Portable Technology for Obtaining Plasma-Activated Water to Stimulate the Growth of Spruce and Strawberry Plants
Abstract
:1. Introduction
2. Materials and Methods
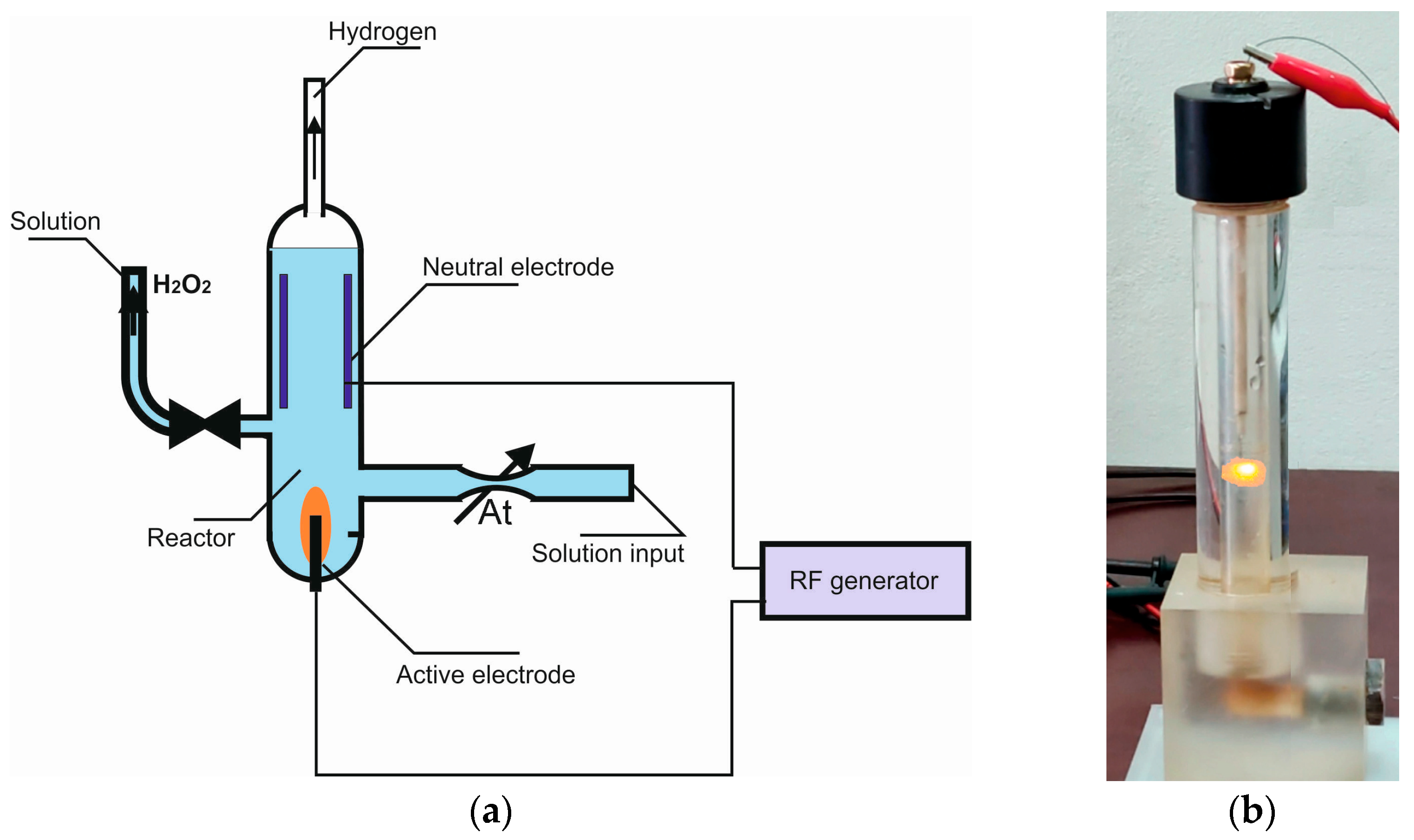
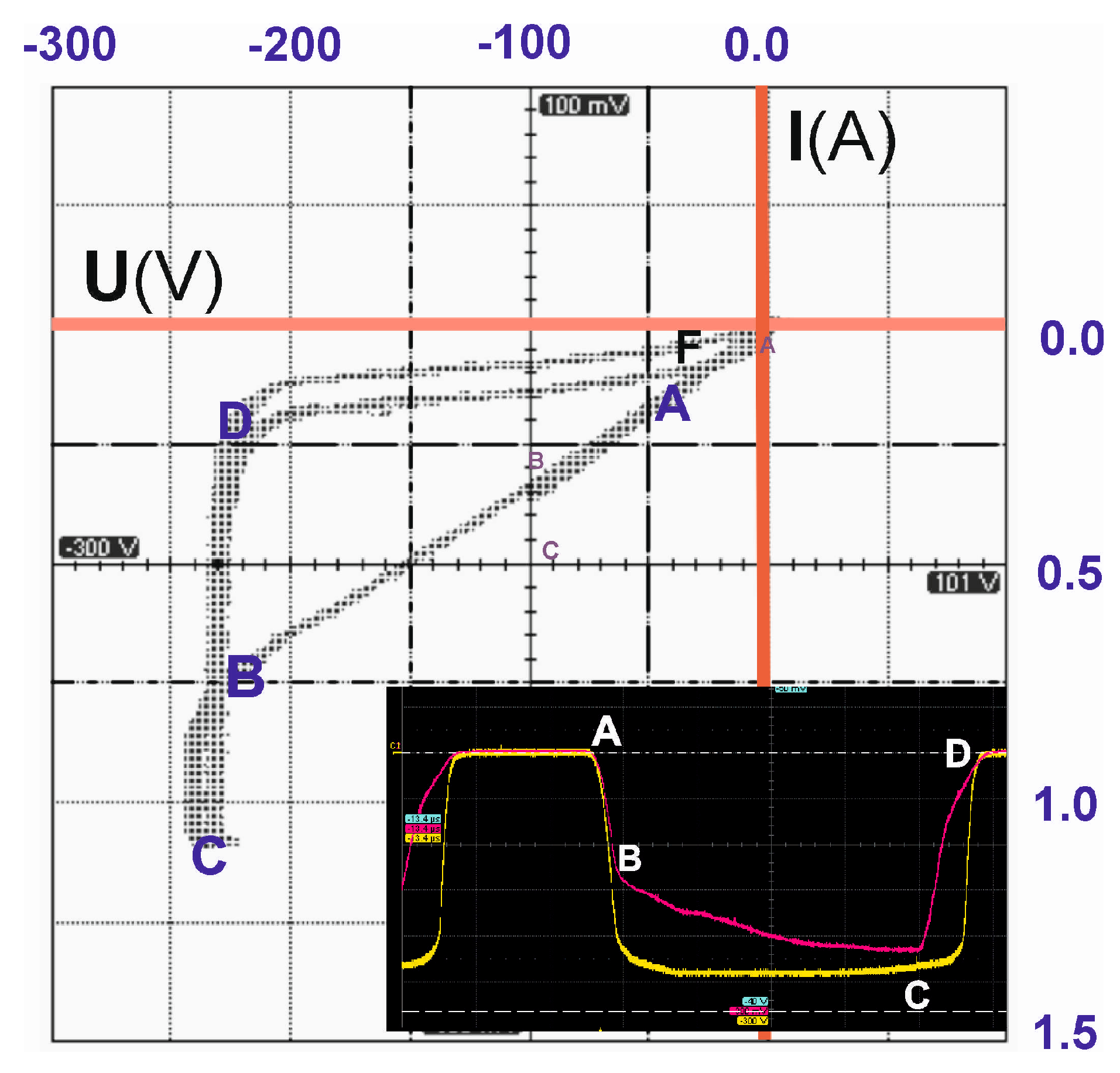
2.1. Portable PAW Reactor
2.2. Investigation of PAW Properties
2.3. Preparation of PAW Complex and Polyvinylpyrrolidone
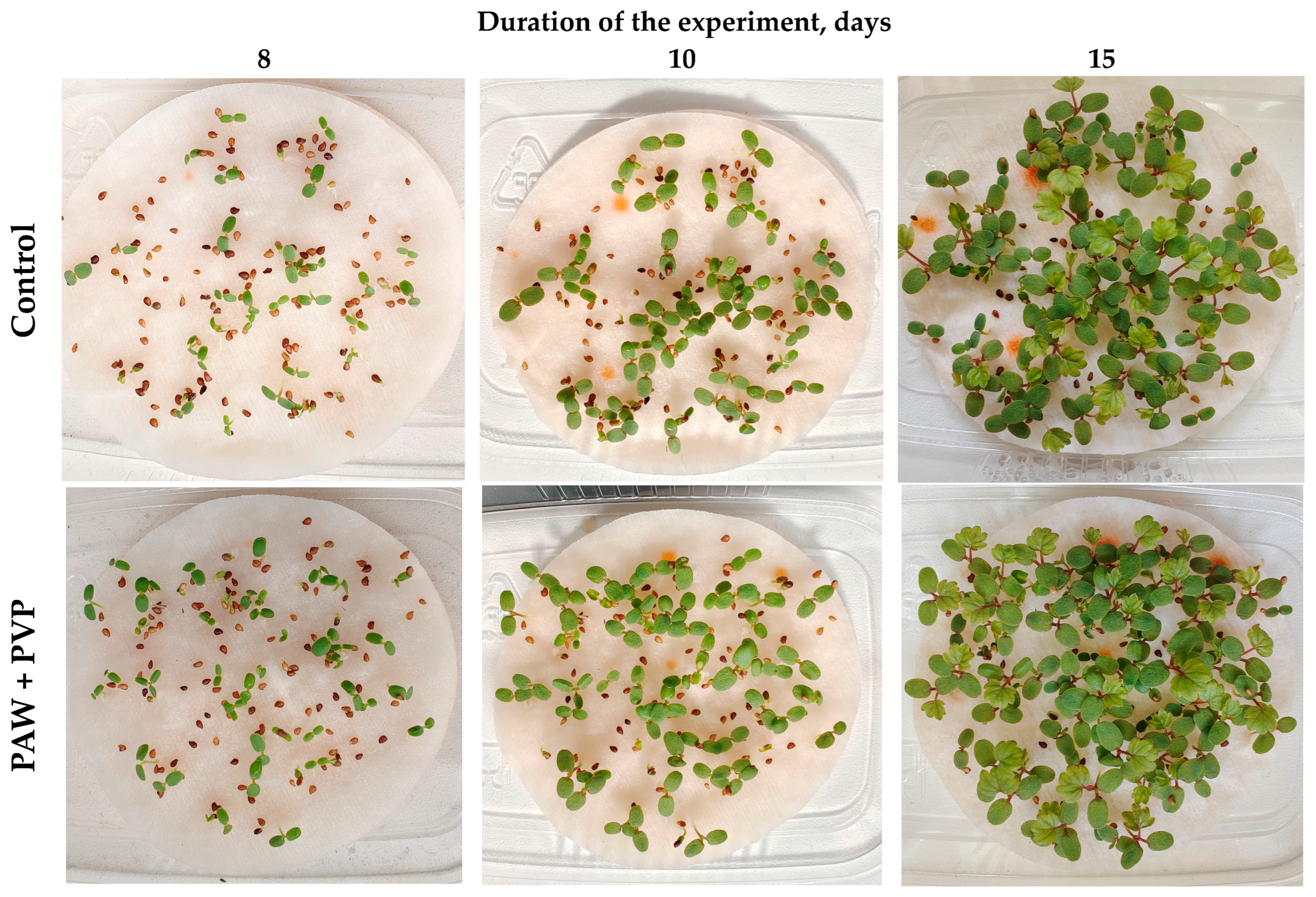
2.4. Growing Plants
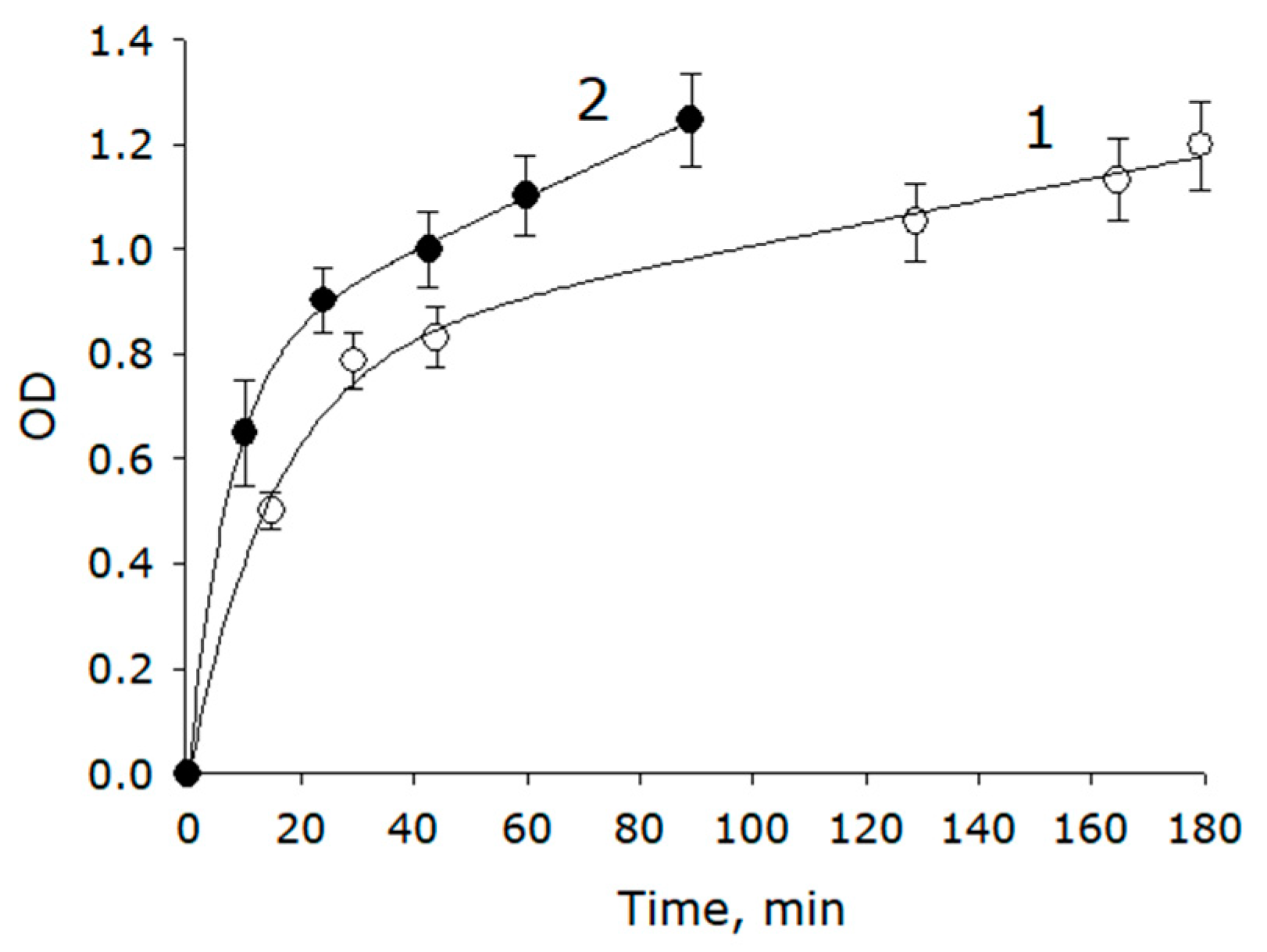
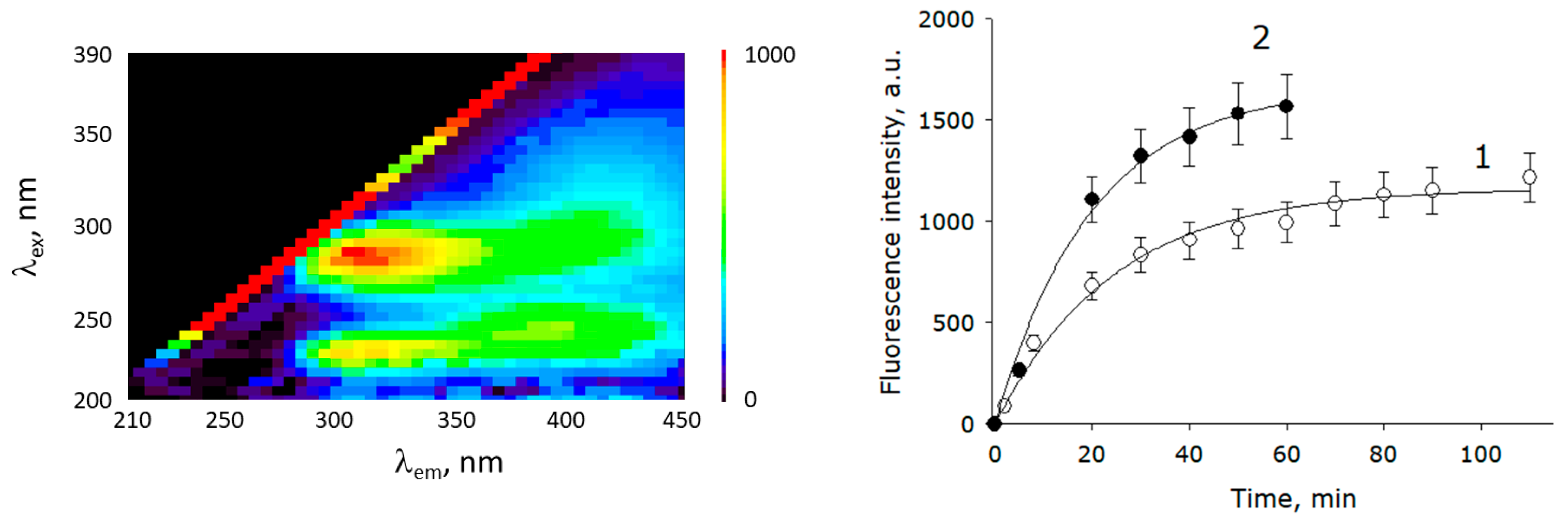
2.5. Evaluation of the Release of Metabolites from Seeds during Soaking
2.6. Markers of Oxidative Stress
2.7. Characterization of Plant Photosynthesis
2.8. Definition of Phytohormones
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castree, N.; Adams, W.M.; Barry, J.; Brockington, D.; Büscher, B.; Corbera, E.; Demeritt, D.; Duffy, R.; Felt, U.; Neves, K.; et al. Changing the Intellectual Climate. Nat. Clim. Chang. 2014, 4, 763–768. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate Change and the Need for Agricultural Adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Selwey, W.A.; Ibrahim, A.A.; Shady, M.; Alsadon, A.A. Foliar Applications of ZnO and SiO2 Nanoparticles Mitigate Water Deficit and Enhance Potato Yield and Quality Traits. Agronomy 2023, 13, 466. [Google Scholar] [CrossRef]
- Pozner, E.; Bar-On, P.; Livne-Luzon, S.; Moran, U.; Tsamir-Rimon, M.; Dener, E.; Schwartz, E.; Rotenberg, E.; Tatarinov, F.; Preisler, Y.; et al. A Hidden Mechanism of Forest Loss under Climate Change: The Role of Drought in Eliminating Forest Regeneration at the Edge of Its Distribution. For. Ecol. Manag. 2022, 506, 119966. [Google Scholar] [CrossRef]
- Istriningsih; Dewi, Y.A.; Yulianti, A.; Hanifah, V.W.; Jamal, E.; Dadang; Sarwani, M.; Mardiharini, M.; Anugrah, I.S.; Darwis, V.; et al. Farmers’ Knowledge and Practice Regarding Good Agricultural Practices (GAP) on Safe Pesticide Usage in Indonesia. Heliyon 2022, 8, e08708. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Bulanov, S.V. Electron Dynamics in the Field of Strong Plasma and Electromagnetic Waves: A Review. Phys. Wave Phenom. 2021, 29, 1–46. [Google Scholar] [CrossRef]
