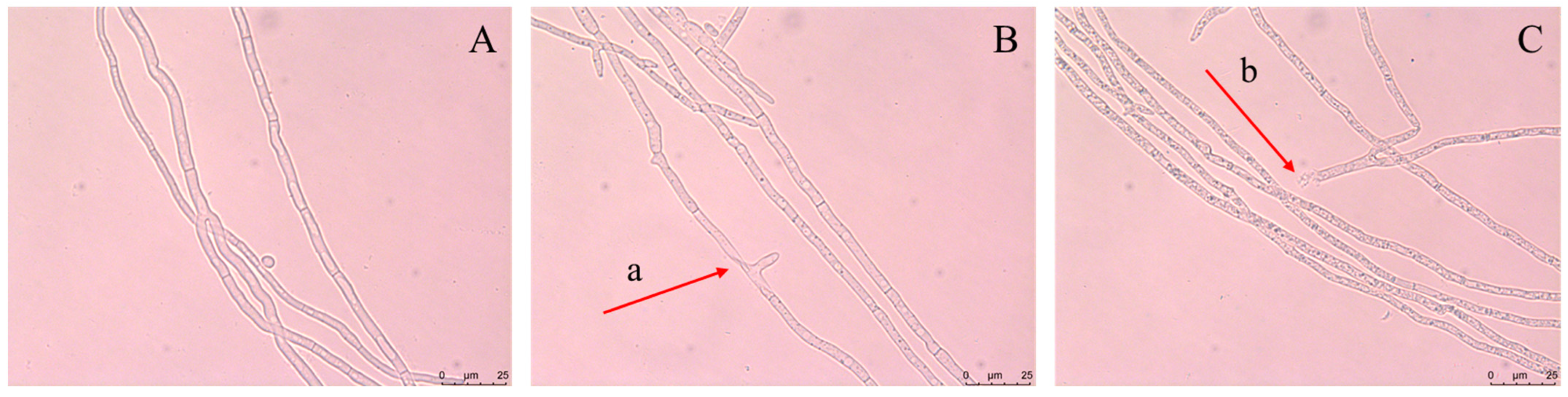
3.6.1. Optical Microscopy Observations
3.6.2. Scanning Electron Microscopy (SEM)
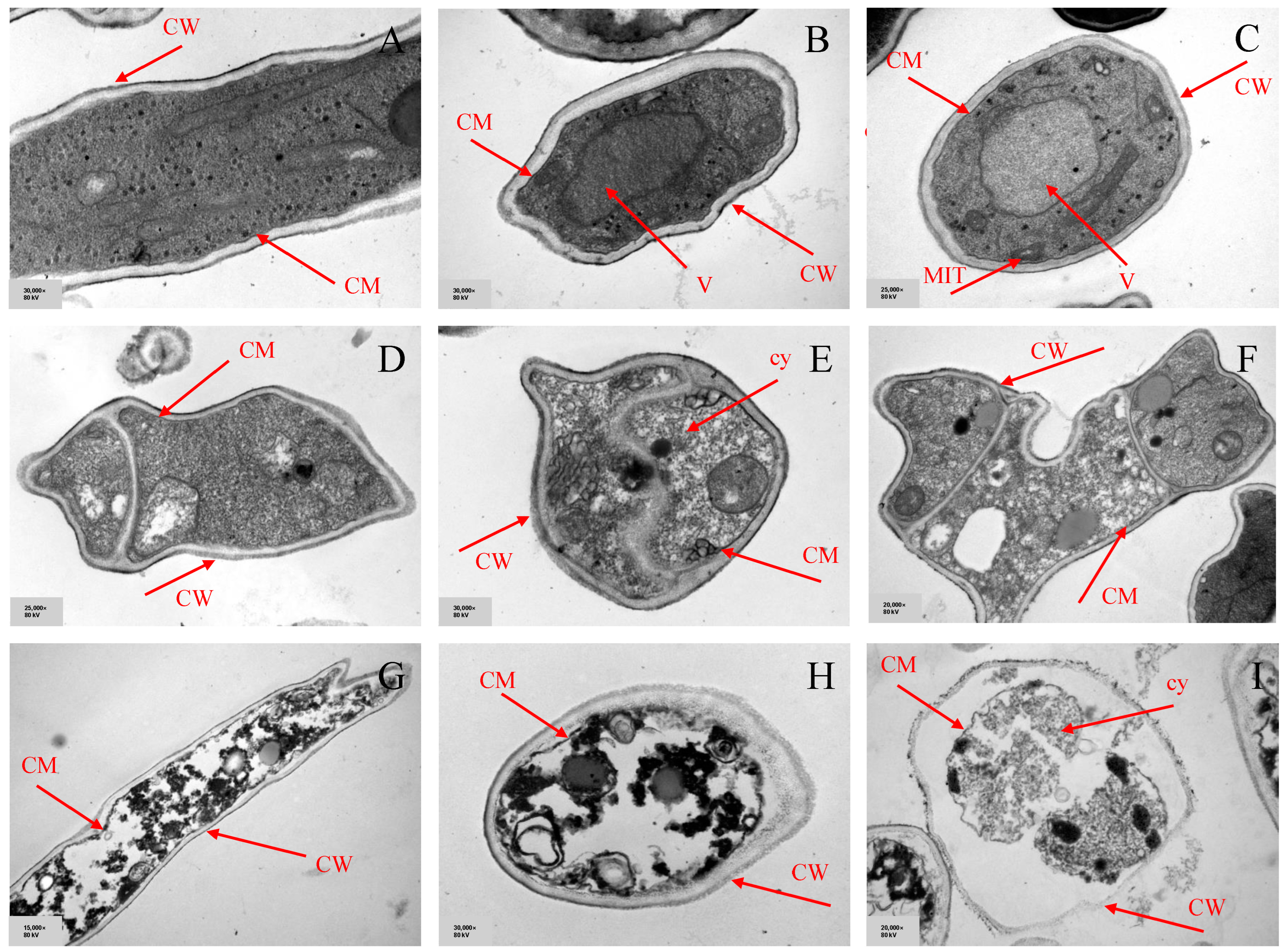
3.6.3. Transmission Electron Microscopy (TEM)
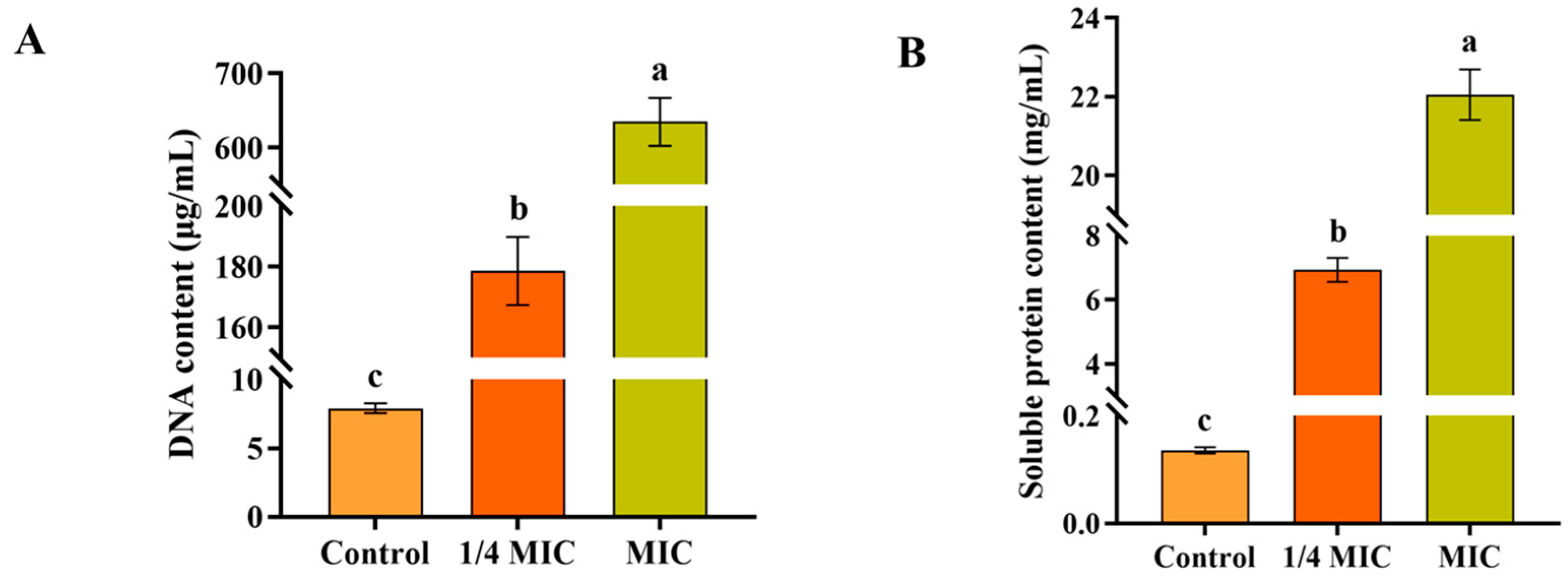
3.7. Effect on Leakage of Cell Contents of F. oxysporum
3.8. The Effect of O. vulgare EO on Endogenous ROS in F. oxysporum
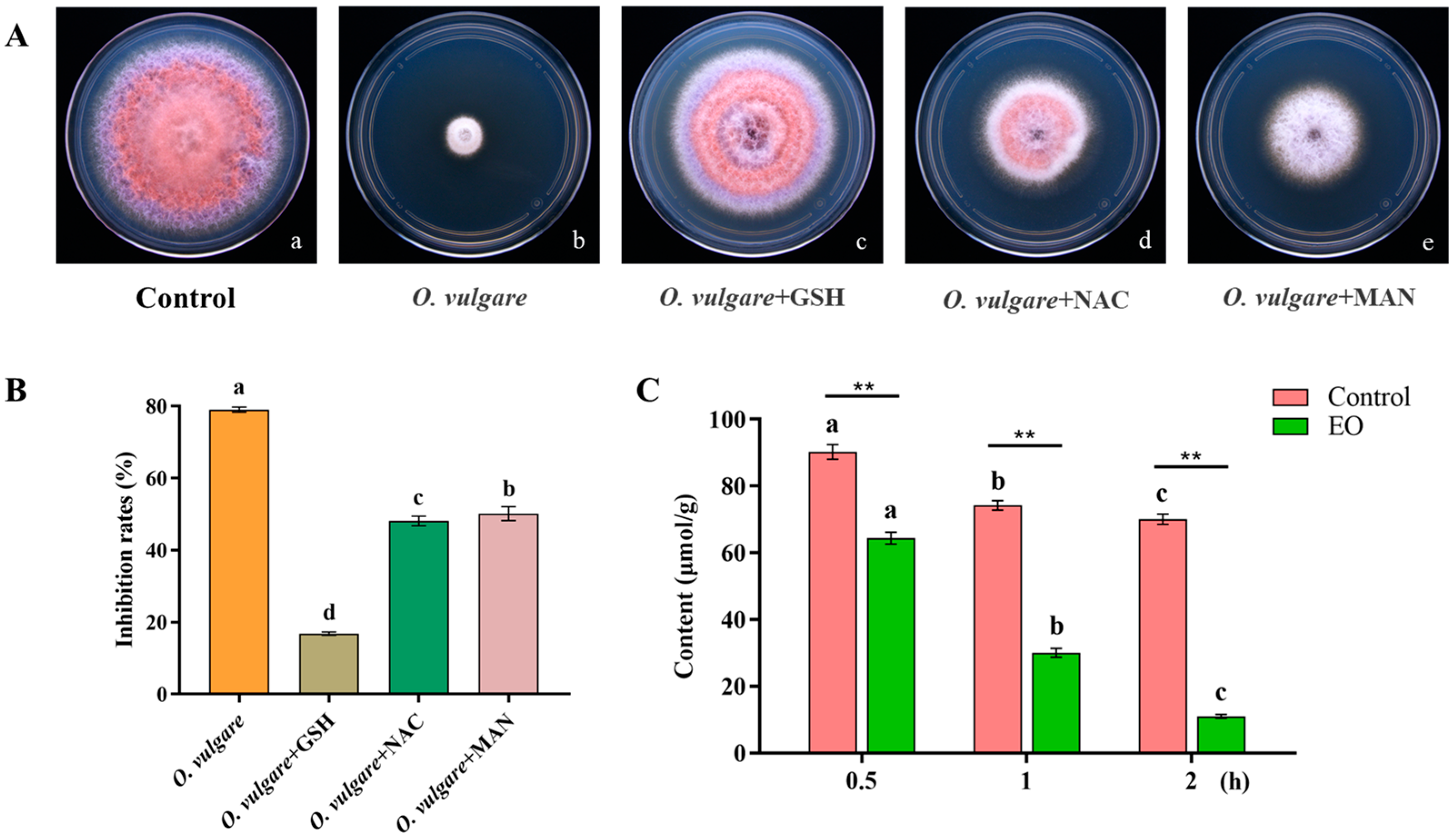
3.9. Effects of Exogenous ROS Scavengers on the Activity of O. vulgare EO
3.10. Intracellular GSH Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yun, C.; Zhao, Z.; Gu, L.; Zhang, Z.; Wang, S.; Shi, Y.; Miao, N.; Ri, I.; Wang, W.; Wang, H. In vitro production of atractylon and β-eudesmol from Atractylodes chinensis by adventitious root culture. Appl. Microbiol. Biotechnol. 2022, 106, 7027–7037. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Wu, X.; Wang, Z.; Fang, W.; Jiang, M.; Chen, H.; Huang, L.; Liu, C. Phylogenetic relationships of Atractylodes lancea, A. chinensis and A. macrocephala, revealed by complete plastome and nuclear gene sequences. PLoS ONE 2020, 15, e0227610. [Google Scholar] [CrossRef]
- Lei, H.; Yue, J.; Yin, X.Y.; Fan, W.; Tan, S.H.; Qin, L.; Zhao, Y.N.; Bai, J.H. HS-SPME coupled with GC-MS for elucidating differences between the volatile components in wild and cultivated Atractylodes chinensis. Phytochem. Anal. 2023, 34, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, C.; Shi, F.; Ma, S.; Zheng, J.; Du, X.; Zhang, L. Comparative transcriptome analysis reveals sesquiterpenoid biosynthesis among 1-, 2- and 3-year old Atractylodes chinensis. BMC Plant Biol. 2021, 21, 354. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, G.; Yang, Z.; Zhang, G.; Sun, L.; Wang, M.; Ren, X. Species Differentiation and Quality Evaluation for Atractylodes Medicinal Plants by GC-MS coupled with Chemometric Analysis. Chem. Biodivers. 2023, 20, e202300793. [Google Scholar] [CrossRef]
