Research Progress of Arbuscular Mycorrhizal Fungi Promoting Citrus Growth
Abstract
:1. Introduction
2. Diversity of Symbiotic AMF Present in Citrus
3. AMF Promotes the Mineral Nutrient Absorption of Citrus
4. Effects of AMF on the Growth of Citrus Roots
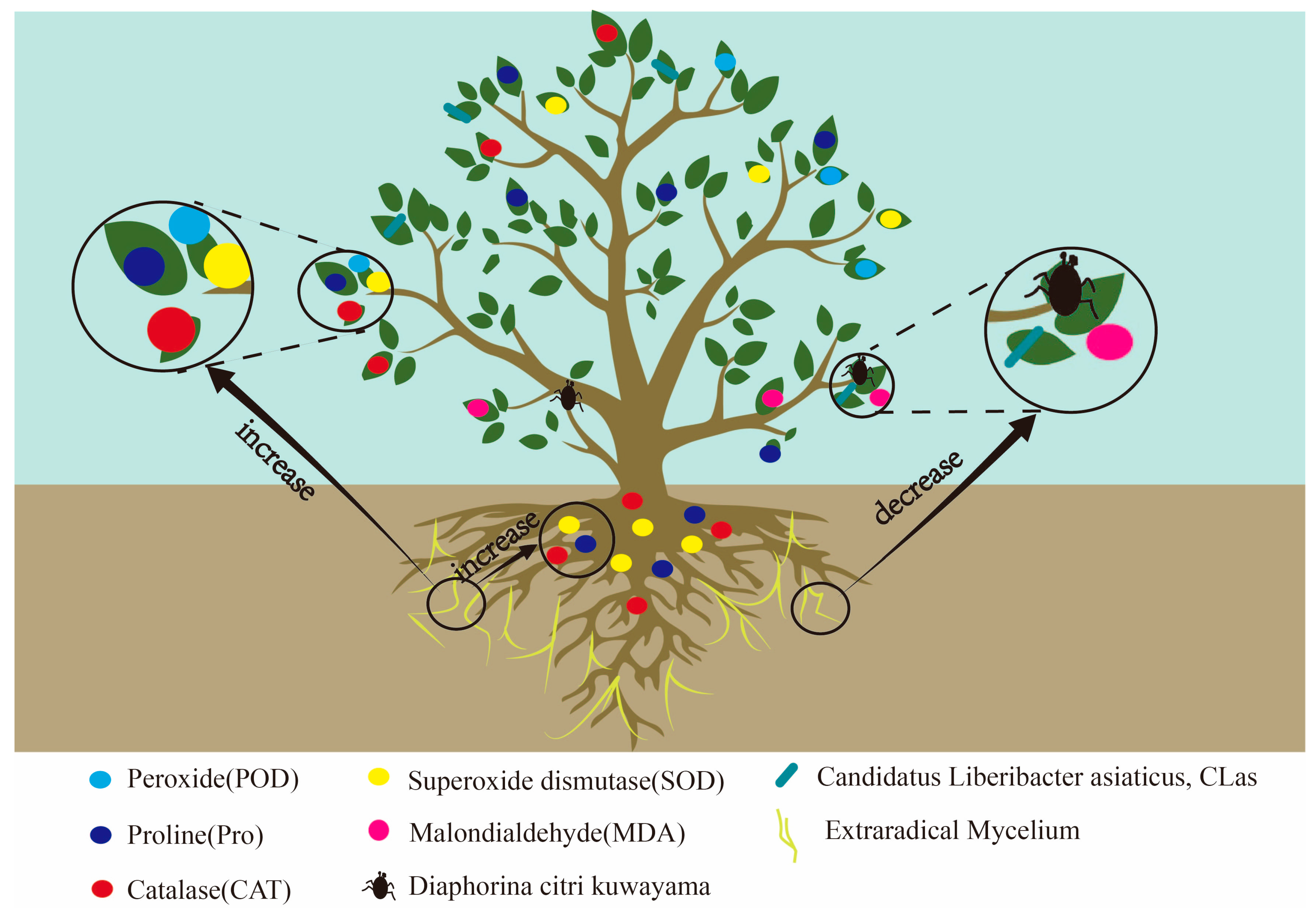
5. Effect and Mechanism of AMF on Citrus Disease Resistance
6. AMF Induces the Expression of Citrus Defense Genes
7. Effect of AMF Inoculation on the Response of Citrus to Abiotic Stress
7.1. AMF Improves Drought Tolerance in Citrus
7.2. AMF Improves the Tolerance of Citrus to Salt and Alkali
7.3. AMF Impacts the Response of Citrus Plants to Temperature Stress
8. Application of AMF in Citrus Cultivation
9. Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, X.M.; Xiang, Q.; Tang, T.T.; Tian, Z.K.; Chen, C. Research on Soil Lead and Cadmium Pollution Control by Arbuscular Mycorrhizal Fung. Biol. Chem. Eng. 2017, 3, 91–93+97. [Google Scholar]
- Gong, Y.L.; Bi, Y.L.; Hu, J.J.; Guo, C. Effect of Inoculation with AM Fungi on Maize Growth and Hyperspectral Estimation of Total Nitrogen Content in Maize Leaves. Environ. Manag. 2020, 38, 210–214. [Google Scholar] [CrossRef]
- Lei, M.; Ding, C.; Gan, Z.Y.; Qiu, Q.Y. Effects of Arbuscular Mycorrhizal Fungi and Application of Different Nitrogen Fertilizers on Nutrient Absorption of Chinese Fir Seedlings. J. Trop. Subtrop. Bot. 2022, 30, 518–527. [Google Scholar] [CrossRef]
- Ma, X.N.; Luo, W.Q.; Xu, F.F.; Wu, F.Y. Effects of two AM fungi on zinc uptake of winter wheat roots. Mycosystema 2017, 36, 933–941. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, D.; Song, L.L.; Han, S.Y.; Chen, T.S. Effects of Arbuscular Mycorrhizal Fungi on Nutrient Exchange in Mulberry Plant in Rocky Desertification Areas. Chin. J. Trop. Crops 2020, 41, 7–14. [Google Scholar] [CrossRef]
- Jiang, L.; Li, H.Y.; Zhang, Q.; Zhang, H.L.; Qiao, Y.H.; Zhang, H.X.; Yang, X.Y. Effects of arbuscular mycorrhiza fungi on the growth and physiological metabolism of Pyrus betulaefolia Bunge seedlings under saline-alkaline stress. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 152–160. [Google Scholar] [CrossRef]
- Hou, S.J.; Li, T.; Lin, G.; Chen, B.D. Effects of biogas slurry and AM fungi on growth and heavy metal accumulation of licorice plants. J. Agro-Environ. Sci. 2016, 35, 1465–1472. [Google Scholar] [CrossRef]
- Li, Y.D.; Duan, T.Y. AM Effects of AM fungi on alfalfa leaf spot caused by Phoma medicaginis and pea aphids Acyrthosiphon pisum. Chin. J. Ecol. 2020, 39, 1214–1221. [Google Scholar] [CrossRef]
- Qu, M.H.; Yu, Y.C.; Li, S.; Zhang, J.C. Advances in research on activation of mineral nutrients by arbuscular mycorrhizal fungi. J. Zhejiang Agric. For. Univ. 2019, 36, 394–405. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, Z.Y.; Jiang, X.L.; Xing, X.K. Review and prospect of researches on the mechanisms of mycorrhizal fungi in improving plant drought resistance. Mycosystema 2021, 40, 851–872. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N.; Malhotra, S.K. Mycorrhizas in citrus: Beyond soil fertility and plant nutrition. Indian J. Agric. Sci. 2017, 87, 427–443. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. New Advances in the Research of Arbuscular Mycorrhizas in Citrus. Acta. Agric. Univ. Jiangxi 2014, 36, 279–284. [Google Scholar]