- Piskarev, I.M. Nitration of Phenol with Water Activated by Pulsed Hot Plasma Radiation. High Energy Chem. 2023, 57, 379–383. [Google Scholar] [CrossRef]
- Simakin, A.V.; Baymler, I.V.; Smirnova, V.V.; Astashev, M.E.; Voronov, V.V.; Dorokhov, A.S.; Gudkov, S.V. Comparison of the Intensities of Chemical and Physical Processes Occurring during Laser-Induced Breakdown of Colloidal Solutions of Tb Nanoparticles with Different Oxidation States. Dokl. Phys. 2022, 67, 465–471. [Google Scholar] [CrossRef]
- Sergeichev, K.F.; Lukina, N.A.; Apasheva, L.M.; Ovcharenko, E.N.; Lobanov, A.V. Water Activated by a Microwave Plasma Argon Jet as a Factor Stimulating the Germination of Plant Seeds. Russ. J. Phys. Chem. B 2022, 16, 84–89. [Google Scholar] [CrossRef]
- Ivanov, V.E.; Usacheva, A.M.; Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Formation of Long-Lived Reactive Species of Blood Serum Proteins Induced by Low-Intensity Irradiation of Helium-Neon Laser and Their Involvement in the Generation of Reactive Oxygen Species. J. Photochem. Photobiol. B Biol. 2017, 176, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zenkov, N.K.; Kozhin, P.M.; Vcherashnyaya, A.V.; Martinovich, G.G.; Kandalintseva, N.V.; Menshchikova, E.B. Features of redox regulation in tumor cells. Sib. Sci. Med. J. 2019, 39, 11–26. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The Role of Peroxiredoxin 6 in Neutralization of X-ray Mediated Oxidative Stress: Effects on Gene Expression, Preservation of Radiosensitive Tissues and Postradiation Survival of Animals. Free. Radic. Res. 2017, 51, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Forysenkova, A.A.; Trofimchuk, E.S.; Gafurov, M.R.; Ahmed, A.I.; Davidova, G.A.; Antonova, O.S.; Barinov, S.M. Porous Matrixes Based on Polyvinylpyrrolidone Containing Calcium Phosphates for Medical Application. Russ. Chem. Bull. 2022, 71, 543–548. [Google Scholar] [CrossRef]
- Kirsh, Y.E. Poly-N-vinylpyrrolidone and Other Poly-N-vinylamides: Synthesis and Physico-Chemical Properties; Nauka: Moscow, Russia, 1998; p. 252. ISBN 5-02-004498-9. [Google Scholar]