- Park, Y.J.; Seo, M.G.; Cominguez, D.C.; Han, I.; An, H.J. Atractylodes chinensis Water Extract Ameliorates Obesity via Promotion of the SIRT1/AMPK Expression in High-Fat Diet-Induced Obese Mice. Nutrients 2021, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, G.Y.; Dai, R.H.; Ma, Y.P.; Zhang, K.; Zhang, C.; Li, X.; Wang, J.H. Two new polyacetylenic compounds from Atractylodes chinensis (DC.) Koidz. J. Asian Nat. Prod. Res. 2011, 13, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and Antinociceptive Constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Nasir, F.; Tian, L.; Chang, J.; Sun, Y.; Zhang, J.; Li, X.; Tian, C. Outbreaks of Root Rot Disease in Different Aged American Ginseng Plants Are Associated with Field Microbial Dynamics. Front. Microbiol. 2021, 12, 676880. [Google Scholar] [CrossRef] [PubMed]
- La Placa, L.; Giorni, P.; Mondani, L.; Magan, N.; Battilani, P. Comparison of Different Physical Methods and Preservatives for Control of Fusarium proliferatum Rot in Garlic. Horticulturae 2022, 8, 1203. [Google Scholar] [CrossRef]
- Haus, M.J.; Wang, W.; Jacobs, J.L.; Peplinski, H.; Chilvers, M.I.; Buell, C.R.; Cichy, K. Root Crown Response to Fungal Root Rot in Phaseolus vulgaris Middle American × Andean Lines. Plant Dis. 2020, 104, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L. Fusarium Species Associated with Diseases of Major Tropical Fruit Crops. Horticulturae 2023, 9, 322. [Google Scholar] [CrossRef]
- Takeda, O.; Miki, E.; Terabayashi, S.; Okada, M.; Lu, Y.; He, H.S.; He, S.A. A comparative study on essential oil components of wild and cultivated Atractylodes lancea and A. chinensis. Planta Med. 1996, 62, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.H.; Wen, X.L.; Li, S.M.; Feng, L.N.; Lan, S.H.; Dong, L.X.; Guo, S.R.; Li, J.N.; Wang, J.H.; Qi, H.X. ldentification and Biological Characteristics of the Pathogen Causing Root Rot of Atractylodes chinensis. J. Agric. Sci. Technol. 2022, 24, 137–144. [Google Scholar] [CrossRef]
- Pietro, A.D.; Madrid, M.P.; Caracuel, Z.; Delgado-Jarana, J.; Roncero, M.I. Fusarium oxysporum: Exploring the molecular arsenal of a vascular wilt fungus. Mol. Plant. Pathol. 2003, 4, 315–325. [Google Scholar] [CrossRef]
- Li, C.N.; Li, H.T.; Li, Y.C.; Li, J.H.; Ji, H.; Zhang, L.; Wang, L. Research progress and control of root rot in Atractylodes lancea. J. Agro-Environ. Sci. 2022, 41, 2840–2846. [Google Scholar] [CrossRef]