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Srivastava, A.; Wu, Q.S.; Zou, Y.N. Mycorrhiza Stimulates Root-Hair Growth and IAA Synthesis and Transport in Trifoliate Orange under Drought Stress. Sci. Rep. 2018, 8, 1978. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Telajin, N.S.E.; Li, Y.B.; Huang, J.G. Effects of arbuscular mycorrhizal fungi on growth, nutrition and water use of jujube seedlings in Xinjiang. Agric. Technol. Serv. 2014, 31, 63–64. [Google Scholar]
- Liu, C.Y.; Zou, Y.N.; Zhang, D.J.; Shu, B.; Wu, Q.S. Mycorrhizae and Tolerance of Abiotic Stress in Citrus Plants. Soil Biol. 2019. [Google Scholar] [CrossRef]
- Ortas, I. Role of mycorrhizae on mineral nutrition of fruit trees. Acta Hortic. 2018, 271–284. [Google Scholar] [CrossRef]
- Senes-Guerrero, C.; Gimenez, S.; Pacheco, A.; Gradilla-Hernandez, M.S.; Schuessler, A. New MiSeq based strategy exposed plant-preferential arbuscular mycorrhizal fungal communities in arid soils of Mexico. Symbiosis 2020, 81, 235–246. [Google Scholar] [CrossRef]
- Li, G.G.; Liu, J.H.; Song, J.; Li, M.Y.; Li, D.P.; Liu, S.Q.; Zhang, J.L.; Chen, Y.S. Research on Arbuscular Mycorrhizal Fungi on the Growth of Ziyang Xiangcheng Seedling. J. Yunnan Agric. Univ. (Nat. Sci.) 2021, 36, 1022–1027. [Google Scholar]
- Zhang, J.L.; Li, M.Y.; Kang, Y.H.; Liu, J.H.; Chen, Y.S.; Li, X.L.; Song, J.; Liu, S.Q. Effect of Fourteen Arbuscular Mycorrhizal Fungi on Growth of Fructus aurantii. Chin. J. Trop. Crops 2021, 42, 3278–3283. [Google Scholar]
- Song, F.; Wu, L.M.; Li, H.F.; He, L.G.; Wang, Z.J.; Huang, Y.M.; Jiang, Y.C. Identification of root endophytic arbuscular mycorrhizal fungi community diversity and its variations under the infection of Candidatus Liberibacter asiaticus in the citrus orchard of Ganzhou city. J. Fruit Sci. 2019, 36, 892–902. [Google Scholar] [CrossRef]
- Liu, R.J.; Jiao, H.; Li, Y.; Li, M.; Zhu, X.C. Research advances in species diversity of arbuscular mycorrhizal fungi. Chin. J. Appl. Ecol. 2009, 20, 2301–2307. [Google Scholar]
- del Mar Alguacil, M.; Torrecillas, E.; Torres, P.; Garcia-Orenes, F.; Roldan, A. Long-Term Effects of Irrigation with Waste Water on Soil AM Fungi Diversity and Microbial Activities: The Implications for Agro-Ecosystem Resilience. PLoS ONE 2012, 7, e47680. [Google Scholar] [CrossRef]
- Wang, P.; Shu, B.; Wang, Y.; Zhang, D.J.; Liu, J.F.; Xia, R.X. Diversity of arbuscular mycorrhizal fungi in red tangerine (Citrus reticulata Blanco) rootstock rhizospheric soils from hillside citrus orchards. Pedobiologia 2013, 56, 161–167. [Google Scholar] [CrossRef]
- Xi, M.Y.; Deyett, E.; Ginnan, N.; Ashworth, V.E.T.M.; Dang, T.; Bodaghi, S.; Vidalakis, G.; Roper, M.C.; Glassman, S.I.; Rolshausen, P.E. Geographic Location, Management Strategy, and Huanglongbing Disease Affect Arbuscular Mycorrhizal Fungal Communities Across U.S. Citrus Orchards. Phytobiomes J. 2022, 6, 342–353. [Google Scholar] [CrossRef]
- Mullath, S.K.; Blaszkowski, J.; Govindan, B.N.; Al Dhaheri, L.; Symanczik, S.; Al-Yahya’ei, M.N. Organic farming practices in a desert habitat increased the abundance, richness, and diversity of arbuscular mycorrhizal fungi. Emir. J. Food Agric. 2019, 31, 969–979. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.J.; Shu, B.; Xia, R.X. Arbuscular mycorrhizal fungi associated with citrus orchards under different types of soil management, southern China. Plant Soil Environ. 2012, 58, 302–308. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuca, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Rahimi, S.; Baninasab, B.; Talebi, M.; Gholami, M.; Zarei, M. Arbuscular mycorrhizal fungi inoculation improves iron deficiency in quince via alterations in host root phenolic compounds and expression of genes. Sci. Hortic. 2021, 285, 110165. [Google Scholar] [CrossRef]