- Chambers, L.I.; Yufit, D.S.; Fox, M.A.; Musa, O.M.; Steed, J.W. Structure and Hydration of Polyvinylpyrrolidone–Hydrogen Peroxide. Chem. Commun. 2022, 58, 80–83. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Mudretsova, S.N.; Sinko, G.V.; Komissarov, G.G.; Stoyanov, O.V.; Zaikov, G.E. On the nature of the anomal effect of stabilization of tetrapyrroles in complexes with poly-n-vinylpyrrolidone and hydrogen peroxide. Vestn. Kaz. Technol. Univ. 2014, 17, 20–22. [Google Scholar]
- Panarin, E.F.; Kalninsh, K.K.; Pestov, D.V. Complexation of Hydrogen Peroxide with Polyvinylpyrrolidone: Ab Initio Calculations. Eur. Polym. J. 2001, 37, 375–379. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; et al. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 616. [Google Scholar] [CrossRef]
- Danileiko, Y.K.; Lukanin, V.I.; Altukhov, E.L.; Yakovlev, A.A.; Osmanov, E.G.; Kogan, E.A.; Shulutko, A.M.; Simakin, A.V.; Baimler, I.V.; Serov, D.A.; et al. Therapy of Pressure Sores via Activation of Regenerative Processed in Tissues by Low-Temperature Glow-Type Plasma Discharges of Glow Type. Opera Medica Physiol. 2022, 9, 15–30. [Google Scholar] [CrossRef]
- Danilejko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Apasheva, L.M.; Dushkov, V.Y.; Budnik, M.I.; Belyakov, A.M.; Kulik, K.N.; et al. Increase of Productivity and Neutralization of Pathological Processes in Plants of Grain and Fruit Crops with the Help of Aqueous Solutions Activated by Plasma of High-Frequency Glow Discharge. Plants 2021, 10, 2161. [Google Scholar] [CrossRef]
- Shcherbakov, I.A.; Baimler, I.V.; Gudkov, S.V.; Lyakhov, G.A.; Mikhailova, G.N.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a Constant Magnetic Field on Some Properties of Water Solutions. Dokl. Phys. 2020, 65, 273–275. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shtarkman, I.N.; Chernikov, A.V.; Usacheva, A.M.; Bruskov, V.I. Guanosine and Inosine (Riboxin) Eliminate the Long-Lived Protein Radicals Induced X-ray Radiation. Dokl. Biochem. Biophys. 2007, 413, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Heat-Induced Formation of Nitrogen Oxides in Water. J. Biol. Phys. 2013, 39, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Chernikov, A.V.; Gudkov, S.V.; Masalimov, Z.K. Thermal Activation of the Reducing Properties of Seawater Anions. Biofizika 2003, 48, 1022–1029. [Google Scholar] [PubMed]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-mediated damage to DNA. Biofizika 2007, 52, 244–251. [Google Scholar]