- Huang, X.G.; Li, M.Y.; Yan, X.N.; Yang, J.S.; Rao, M.C.; Yuan, X.F. The potential of Trichoderma brevicompactum for controlling root rot on Atractylodes macrocephala. Can. J. Plant Pathol. 2021, 43, 794–802. [Google Scholar] [CrossRef]
- Wang, L.; Tu, H.; Hou, H.; Zhou, Z.; Yuan, H.; Luo, C.; Gu, Q. Occurrence and Detection of Carbendazim Resistance in Botryosphaeria dothidea from Apple Orchards in China. Plant Dis. 2022, 106, 207–214. [Google Scholar] [CrossRef]
- Liu, S.; Fu, L.; Wang, S.; Chen, J.; Jiang, J.; Che, Z.; Tian, Y.; Chen, G. Carbendazim Resistance of Fusarium graminearum From Henan Wheat. Plant Dis. 2019, 103, 2536–2540. [Google Scholar] [CrossRef]
- Al-Balushi, Z.M.; Agrama, H.; Al-Mahmooli, I.H.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M. Development of Resistance to Hymexazol Among Pythium Species in Cucumber Greenhouses in Oman. Plant Dis. 2018, 102, 202–208. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Li, W.; Shen, T.; He, Z.; Zhang, M.; Zhang, H.; Sun, Y.; Liu, F. Detection of chlorpyrifos and carbendazim residues in the cabbage using visible/near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 257, 119759. [Google Scholar] [CrossRef]
- Anastassiadou, M.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; Miron, I.; et al. Review of the existing maximum residue levels for hymexazol according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2019, 17, e05895. [Google Scholar] [CrossRef] [PubMed]
- Tompros, A.; Wilber, M.Q.; Fenton, A.; Carter, E.D.; Gray, M.J. Efficacy of Plant-Derived Fungicides at Inhibiting Batrachochytrium salamandrivorans Growth. J. Fungi 2022, 8, 1025. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Parikh, L.; Agindotan, B.O.; Burrows, M.E. Antifungal Activity of Plant-Derived Essential Oils on Pathogens of Pulse Crops. Plant Dis. 2021, 105, 1692–1701. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Raja, R. Medicinally Potential Plants of Labiatae (Lamiaceae) Family: An Overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Kot, B.; Wierzchowska, K.; Piechota, M.; Czerniewicz, P.; Chrzanowski, G. Antimicrobial activity of five essential oils from lamiaceae against multidrug-resistant Staphylococcus aureus. Nat. Prod. Res. 2019, 33, 3587–3591. [Google Scholar] [CrossRef]