- Wu, Q.S.; Gao, W.Q.; Srivastava, A.K.; Zhang, F.; Zou, Y.N. Nutrient acquisition and fruit quality of Ponkan mandarin in response to AMF inoculation. Indian J. Agric. Sci. 2020, 90, 1563–1567. [Google Scholar] [CrossRef]
- Ortas, I. Comparison of indigenous and selected mycorrhiza in terms of growth increases and mycorrhizal dependency of sour orange under phosphorus and zinc deficient soils. Eur. J. Hortic. Sci. 2019, 84, 218–225. [Google Scholar] [CrossRef]
- Shu, B. Effects and Mechanism of Arbuscular Mycorrhizal Fungi on Phosphrous Uptake in Trifoliate Orange (Poncirus trifoliata L. Raf). Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2013. [Google Scholar]
- Navarro, J.M.; Morte, A. Mycorrhizal effectiveness in Citrus macrophylla at low phosphorus fertilization. J. Plant Physiol. 2018, 232, 301–310. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.Y.; Wu, S.H.; Wen, M.X.; Lu, L.M.; Ke, F.Z.; Wu, Q.S. Effect of arbuscular mycorrhizal fungi on rhizosphere organic acid content and microbial activity of trifoliate orange under different low P conditions. Arch. Agron. Soil Sci. 2019, 65, 2029–2042. [Google Scholar] [CrossRef]
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Mycorrhiza-Released Glomalin-Related Soil Protein Fractions Contribute to Soil Total Nitrogen in Trifoliate Orange. Plant Soil Environ. 2020, 66, 183–189. [Google Scholar] [CrossRef]
- Liu, P. The Effect of Inoculation of AM Fungi on The Fe Absorption of Citrus. Master’s Dissertation, Southwest University, Chongqing, China, 2010. [Google Scholar]
- Wang, M.Y.; Xia, R.X. Effects of arbuscular mycorrhizal fungi on growth and iron uptake of Poncirus trifoliata under different pH. Acta Microbiol. Sin. 2009, 49, 1374–1379. [Google Scholar] [CrossRef]
- Wang, M.Y.; Xia, R.X.; Wang, Y.S.; Zhou, K.B.; Ni, H.Z. Effects of Arbuscular Mycorrhizal Fungi on Growth of Poncirus trifoliata Seedlings under Iron Deficiency and Heavy Bicarbonate Stresses. Acta Hortic. Sin. 2008, 469–474. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Influence of Arbuscular Mycorrhizal Fungi on Mineral Nutrition in Roots of Red Tangerine Seedlings Exposed to Water Stress. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2009, 22, 526–529+543. [Google Scholar]
- Zhang, F.; Du, P.; Song, C.X.; Wu, Q.S. Alleviation of magnesium deficiency by mycorrhiza in trifoliate orange: Changes in physiological activity. Emir. J. Food Agri. 2015, 27, 763–769. [Google Scholar] [CrossRef]
- Xiao, J.X.; Hu, C.Y.; Chen, Y.Y.; Hua, J.; Yang, B. Growth and Nutrient Content of Trifoliate Orange Seedlings Influenced by Arbuscular Mycorrhizal Fungi Inoculation in Low Magnesium Soil. J. Plant Nutr. 2014, 38, 1516–1529. [Google Scholar] [CrossRef]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuca, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hu, C.Y.; Xiao, J.X. Effects of arbuscular mycorrhizal fungi on the growth and zinc uptake of trifoliate orange (Poncirus trifoliata) seedlings grown in low-zinc soil. J. Plant Nutr. 2016, 40, 324–331. [Google Scholar] [CrossRef]
- Liu, C.Y.; Guo, X.N.; Wu, X.L.; Dai, F.J.; Wu, Q.S. The Comprehensive Effects of Rhizophagus intraradices and P on Root System Architecture and P Transportation in Citrus limon L. Agriculture 2022, 12, 317. [Google Scholar] [CrossRef]