- Sergeichev, K.F.; Lukina, N.A.; Sarimov, R.M.; Smirnov, I.G.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Physicochemical Properties of Pure Water Treated by Pure Argon Plasma Jet Generated by Microwave Discharge in Opened Atmosphere. Front. Phys. 2021, 8, 614684. [Google Scholar] [CrossRef]
- Matveeva, T.A.; Baimler, I.V.; Artemiev, K.V.; Gorudko, I.V.; Sarimov, R.M. Laser Optical Breakdown Modified Physical Properties of Lysozyme in Aqueous Solution. Opera Medica Physiol. 2022, 4, 126–136. [Google Scholar] [CrossRef]
- Serov, D.A.; Khabatova, V.V.; Tikhonova, I.V.; Reut, V.E.; Pobedonostsev, R.V.; Astashev, M.E. Study of the Effects of Selenium Nanoparticles and Their Combination with Immunoglobulins on the Survival and Functional State of Polymorphonuclear Cells. Opera Medica Physiol. 2022, 4, 137–159. [Google Scholar] [CrossRef]
- Yin, D.; Chen, S.; Chen, F.; Guan, Z.; Fang, W. Morphological and Physiological Responses of Two Chrysanthemum Cultivars Differing in Their Tolerance to Waterlogging. Environ. Exp. Bot. 2009, 67, 87–93. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Hu, Y.; Zhao, P.; Xu, T.; Sun, J.; Gao, X. Proline, Sugars, and Antioxidant Enzymes Respond to Drought Stress in the Leaves of Strawberry Plants. Korean J. Hortic. Sci. Technol. 2015, 33, 625–632. [Google Scholar] [CrossRef]
- Grinberg, M.A.; Gudkov, S.V.; Balalaeva, I.V.; Gromova, E.; Sinitsyna, Y.; Sukhov, V.; Vodeneev, V. Effect of Chronic β-Radiation on Long-Distance Electrical Signals in Wheat and Their Role in Adaptation to Heat Stress. Environ. Exp. Bot. 2021, 184, 104378. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Popov, A.V.; Pobedonostsev, R.V.; Kazantseva, D.V.; Dorokhov, A.S.; Izmailov, A.Y.; Vyatchinov, A.A.; Orlovskaya, E.O.; Shaidulin, A.T.; et al. Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants. Horticulturae 2023, 9, 846. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise Estimation of Chlorophyll a, b and Carotenoid Content by Deconvolution of the Absorption Spectrum and New Simultaneous Equations for Chl Determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lai, T.; Huang, Q.