- Coimbra, A.; Ferreira, S.; Duarte, A.P. Biological properties of Thymus zygis essential oil with emphasis on antimicrobial activity and food application. Food Chem. 2022, 393, 133370. [Google Scholar] [CrossRef]
- Carvalho, F.; Coimbra, A.T.; Silva, L.; Duarte, A.P.; Ferreira, S. Melissa officinalis essential oil as an antimicrobial agent against Listeria monocytogenes in watermelon juice. Food Microbiol. 2023, 109, 104105. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, P.; Shi, W.; Zhang, Y. Identification of essential oils with activity against stationary phase Staphylococcus aureus. BMC Complement. Med. Ther. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vázquez, M.A.K.; Pacheco-Hernández, Y.; Lozoya-Gloria, E.; Mosso-González, C.; Ramírez-García, S.A.; Romero-Arenas, O.; Villa-Ruano, N. Peppermint Essential Oil and Its Major Volatiles as Protective Agents against Soft Rot Caused by Fusarium sambucinum in Cera Pepper (Capsicum pubescens). Chem. Biodivers. 2022, 19, e202100835. [Google Scholar] [CrossRef] [PubMed]
- Nafis, A.; Ouedrhiri, W.; Iriti, M.; Mezrioui, N.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Hassani, L. Chemical composition and synergistic effect of three Moroccan lavender EOs with ciprofloxacin against foodborne bacteria: A promising approach to modulate antimicrobial resistance. Lett. Appl. Microbiol. 2021, 72, 698–705. [Google Scholar] [CrossRef]
- Aebisher, D.; Cichonski, J.; Szpyrka, E.; Masjonis, S.; Chrzanowski, G. Essential Oils of Seven Lamiaceae Plants and Their Antioxidant Capacity. Molecules 2021, 26, 3793. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Li, Q.-Q.; Zeng, Z.-Y.; Duan, S.-S.; Wang, W.; Xu, F.-R.; Cheng, Y.-X.; Dong, X. Efficacy and mechanism of Mentha haplocalyx and Schizonepeta tenuifolia essential oils on the inhibition of Panax notoginseng pathogens. Ind. Crops. Prod. 2020, 145, 112073. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Xu, J.; Yang, Y.-Y.; Shao, Y.-Z.; Zhou, F.; Wang, J.-L. Toxicity and Synergistic Effect of Elsholtzia ciliata Essential Oil and Its Main Components against the Adult and Larval Stages of Tribolium castaneum. Foods 2020, 9, 345. