Phytochemical Profile and Antioxidant Activity of Some Open-Field Ancient-Tomato (Solanum lycopersicum L.) Genotypes and Promising Breeding Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Sampling
2.2. Evaluation of the Main Agronomic Characteristics
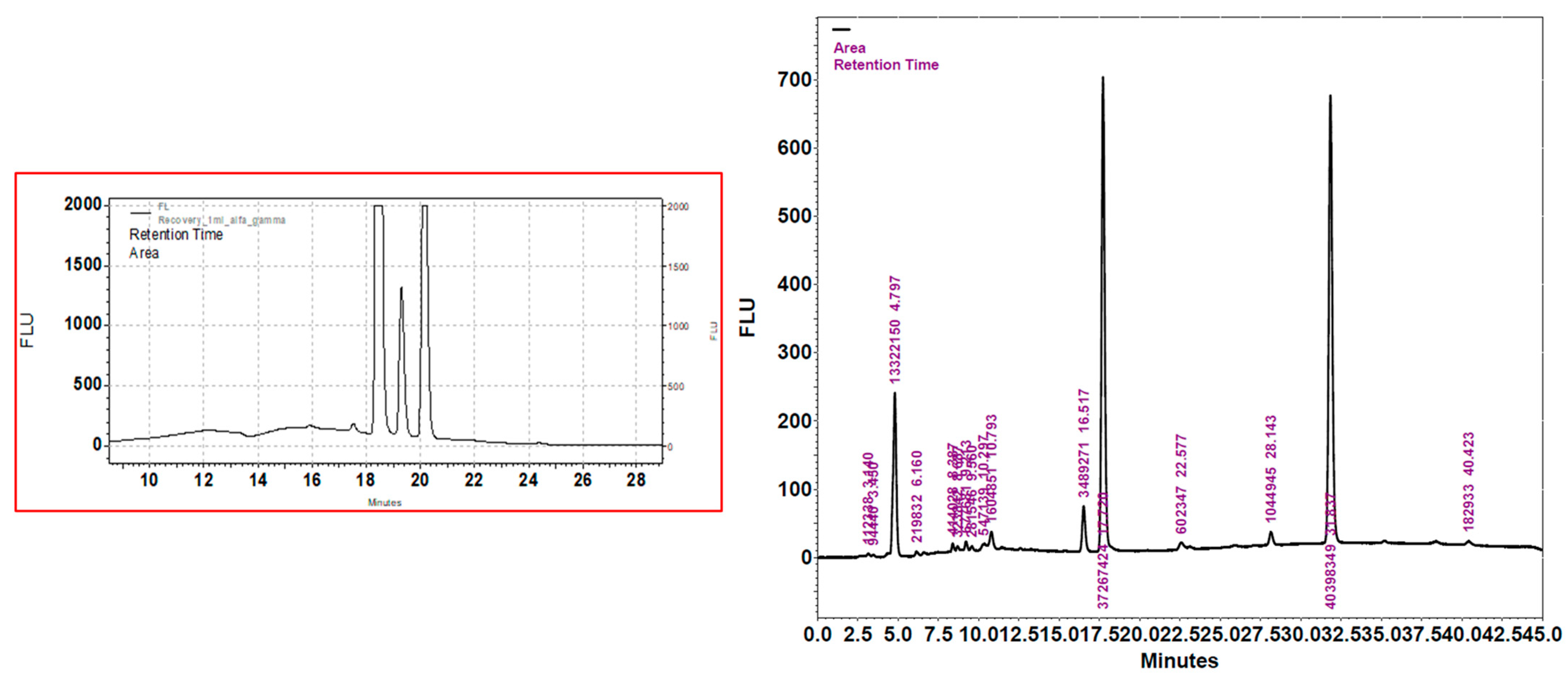
2.3. Evaluation of Carotenoid Content
2.4. Evaluation of Total Phenolic and Flavonoid Contents
2.5. Evaluation of Total Vitamin C Content
2.6. Evaluation of Tocopherol Content