-W.; Yang, X.-M.; Shen, Q.-R. Effect of N Fertilizers on Root Growth and Endogenous Hormones in Strawberry. Pedosphere 2009, 19, 86–95. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.Z.; Mustafina, A.R.; Kof, E.M. Modified Solvent Partitioning Scheme Providing Increased Specificity and Rapidity of Immunoassay for Indole-3-Acetic Acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Akhtyamova, Z.; Arkhipova, T.; Martynenko, E.; Nuzhnaya, T.; Kuzmina, L.; Kudoyarova, G.; Veselov, D. Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots. Int. J. Mol. Sci. 2021, 22, 10680. [Google Scholar] [CrossRef]
- Shcherbakov, I.A. Current Trends in the Studies of Aqueous Solutions. Phys. Wave Phenom. 2022, 30, 129–134. [Google Scholar] [CrossRef]
- Shcherbakov, I.A. Influence of External Impacts on the Properties of Aqueous Solutions. Phys. Wave Phenom. 2021, 29, 89–93. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Ivanov, V.E.; Karp, O.E.; Chernikov, A.V.; Belosludtsev, K.N.; Bobylev, A.G.; Astashev, M.E.; Gapeyev, A.B.; Bruskov, V.I. Impact of Biologically Relevant Anions on Reactive Oxygen Species Formation in Water under the Action of Non-Ionizing Physical Agents. Biophysics 2014, 59, 700–707. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Karmanova, E.E.; Chernikov, A.V.; Usacheva, A.M.; Ivanov, V.E.; Emel’yanenko, V.I. Formation of Hydrated Electrons in Water under Thermal Electromagnetic Exposure. Phys. Wave Phenom. 2021, 29, 94–97. [Google Scholar] [CrossRef]
- Singh, S. A Review on Possible Elicitor Molecules of Cyanobacteria: Their Role in Improving Plant Growth and Providing Tolerance against Biotic or Abiotic Stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.M.; Nagaev, E.I.; Matveyeva, T.A.; Binhi, V.N.; Burmistrov, D.E.; Serov, D.A.; Astashev, M.E.; Simakin, A.V.; Uvarov, O.V.; Khabatova, V.V.; et al. Investigation of Aggregation and Disaggregation of Self-Assembling Nano-Sized Clusters Consisting of Individual Iron Oxide Nanoparticles upon Interaction with HEWL Protein Molecules. Nanomaterials 2022, 12, 3960. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-L. Detection and Characterization of Biological and Other Organic-Carbon Aerosol Particles in Atmosphere Using Fluorescence. J. Quant. Spectrosc. Radiat. Transf. 2015, 150, 12–35. [Google Scholar] [CrossRef]