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Wu, T.-L.; Zhang, B.-Q.; Luo, X.-F.; Li, A.-P.; Zhang, S.-Y.; An, J.-X.; Zhang, Z.-J.; Liu, Y.-Q. Antifungal efficacy of sixty essential oils and mechanism of oregano essential oil against Rhizoctonia solani. Ind. Crops. Prod. 2023, 191, 115975. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Su, Y.; Li, Y.; Zuo, D.; Liu, H.; Liu, Y.; Mei, X.; Huang, H.; Yang, M.; et al. Allyl Isothiocyanate in the Volatiles of Brassica juncea Inhibits the Growth of Root Rot Pathogens of Panax notoginseng by Inducing the Accumulation of ROS. J. Agric. Food Chem. 2021, 69, 13713–13723. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.X.; Liu, Y.; Wang, S.Y.; Sun, Y.; Pan, J.; Guan, W.; Hao, Z.C.; Kuang, H.X.; Yang, B.Y. Cytotoxic Sesquiterpenoids from Atractylodes chinensis (DC.) Koidz. Chem. Biodivers. 2022, 19, e202200812. [Google Scholar] [CrossRef] [PubMed]
- Brugel, M.; Carlier, C.; Reyes-Castellanos, G.; Callon, S.; Carrier, A.; Bouché, O. Pesticides and pancreatic adenocarcinoma: A transversal epidemiological, environmental and mechanistic narrative review. Dig. Liver Dis. 2022, 54, 1605–1613. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Metal nanoparticles against fungicide resistance: Alternatives or partners? Pest Manag. Sci. 2022, 78, 3953–3956. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Çalış, İ.; Başer, K.H.C. Review of Studies on Phlomis and Eremostachys Species (Lamiaceae) with Emphasis on Iridoids, Phenylethanoid Glycosides, and Essential Oils. Planta Med. 2021, 87, 1128–1151. [Google Scholar] [CrossRef] [PubMed]
- Regnier, T.; Combrinck, S.; Veldman, W.; Du Plooy, W. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind. Crops. Prod. 2014, 61, 151–159. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Med. 2004, 70, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Simal-Gandara, J.; Murugan, M.; Dhanya, M.K.; Pandian, A. Nutmeg (Myristica fragrans Houtt.) essential oil: A review on its composition, biological, and pharmacological activities. Phytother. Res. 2022, 36, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Netopilova, M.; Houdkova, M.; Rondevaldova, J.; Kmet, V.; Kokoska, L. Evaluation of in vitro growth-inhibitory effect of carvacrol and thymol combination against Staphylococcus aureus in liquid and vapour phase using new broth volatilization chequerboard method. Fitoterapia 2018, 129, 185–190. [Google Scholar] [CrossRef]