- Xu, P.Y. Regulation of Arbuscular Mycorrhizal Fungi and Phosphorus on Plant Lateral Root Formation and Involved Auxin Signaling Pathways. Master’s Dissertation, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- Deng, L. Effects of Arbuscular Mycorrhizal Fungi on Plant Growth and Calcium Uptake in Citrus Seedlings. Master’s Dissertation, Southwest University, Chongqing, China, 2016. [Google Scholar]
- Li, J.F. Study on Phenolis and Key Gene in Citrus Mycorrhizal Symbiont under Iron deficiency. Master’s Dissertation, Huaqiao University, Quanzhou, China, 2015. [Google Scholar]
- Song, Y.L. Effects and Mechanisms of Arbuscular Mycorrhizal Fungi on Zinc Uptake by Citrus. Master’s Dissertation, Hunan Agricultural University, Changsha, China, 2015. [Google Scholar]
- Seguel, A.; Cumming, J.R.; Klugh-Stewart, K.; Cornejo, P.; Borie, F. The role of arbuscular mycorrhizas in decreasing aluminium phytotoxicity in acidic soils: A review. Mycorrhiza 2013, 23, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Borie, F.; Bolan, N.; Cornejo, P. Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit. Rev. Environ. Sci. Technol. 2011, 42, 741–775. [Google Scholar] [CrossRef]
- Chen, W.L.; Li, J.; Zhu, H.H.; Xu, P.Y.; Chen, J.Z.; Yao, Q. Arbuscular Mycorrhizal Fungus Enhances Lateral Root Formation in Poncirus trifoliata (L.) as Revealed by RNA-Seq Analysis. Front. Plant Sci. 2017, 8, 2039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Wang, M.Y.; Hou, S.Z.; Liu, J.F.; Lin, P.; Li, Y.Q. Effects of Arbuscular Mycorrhizal Fungi on Plant Growth and Secondary Metabolism in Citrus reticulata. J. Trop. Subtrop. Bot. 2020, 28, 78–83. [Google Scholar]
- Bi, Y.L.; Sun, J.H.; Zhang, J.; Song, Z.H.; Cai, Y.; Sun, H. Remediation effects of plant root growth inoculated with AM fungi on simulation subsidence injured. J. China Coal Soc. 2017, 42, 1013–1020. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Liu, C.Y.; Lu, T. Interacted Effect of Arbuscular Mycorrhizal Fungi and Polyamines on Root System Architecture of Citrus Seedlings. J. Integr. Agric. 2012, 11, 1675–1681. [Google Scholar] [CrossRef]
- Liu, R.C.; Yang, L.; Zou, Y.N.; Wu, Q.S. Root-associated endophytic fungi modulate endogenous auxin and cytokinin levels to improve plant biomass and root morphology of trifoliate orange. Hortic. Plant J. 2023, 9, 463–472. [Google Scholar] [CrossRef]
- Chai, Y.J. Effect of Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhizal Fungi on Growth and Development of Trifoliate Orange. Master’s Dissertation, Southwest University, Chongqing, China, 2022. [Google Scholar] [CrossRef]
- Tian, L.; Nasrullah, N.; Huang, X.Y.; Wu, Q.S. Nitric Oxide Accelerates Mycorrhizal Effects on Plant Growth and Root Development of Trifoliate Orange. Sains Malays. 2017, 46, 1687–1691. [Google Scholar] [CrossRef]
- Wu, X.Y.; Cui, X.Y. Effects of Arbuscular Mycorrhizal Fungi on Plant Growth and Fruit Quality. Tianjin Agric. Sci. 2016, 22, 116–119. [Google Scholar] [CrossRef]
- Song, F. Characterization of Roof Microbiome and Identification of miRNAs Involved in Arbuscular Mycorrhizal Symbiosis in Citrus. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Kang, Y.H. Study on the Defense Effect of Inoculation with Mycorrhizal Fungi on Citrus Huanglongbing and Its Vector Diaphorina Citri. Master’s Dissertation, Fujian Agriculture and Forestry University, Fuzhou, China, 2022. [Google Scholar]
- Quan, D.W.; Li, D.; Zhang, J.L.; Song, J.; Hu, L.; Cheng, T.; Huang, J.H.; Chen, T.S. Effects of Inhibition Citrus Huanglongbing on Catharanthus roseus with Different Arbuscular Mycorrhizal Fungi Species. Chin. J. Trop. Crops 2020, 41, 2259–2266. [Google Scholar] [CrossRef]