2.7. Evaluation of the Antioxidant Activity
2.8. Statistical Analysis
3. Results
3.1. Agronomic Characteristics
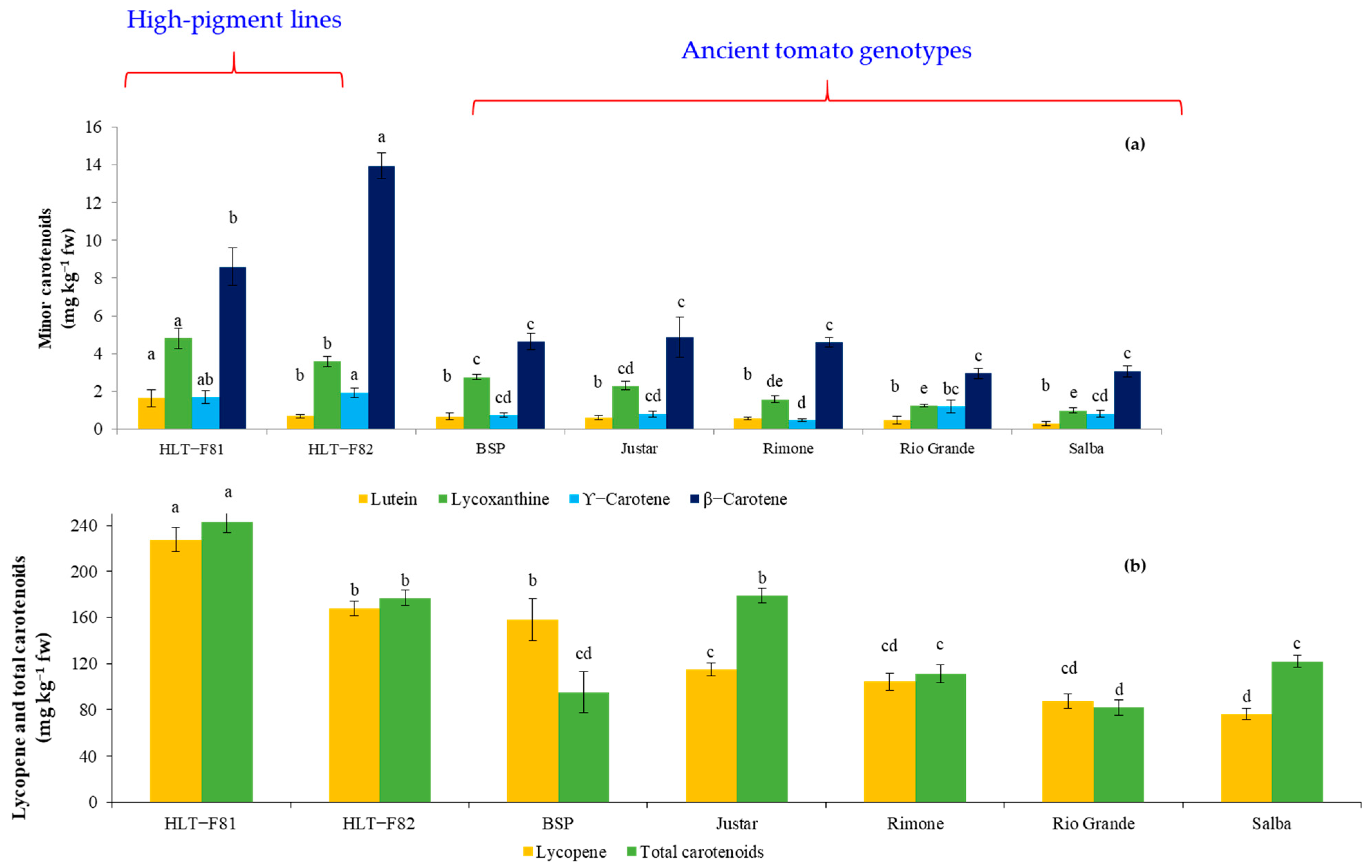
3.2. Carotenoid Content
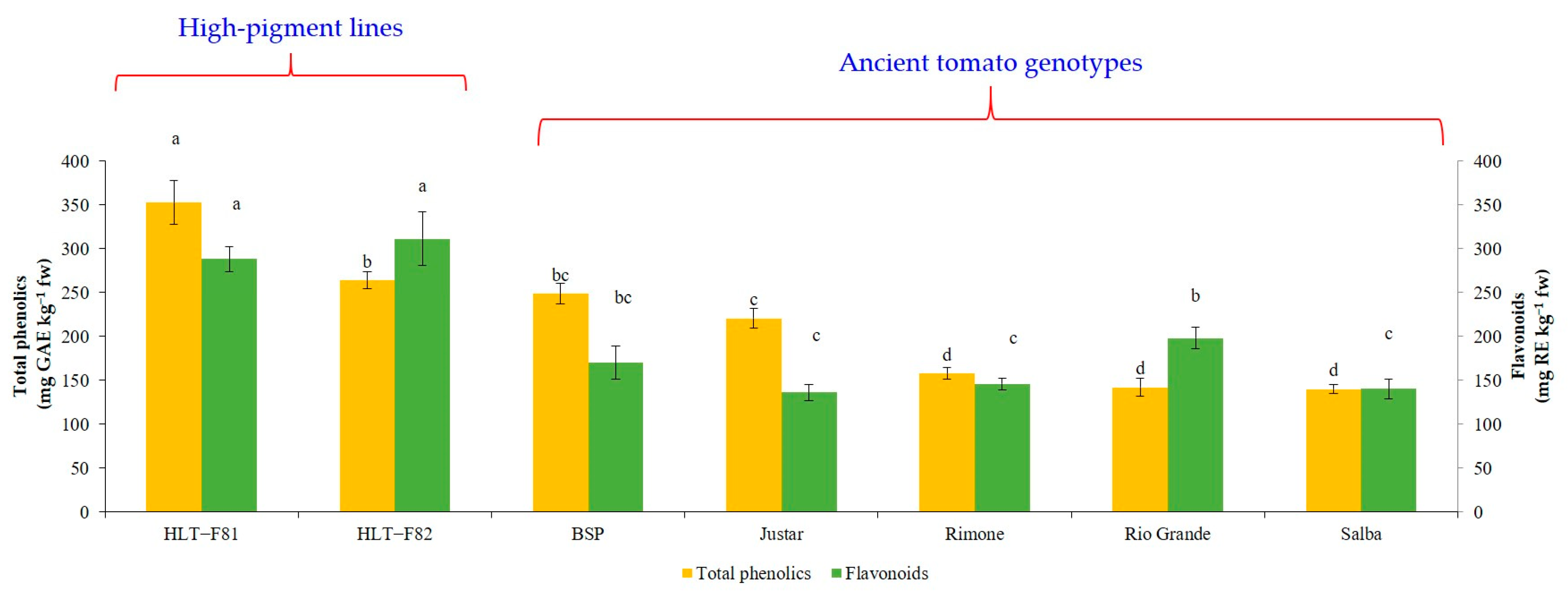
3.3. Total Phenolic and Flavonoid Contents