- Jesus, F.P.; Ferreiro, L.; Bizzi, K.S.; Loreto, É.S.; Pilotto, M.B.; Ludwig, A.; Alves, S.H.; Zanette, R.A.; Santurio, J.M. In vitro activity of carvacrol and thymol combined with antifungals or antibacterials against Pythium insidiosum. J. Mycol. Med. 2015, 25, e89–e93. [Google Scholar] [CrossRef]
- Gallucci, M.N.; Carezzano, M.E.; Oliva, M.M.; Demo, M.S.; Pizzolitto, R.P.; Zunino, M.P.; Zygadlo, J.A.; Dambolena, J.S. In vitro activity of natural phenolic compounds against fluconazole-resistant Candida species: A quantitative structure–activity relationship analysis. J. Appl. Microbiol. 2014, 116, 795–804. [Google Scholar] [CrossRef]
- Hakalová, E.; Čechová, J.; Tekielska, D.A.; Eichmeier, A.; Pothier, J.F. Combined effect of thyme and clove phenolic compounds on Xanthomonas campestris pv. campestris and biocontrol of black rot disease on cabbage seeds. Front. Microbiol. 2022, 13, 1007988. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.-H.; Ye, M.; Wang, K.-B.; Fan, L.-M.; Su, F.-W. Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- da Silva, R.S.; de Oliveira, M.M.G.; de Melo, J.O.; Blank, A.F.; Corrêa, C.B.; Scher, R.; Fernandes, R.P.M. Antimicrobial activity of Lippia gracilis essential oils on the plant pathogen Xanthomonas campestris pv. campestris and their effect on membrane integrity. Pestic. Biochem. Physiol. 2019, 160, 40–48. [Google Scholar] [CrossRef]
- Ma, Y.-N.; Xu, F.-R.; Chen, C.-J.; Li, Q.-Q.; Wang, M.-Z.; Cheng, Y.-X.; Dong, X. The beneficial use of essential oils from buds and fruit of Syzygium aromaticum to combat pathogenic fungi of Panax notoginseng. Ind. Crops Prod. 2019, 133, 185–192. [Google Scholar] [CrossRef]