- Cheng, S.; Tian, L.; Zou, Y.N.; Wu, Q.S.; Kuca, K.; Bora, P. Molecular responses of arbuscular mycorrhizal fungi in tolerating root rot of trifoliate orange. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 558–571. [Google Scholar] [CrossRef]
- Xie, M.M.; Zhang, Y.C.; Liu, L.P.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Mycorrhiza regulates signal substance levels and pathogen defense gene expression to resist citrus canker. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1161–1167. [Google Scholar] [CrossRef]
- Tian, L.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhiza-induced plant defence responses in trifoliate orange infected by Phytophthora parasitica. Acta Physiol. Plant. 2021, 43, 45. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zou, Y.N.; Liu, L.P.; Wu, Q.S. Common mycorrhizal networks activate salicylic acid defense responses of trifoliate orange (Poncirus trifoliata). J. Integr. Plant Biol. 2018, 61, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, Z.X.; Wang, C.; Wang, Z.J.; He, L.G.; Jiang, Y.C.; Wu, L.M.; Bai, F.X. Cloning and Function Analysis of Mycorrhizal Signaling Receptor Protein Lysin Motif Receptor-like Kinases 2 Gene (LYK2) in Citrus. Acta Hortic. Sin. 2022, 49, 281–292. [Google Scholar] [CrossRef]
- Sun, M.Q. Functional Analysis of Mycorrhizal-Induced Gene EXO70I and Lipase3 from Citrus and Their Homolog Genes in Medicago. Master’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Shu, B.; Cai, D.; Zhang, F.; Zhang, D.J.; Liu, C.Y.; Wu, Q.S.; Luo, C. Identifying citrus CBL and CIPK gene families and their expressions in response to drought and arbuscular mycorrhizal fungi colonization. Biol. Plant 2020, 64, 773–783. [Google Scholar] [CrossRef]
- Cheng, H.Q.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in Trifoliate Orange by Regulating H+-ATPase Activity and Gene Expression. Front. Plant Sci. 2021, 12, 659694. [Google Scholar] [CrossRef]
- Huang, S.Y. Expression and Function Analysis of A Nitrate Transpoter Gene PtrNPF5.2 Associated with Arbuscular Mycorrhizal Symbiosis in Poncirus Trifolata. Master’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Liu, Z.; Cheng, S.; Liu, X.Q.; Kuca, K.; Hashem, A.; Al-Arjani, A.F.; Almutairi, K.F.; Abd Allah, E.F.; Wu, Q.S.; Zou, Y.N. Cloning of a CHS gene of Poncirus trifoliata and its expression in response to soil water deficit and arbuscular mycorrhizal fungi. Front. Plant Sci. 2022, 13, 1101212. [Google Scholar] [CrossRef]
- Shu, B.; Xia, R.X.; Wang, P. Differential regulation of Pht1 phosphate transporters from trifoliate orange (Poncirus trifoliata L. Raf) seedlings. Sci. Hortic. 2012, 146, 115–123. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, S.; Xu, Q.L.; Xiao, J.X. Transcriptome responses of grafted Citrus sinensis plants to inoculation with the arbuscular mycorrhizal fungus Glomus versiforme. Trees-Struct. Funct. 2016, 30, 1073–1082. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression. Plant Physiol. Biochem. 2021, 162, 27–35. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wu, Q.L.; Wan, Q.; Shu, B. Research progress of arbuscular mycorrhizal fungi. Mod. Agric. 2019, 12, 9–12. [Google Scholar] [CrossRef]
- Li, X. Effects of Arbuscular Mycorrhizal Fungi on Growth and Drought Resistance of Citrus Seedlings under Water Stress. Master’s Dissertation, Southwest University, Chongqing, China, 2022. [Google Scholar]