3.4. Tocopherol Content
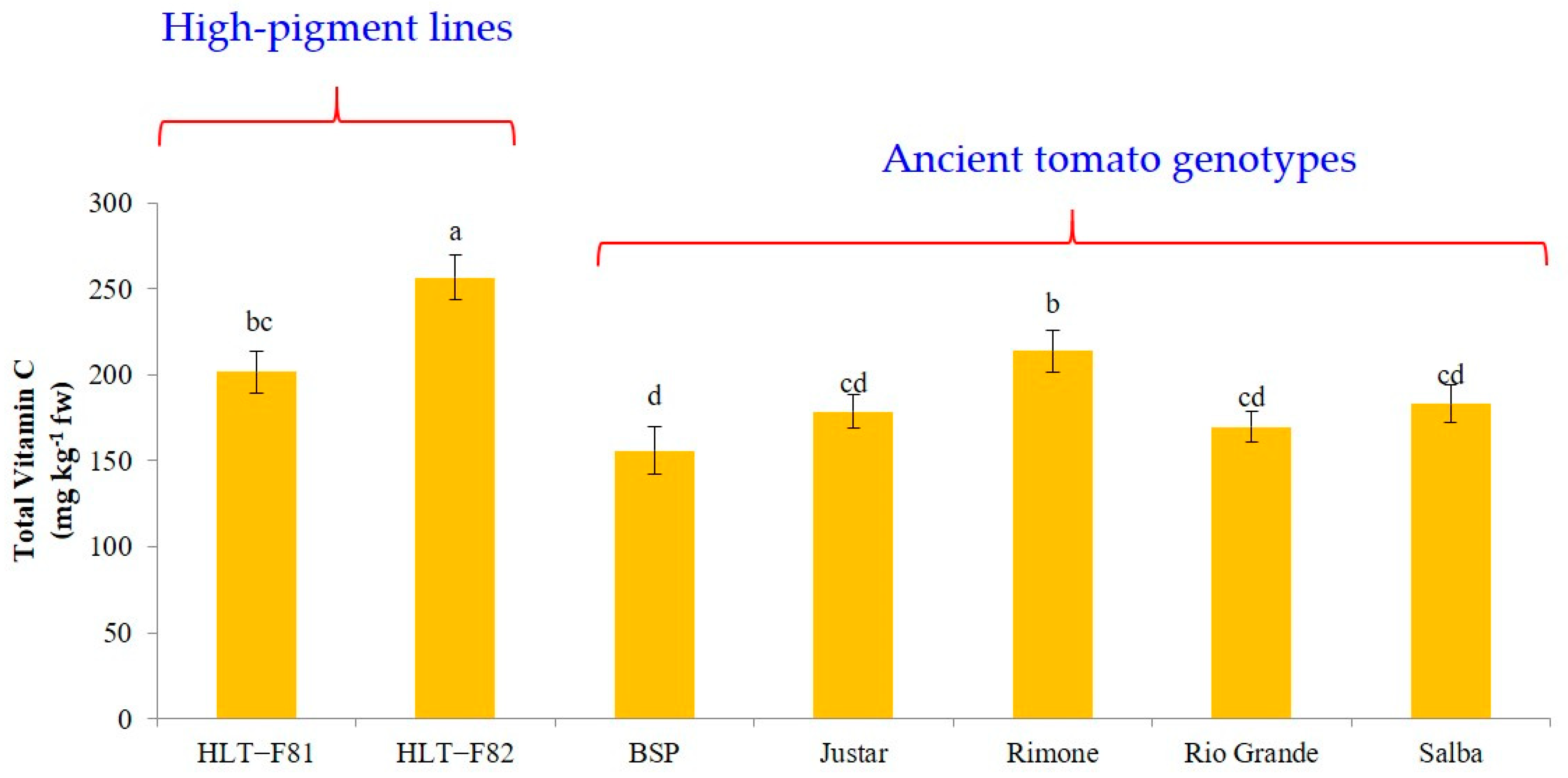
3.5. Total Vitamin C Content
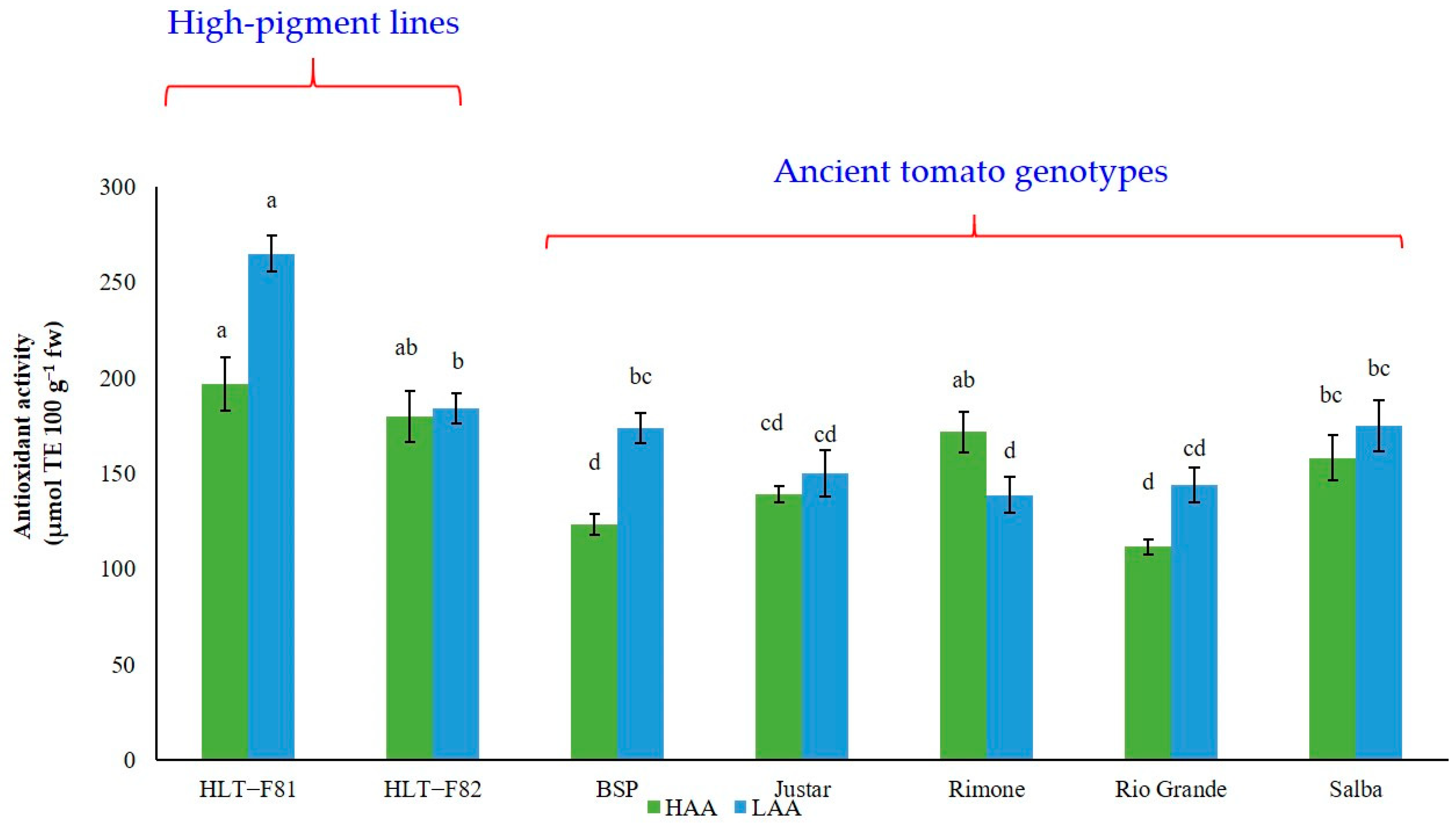
3.6. The Antioxidant Activity (the Trolox Equivalent Antioxidant Capacity, TEAC Assay)
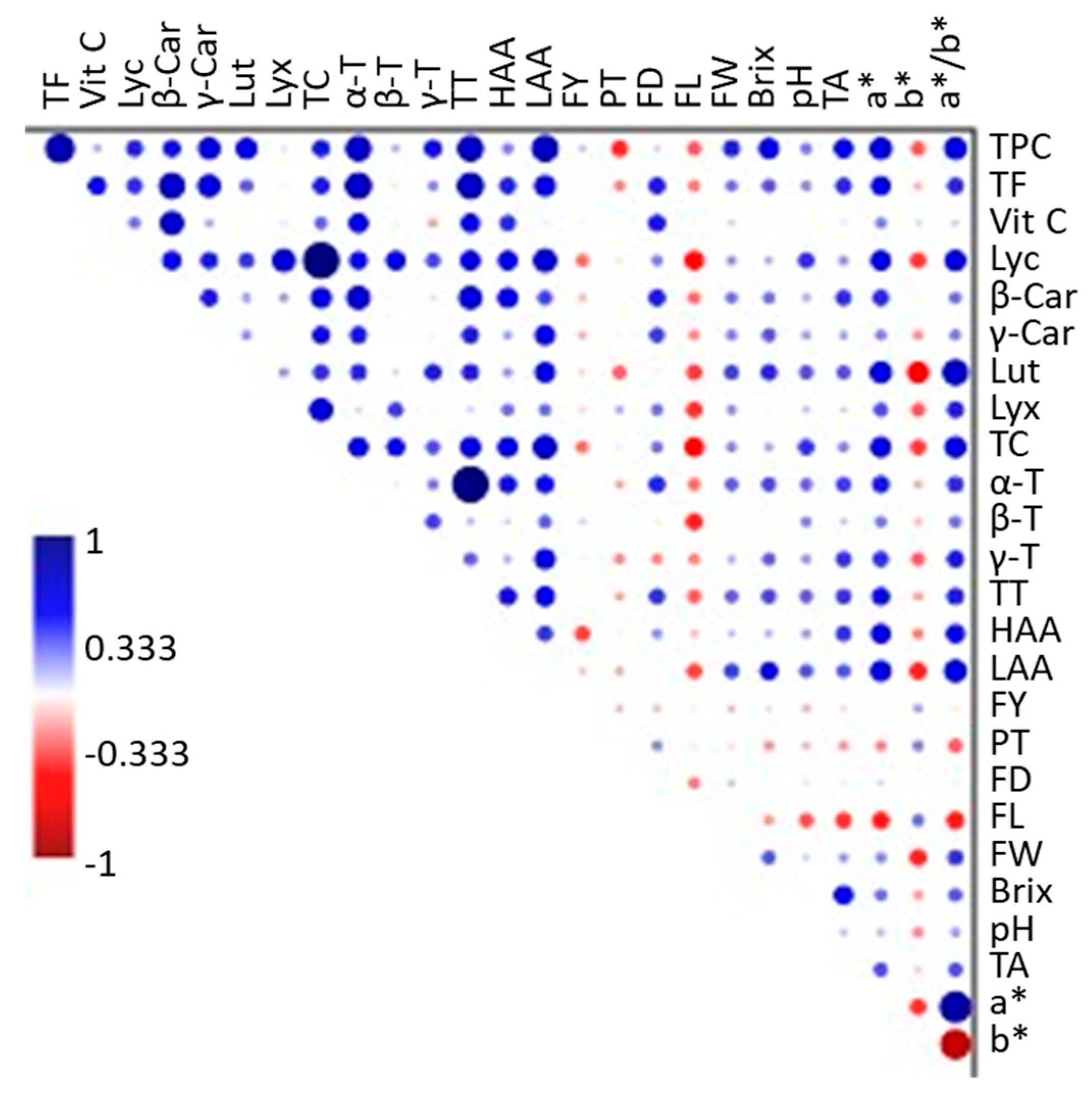
3.7. Correlation Analysis
3.8. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Harvested Area, Production and Average Yield of Tomato Culture in Tunisia. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 October 2023).