- Zhou, T.; Guo, T.; Wang, Y.; Wang, A.; Zhang, M. Carbendazim: Ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere 2023, 314, 137723. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.; Kanjilal, T.; Das, S. Optimized biodegradation of carcinogenic fungicide Carbendazim by Bacillus licheniformis JTC-3 from agro-effluent. Biotechnol. Res. Innov. 2018, 2, 45–57. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Lenardon, M.D. Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 2023, 21, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Gou, L.-J.; Zeng, H.; Zhou, G.; Dong, W.-R.; Cui, Y.; Cai, Q.; Chen, Y.-X. Inhibitory Effect and Mechanism of Dill Seed Essential Oil on Neofusicoccum parvum in Chinese Chestnut. Separations 2022, 9, 296. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Wong, F.; Amir, A. Mechanics and Dynamics of Bacterial Cell Lysis. Biophys J. 2019, 116, 2378–2389. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Li, B.; Xie, J. Antibacterial Activity and Antibacterial Mechanism of Lemon Verbena Essential Oil. Molecules 2023, 28, 3102. [Google Scholar] [CrossRef]
- Saccà, B.; Niemeyer, C.M. Functionalization of DNA nanostructures with proteins. Chem. Soc. Rev. 2011, 40, 5910–5921. [Google Scholar] [CrossRef]
- Minchin, S.; Lodge, J. Understanding biochemistry: Structure and function of nucleic acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Bi, W.; Jiang, C. Relationship between membrane damage, leakage of intracellular compounds, and inactivation of Escherichia coli treated by pressurized CO2. J. Basic. Microbiol. 2014, 54, 858–865. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Tudzynski, P.; Heller, J.; Siegmund, U. Reactive oxygen species generation in fungal development and pathogenesis. Curr. Opin. Microbiol. 2012, 15, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss. Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Schmacht, M.; Lorenz, E.; Senz, M. Microbial production of glutathione. World J. Microbiol. Biotechnol. 2017, 33, 106. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Job, J.T.; Narayanankutty, V. Glutathione, an Antioxidant Tripeptide: Dual Roles in Carcinogenesis and Chemoprevention. Curr. Protein. Pept. Sci. 2019, 20, 907–917. [Google Scholar] [CrossRef]