- Peng, F. Effects of Arbuscular Mycorrhizal Fungi and Nutrient Interaction on the Drought Resistance of Leymus chinensis. Master’s Dissertation, Northeast Normal University, Changchun, China, 2019. [Google Scholar]
- Zou, Y.N.; Wu, H.H.; Giri, B.; Wu, Q.S.; Kuca, K. Mycorrhizal symbiosis down-regulates or does not change root aquaporin expression in trifoliate orange under drought stress. Plant Physiol. Biochem. 2019, 144, 292–299. [Google Scholar] [CrossRef]
- Luo, Y. The Effects of AMF on Cell Membrane Endogenous Polyamines and Salicylic Acid in Cirtus Under Drought Stress. Master’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2009. [Google Scholar]
- Wang, W.X.; Zhang, F.; Chen, Z.L.; Liu, J.; Guo, C.; He, J.D.; Zou, Y.N.; Wu, Q.S. Responses of phytohormones and gas exchange to mycorrhizal colonization in trifoliate orange subjected to drought stress. Arch. Agron. Soil Sci. 2016, 63, 14–23. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X.; Zou, Y.N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 2008, 44, 122–128. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ. Exp. Bot. 2019, 171, 103926. [Google Scholar] [CrossRef]
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2019, 262, 108745. [Google Scholar] [CrossRef]
- Liang, S.M.; Zhang, F.; Zou, Y.N.; Kuca, K.; Wu, Q.S. Metabolomics Analysis Reveals Drought Responses of Trifoliate Orange by Arbuscular Mycorrhizal Fungi with a Focus on Terpenoid Profile. Front. Plant Sci. 2021, 12, 740524. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Chen, Z.L.; He, J.D.; Zou, Y.N. Mycorrhizal Inoculation Modulates Root Morphology and Root Phytohormone Responses in Trifoliate Orange under Drought Stress. Emir. J. Food Agric. (EJFA) 2016, 28, 251–256. [Google Scholar] [CrossRef]
- Wu, H.H.; Zou, Y.N.; Rahman, M.M.; Ni, Q.D.; Wu, Q.S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef]
- Zhang, F.; He, J.D.; Ni, Q.D.; Wu, Q.S.; Zou, Y.N. Enhancement of Drought Tolerance in Trifoliate Orange by Mycorrhiza: Changes in Root Sucrose and Proline Metabolisms. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 46, 270–276. [Google Scholar] [CrossRef]
- Wu, Q.S.; He, J.D.; Srivastava, A.K.; Zou, Y.N.; Kuca, K. Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol. 2019, 39, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Wu, Q.S.; Huang, Y.M.; Ni, Q.D.; He, X.H. Mycorrhizal-Mediated Lower Proline Accumulation in Poncirus trifoliata under Water Deficit Derives from the Integration of Inhibition of Proline Synthesis with Increase of Proline Degradation. PLoS ONE 2013, 8, e80568. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Zhang, F.; Srivastava, A.K.; Wu, Q.S.; Kuca, K. Arbuscular Mycorrhizal Fungi Regulate Polyamine Homeostasis in Roots of Trifoliate Orange for Improved Adaptation to Soil Moisture Deficit Stress. Front. Plant Sci. 2021, 11, 600792. [Google Scholar] [CrossRef]
- He, J.D.; Li, J.L.; Wu, Q.S. Effects of Rhizoglomus intraradices on plant growth and root endogenous hormones of trifoliate orange under salt stress. J. Anim. Plant Sci. 2019, 29, 245–250. [Google Scholar]
- Wu, Q.S.; Zou, Y.N. Mycorrhizal Symbiosis Alters Root H+ Effluxes and Root System Architechure of Trifollate Orange Seedlings Under Salt Stress. J. Anim. Plant Sci. 2013, 23, 143–148. [Google Scholar]
- Ding, Y.E.; Fan, Q.F.; He, J.D.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Effects of mycorrhizas on physiological performance and root TIPs expression in trifoliate orange under salt stress. Arch. Agron. Soil Sci. 2019, 66, 182–192. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, P.; Wu, Q.H.; Zou, Y.N.; Bao, Q.; Wu, Q.S. Arbuscular mycorrhizas improve plant growth and soil structure in trifoliate orange under salt stress. Arch. Agron. Soil Sci. 2017, 63, 491–500. [Google Scholar] [CrossRef]