- Ashurov, M.K.; Ashurov, E.M.; Astashev, M.E.; Baimler, I.V.; Gudkov, S.V.; Konchekov, E.M.; Lednev, V.N.; Lukina, N.A.; Matveeva, T.A.; Markendudis, A.G.; et al. Development of an Environmentally Friendly Technology for the Treatment of Aqueous Solutions with High-Purity Plasma for the Cultivation of Cotton, Wheat and Strawberries. ChemEngineering 2022, 6, 91. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Plyusnina, E.N.; Shaposhnikov, M.V. Radiation Hormesis and Radioadaptive Response in Drosophila Melanogaster Flies with Different Genetic Backgrounds: The Role of Cellular Stress-Resistance Mechanisms. Biogerontology 2011, 12, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Volkova, P.Y.; Bondarenko, E.V.; Kazakova, E.A. Radiation Hormesis in Plants. Curr. Opin. Toxicol. 2022, 30, 100334. [Google Scholar] [CrossRef]
- Geras’kin, S.; Churyukin, R.; Volkova, P. Radiation Exposure of Barley Seeds Can Modify the Early Stages of Plants’ Development. J. Environ. Radioact. 2017, 177, 71–83. [Google Scholar] [CrossRef]
- Ushakov, I.; Vasin, M. The Drugs and Natural Antioxidants as the Components of Anti-Radiation Countermeasures during Cosmic Flights. Med. Radiol. Radiat. Saf. 2017, 62, 66–73. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and Application of Photoconversion Fluoropolymer Films for Greenhouses Located at High or Polar Latitudes. J. Photochem. Photobiol. B Biol. 2020, 213, 112056. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Semenova, N.A.; Dorokhov, A.S.; Proshkin, Y.A.; Godyaeva, M.M.; Vodeneev, V.; Sukhov, V.; Panchenko, V.; Chilingaryan, N.O. Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) While Cultivated in Vertical Farms. Agriculture 2022, 12, 1988. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Gudkov, S.V. Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review. Horticulturae 2022, 8, 885. [Google Scholar] [CrossRef]
- Solano, C.; Artola, A.; Barrena, R.; Ballardo, C.; Sánchez, A. Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation. Agronomy 2023, 13, 2204. [Google Scholar] [CrossRef]