- Wangsanut, T.; Pongpom, M. The Role of the Glutathione System in Stress Adaptation, Morphogenesis and Virulence of Pathogenic Fungi. Int. J. Mol. Sci. 2022, 23, 10645. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paudel, V.; Gupta, V. Effect of some Essential Oils on Seed Germination and Seedling Length of Parthenium hysterophorous L. Ecoprint Int. J. Ecol. 2009, 15, 1945. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X.; Li, Y.; Zhang, S. Microencapsulation of essential oils by complex coacervation method: Preparation, thermal stability, release properties and applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]



| F. oxysporum | F. solani | F. redolens | |
|---|---|---|---|
| O. vulgare | 2.60 ± 0.90 a | 3.13 ± 0.00 a | 1.56 ± 0.00 b |
| T. mongolicus Carvacrol | 3.13 ± 0.00 a | 2.60 ± 0.90 a | 2.60 ± 0.90 a |
| 0.83 ± 0.36 bc | 1.04 ± 0.36 b | 0.63 ± 0.00 cd | |
| Thymol | 1.04 ± 0.36 b | 2.60 ± 0.90 a | 1.25 ± 0.00 bc |
| Carbendazim | 0.07 ± 0.02 c | 0.05 ± 0.02 c | 0.07 ± 0.02 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Si, H.; Zhang, S.; Zhou, R.; Xue, Y.; Wang, S.; Wang, S.; Duan, Y.; Niu, J.; Wang, Z. Efficacy and Potential Mechanism of Essential Oils of Three Labiatae Plants against the Pathogenic Fungi of Root Rot Disease in Atractylodes chinensis. Horticulturae 2023, 9, 1136. https://doi.org/10.3390/horticulturae9101136
Xie S, Si H, Zhang S, Zhou R, Xue Y, Wang S, Wang S, Duan Y, Niu J, Wang Z. Efficacy and Potential Mechanism of Essential Oils of Three Labiatae Plants against the Pathogenic Fungi of Root Rot Disease in Atractylodes chinensis. Horticulturae. 2023; 9(10):1136. https://doi.org/10.3390/horticulturae9101136
Chicago/Turabian StyleXie, Siyuan, He Si, Shenfei Zhang, Ru Zhou, Yuyan Xue, Shijie Wang, Shiqiang Wang, Yizhong Duan, Junfeng Niu, and Zhezhi Wang. 2023. "Efficacy and Potential Mechanism of Essential Oils of Three Labiatae Plants against the Pathogenic Fungi of Root Rot Disease in Atractylodes chinensis" Horticulturae 9, no. 10: 1136. https://doi.org/10.3390/horticulturae9101136
APA StyleXie, S., Si, H., Zhang, S., Zhou, R., Xue, Y., Wang, S., Wang, S., Duan, Y., Niu, J., & Wang, Z. (2023). Efficacy and Potential Mechanism of Essential Oils of Three Labiatae Plants against the Pathogenic Fungi of Root Rot Disease in Atractylodes chinensis. Horticulturae, 9(10), 1136. https://doi.org/10.3390/horticulturae9101136