- Xie, X.J. Effects of Arbuscular Mycorrhizal Fungi on Alkaline Tolerance of Citrus and Its Alleviative Mechanism. Master’s Dissertation, Southwest University, Chongqing, China, 2022. [Google Scholar]
- Cheng, H.Q.; Giri, B.; Wu, Q.S.; Zou, Y.N.; Kuca, K. Arbuscular mycorrhizal fungi mitigate drought stress in citrus by modulating root microenvironment. Arch. Agron. Soil Sci. 2021, 68, 1217–1228. [Google Scholar] [CrossRef]
- Yang, X.H.; Zeng, B.; Li, X.G.; Sun, Z.H. The effects of inter-species different of arbuscular mycorrhizal fungi on growth and heat-resistant of trifoliate (Poncirus trifoliata Raf.) seedlings. Mycosystema 2005, 24, 582–589. [Google Scholar]
- Wu, Q.S.; Zou, Y.N. Beneficial roles of arbuscular mycorrhizas in citrus seedlings at temperature stress. Sci. Hortic. 2010, 125, 289–293. [Google Scholar] [CrossRef]
- Cao, M.A.; Zhang, F.; Abd, A.E.F.; Wu, Q.S. Mycorrhiza improves cold tolerance of Satsuma orange by inducing antioxidant enzyme gene expression. Biocell 2022, 46, 1959–1966. [Google Scholar] [CrossRef]
- Pan, C.W.; Liu, X.F.; Qu, P.F.; Wu, Q.S. Enhancement of Arbuscular Mycorrhizal Fungi on Antioxidant Capacity of Roots of Trifoliate Orange under Temperature Conditions. J. Yangtze Univ. (Nat. Sci. Ed.) 2011, 8, 245–247+18. [Google Scholar]
- Zeng, B. Effects of Arbuscular Mycorrhizal Fungi on Growths and High Temperature Tolerance of Trifoliate Orange Seedling. Master’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2005. [Google Scholar]
- Cao, M.A.; Wang, P.; Hashem, A.; Wirth, S.; Abd Allah, E.F.; Wu, Q.S. Field Inoculation of Arbuscular Mycorrhizal Fungi Improves Fruit Quality and Root Physiological Activity of Citrus. Agriculture 2021, 11, 1297. [Google Scholar] [CrossRef]
- Cao, S.C. Effect of interaction between Rhizobia and Arbuscular Mycorrhizal Fungi on Nutrient Absorption and Physiological Effect of Hongjiang Orange. Master’s Dissertation, Southwest University, Chongqing, China, 2020. [Google Scholar]
- Fang, L.F.; He, X.H.; Zhang, X.L.; Yang, Y.H.; Liu, R.; Shi, S.M.; Shi, X.J.; Zhang, Y.T. A Small Amount of Nitrogen Transfer from White Clover to Citrus Seedling via Common Arbuscular Mycorrhizal Networks. Agronomy 2020, 11, 32. [Google Scholar] [CrossRef]
- Sugiura, Y.; Akiyama, R.; Tanaka, S.; Yano, K.; Kameoka, H.; Marui, S.; Saito, M.; Kawaguchi, M.; Akiyama, K.; Saito, K. Myristate can be used as a carbon and energy source for the asymbiotic growth of arbuscular mycorrhizal fungi. BioRxiv 2020, 117, 25779–25788. [Google Scholar] [CrossRef]
| AMF Species | Host Plant | Effect of AMF on the Uptake of Mineral Nutrients | Reference |
|---|---|---|---|
| Glomus intraradices, Glomus versiforme | Poncirus trifoliata (L.) Raf. | Concentrations of Zn, P, K, and Mg increase in branches and roots. | [44] |
| Rhizophagus intraradices | Citrus limon L. | P content and root acid phosphatase activity increase. | [45] |
| Rhizophagus irregularis | Poncirus trifoliata (L.) Raf. | P absorption by citrus increases. | [46] |
| Glomus mosseae, Glomus etunicatum | Tarocco Xinxi | The content of inorganic calcium, water-soluble calcium, and pectin calcium in the root system of citrus seedlings increases. | [47] |