- Korobova, A.; Ivanov, R.; Timergalina, L.; Vysotskaya, L.; Nuzhnaya, T.; Akhiyarova, G.; Kusnetsov, V.; Veselov, D.; Kudoyarova, G. Effect of Low Light Stress on Distribution of Auxin (Indole-3-Acetic Acid) between Shoot and Roots and Development of Lateral Roots in Barley Plants. Biology 2023, 12, 787. [Google Scholar] [CrossRef]
- Bispo, R.L.B.; Ceccato-Antonini, S.R.; Takita, M.A.; Rosa-Magri, M.M. Exogenous Indole-3-Acetic Acid Production and Phosphate Solubilization by Chlorella vulgaris Beijerinck in Heterotrophic Conditions. Fermentation 2023, 9, 116. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic Pathways and Functions of Indole-3-Acetic Acid in Microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Huang, W.; Zhang, D.; Wu, J.; Li, B.; Li, M.; Liu, L.; Yan, M. Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants. Plants 2023, 12, 1023. [Google Scholar] [CrossRef]
- Pizzio, G.A. Abscisic Acid Perception and Signaling in Chenopodium Quinoa. Stresses 2022, 3, 22–32. [Google Scholar] [CrossRef]
- Ortiz-García, P.; González Ortega-Villaizán, A.; Onejeme, F.C.; Müller, M.; Pollmann, S. Do Opposites Attract? Auxin-Abscisic Acid Crosstalk: New Perspectives. Int. J. Mol. Sci. 2023, 24, 3090. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current Advancements in the Molecular Mechanism of Plasma Treatment for Seed Germination and Plant Growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Advancements in Plasma Agriculture: A Review of Recent Studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef]
- Meier, A.; Essumang, D.; Hummerick, M.; Johnson, C.; Kruger, M.; Massa, G.; Engeling, K. Reviewing Plasma Seed Treatments for Advancing Agriculture Applications on Earth and Into the Final Frontier. Gravitational Space Res. 2021, 9, 133–158. [Google Scholar] [CrossRef]





| Group | Physicochemical Characteristics | |||||
|---|---|---|---|---|---|---|
| EC **, mS/cm | pH | [O2], μM | NO3−, mM | Redox, mV | H2O2, mM | |
| Control | 7.9 ± 0.7 | 6.7 ± 0.2 | 273 ± 8 | <0.01 | 315 ± 12 | <0.01 |
| PAW | 20.4 ± 1.1 * | 8.0 ± 0.2 * | 262 ± 7 | 15.73 ± 0.81 * | 556 ± 28 * | 5.20 ± 0.31 * |
| PAW + PVP | 17.7 ± 1.0 * | 7.7 ± 0.2 * | 271 ± 6 | 14.31 ± 0.68 * | 509 ± 25 * | 4.81 ± 0.28 * |