| Glomus versiforme | Poncirus trifoliata (L.) Raf., Citrus reticulate Blanco | Phenolic secretion by citrus roots is enhanced. It chelates Fe, facilitating its binding and retention in the root cell walls for later reuse. | [48] |
| Glomus versiforme, Glomus intraradices | Poncirus trifoliata (L.) Raf. “Ponkan”, “Newhall” trifoliate orange | Levels of Zn, P, K, Mg, Ca, and Cu significantly increased in various parts of citrus. | [49] |
| AMF Species | Host Plant | AMF Effect on the Expression of Defense Genes | Reference |
|---|---|---|---|
| Glomus versiforme | Poncirus trifoliata L., Citrus reticulate Blanco | Significant upregulation of phenylalanine deaminase PAL1 gene expression in the roots. | [48] |
| Funneliformis mosseae | Poncirus trifoliata | The significant expression of PtAHA2 was induced to resist soil drought. | [70] |
| Rhizophagus irregularis | Poncirus trifoliata (L.) Raf. | Nitrate transporter protein gene PtrNPF5.2 significantly upregulated expression. | [71] |
| Glomus versiforme | Poncirus trifoliata (L.) Raf. | Increased CHS activity and PtCHS expression. | [72] |
| five Glomus species | Poncirus trifoliata L. Raf. | The genes PtaPT4 and PtaPT5 were expressed upstream after AMF infection, while the genes PtaPT1, PtaPT2, PtaPT3, and PtaPT7 were expressed downstream. | [73] |
| Glomus versiforme | “Newhall” (Citrus sinensis), Poncirus trifoliata | Upregulation of chlorophyll and transport-related gene expression at protein level and downregulation of protease inhibitor gene expression. | [74] |
| AMF Species | Host Plant | Effect of AMF Inoculation on Citrus Growth under Drought Stress | Reference |
|---|---|---|---|
| Glomus mosseae | Poncirus trifoliata/Citrus sinensis | The plant height, spike thickness, leaf area, and shoot growth of citrus were significantly increased. | [40] |
| Funneliformis mosseae | Poncirus trifoliata | Root growth and leaf gas exchange are enhanced. | [70] |
| Rhizoglomus intraradices | Trifoliate orange | Aboveground and root biomass with greater metabolic activity increase. | [85] |
| Funneliformis mosseae | Trifoliate orange | It regulates root phytohormones and root morphology. | [86] |
| Funneliformis mosseae and Paraglomus occultum | Trifoliate orange | Leaf sucrose, fructose, and glucose concentrations increased, while proline levels decreased. | [87,88] |
| Funneliformis mosseae | Poncirus trifoliata | The unsaturation index of root FAs increased, and the composition of root FAs significantly changed. | [89] |
| Funneliformis mosseae | Trifoliate orange | The root volume increased while proline concentration and content in leaves, roots, and total plants decreased. | [90] |
| Funneliformis mosseae | Trifoliate orange | Plant height and chlorophyll and water content increased. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Zhang, Z.; Yu, L.; Li, Y. Research Progress of Arbuscular Mycorrhizal Fungi Promoting Citrus Growth. Horticulturae 2023, 9, 1162. https://doi.org/10.3390/horticulturae9111162
Tang C, Zhang Z, Yu L, Li Y. Research Progress of Arbuscular Mycorrhizal Fungi Promoting Citrus Growth. Horticulturae. 2023; 9(11):1162. https://doi.org/10.3390/horticulturae9111162
Chicago/Turabian StyleTang, Chungui, Zhongfeng Zhang, Limin Yu, and Ying Li. 2023. "Research Progress of Arbuscular Mycorrhizal Fungi Promoting Citrus Growth" Horticulturae 9, no. 11: 1162. https://doi.org/10.3390/horticulturae9111162
APA StyleTang, C., Zhang, Z., Yu, L., & Li, Y. (2023). Research Progress of Arbuscular Mycorrhizal Fungi Promoting Citrus Growth. Horticulturae, 9(11), 1162. https://doi.org/10.3390/horticulturae9111162