| Group | Percentage of Seeds Germinated by the 6th Day | Percentage of Plants with Free Needles by the 9th Day | Percentage of Live Plants on the 15th Day |
|---|---|---|---|
| Control | 21 | 21 | 11 |
| PVP | 25 | 30 | 22 |
| PAW | 67 | 52 | 37 |
| PAW + PVP | 80 | 61 | 49 |
| Groups | Duration of the Experiment, Days | ||||
|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 15 | |
| Control | 98 ± 6 | 238 ± 14 | 388 ± 16 | 738 ± 5 | 1295 ± 27 |
| PAW + PVP | 135 ± 8 * | 318 ± 13 * | 642 ± 15 * | 877 ± 6 * | 1450 ± 30 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danileyko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Apasheva, L.M.; Ovcharenko, E.N.; Lobanov, A.V.; Astashev, M.E.; Simakin, A.V.; Shkirin, A.V.; et al. Portable Technology for Obtaining Plasma-Activated Water to Stimulate the Growth of Spruce and Strawberry Plants. Horticulturae 2023, 9, 1142. https://doi.org/10.3390/horticulturae9101142
Danileyko YK, Belov SV, Egorov AB, Lukanin VI, Apasheva LM, Ovcharenko EN, Lobanov AV, Astashev ME, Simakin AV, Shkirin AV, et al. Portable Technology for Obtaining Plasma-Activated Water to Stimulate the Growth of Spruce and Strawberry Plants. Horticulturae. 2023; 9(10):1142. https://doi.org/10.3390/horticulturae9101142
Chicago/Turabian StyleDanileyko, Yury K., Sergej V. Belov, Aleksej B. Egorov, Vladimir I. Lukanin, Ludmila M. Apasheva, Elena N. Ovcharenko, Anton V. Lobanov, Maxim E. Astashev, Alexander V. Simakin, Alexey V. Shkirin, and et al. 2023. "Portable Technology for Obtaining Plasma-Activated Water to Stimulate the Growth of Spruce and Strawberry Plants" Horticulturae 9, no. 10: 1142. https://doi.org/10.3390/horticulturae9101142
APA StyleDanileyko, Y. K., Belov, S. V., Egorov, A. B., Lukanin, V. I., Apasheva, L. M., Ovcharenko, E. N., Lobanov, A. V., Astashev, M. E., Simakin, A. V., Shkirin, A. V., Konchekov, E. M., Zakharov, D. A., Stepanova, E. V., Paskhin, M. O., Kazantseva, D. V., Pobedonostsev, R. V., Sukhov, V., Dorokhov, A. S., & Izmailov, A. Y. (2023). Portable Technology for Obtaining Plasma-Activated Water to Stimulate the Growth of Spruce and Strawberry Plants. Horticulturae, 9(10), 1142. https://doi.org/10.3390/horticulturae9101142