- Siddiqui, M.W.; Lara, I.; Ilahy, R.; Tlili, I.; Ali, A.; Homa, F.; Prasad, K.; Deshi, V.; Lenucci, M.S.; Hdider, C. Dynamic changes in health-promoting properties and eating quality during off-vine ripening of tomatoes. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1540–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, D.; Yu, L.; Aluo, Z.; Zhang, Z.; Qi, Y.; Li, T.; Song, Z.; Xu, G.; Zhou, L. Effects of lycopene on skeletal muscle-fiber type and high-fat diet-induced oxidative stress. J. Nutr. Biochem. 2021, 87, 108523. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Li, X.W.; Zhu, S.Y.; Li, M.Z.; Zhao, Y.; Talukder, M.; Li, Y.-H.; Li, J.-L. Lycopene ameliorates di (2-ethylhexyl) phthalate-Induced pyroptosis in spleen via suppression of classic caspase-1/NLRP3 pathway. J. Agric. Food Chem. 2021, 69, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Liu, Z.; Wang, Q.; Liu, J.; Feng, D.; Zou, J. Lycopene reduces cholesterol absorption and prevents atherosclerosis in ApoE–/–Mice by Downregulating HNF-1α and NPC1L1 Expression. J. Agric. Food Chem. 2021, 69, 10114–10120. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Siddiqui, M.W.; Tlili, I.; Montefusco, A.; Piro, G.; Hdider, C.; Lenucci, M.S. When color really matters: Horticultural performance and functional quality of high-lycopene tomatoes. Crit. Rev. Plant Sci. 2018, 37, 15–53. [Google Scholar] [CrossRef]
- Laranjeira, T.; Costa, A.; Faria-Silva, C.; Ribeiro, D.; de Oliveira, J.M.P.F.; Simões, S.; Ascenso, A. Sustainable valorization of tomato by-products to obtain bioactive compounds: Their potential in inflammation and cancer management. Molecules 2022, 27, 1701. [Google Scholar] [CrossRef]
- Yong, K.T.; Yong, P.H.; Ng, Z.X. Tomato and human health: A perspective from post-harvest processing, nutrient bio-accessibility, and pharmacological interaction. Food Front. 2023, 1–18. [Google Scholar] [CrossRef]
- Corrado, G.; Caramante, M.; Piffanelli, P.; Rao, R. Genetic diversity in Italian tomato landraces: Implications for the development of a core collection. Sci. Hortic. 2014, 168, 138–144. [Google Scholar] [CrossRef]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a Tomato Landraces Collection for Fruit-Related Traits by the Aid of a High-Throughput Genomic Platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.; Douthe, C.; El Aou-ouad, H.; Ribas-Carbó, M.; Galmés, J. Tomato landraces as a source to minimize yield losses and improve fruit quality under water deficit conditions. Agric. Water Manag. 2019, 223, 105722. [Google Scholar] [CrossRef]
- Scarano, A.; Olivieri, F.; Gerardi, C.; Liso, M.; Chiesa, M.; Chieppa, M.; Frusciante, L.; Barone, A.; Santino, A.; Rigano, M.M. Selection of tomato landraces with high fruit yield and nutritional quality under elevated temperatures. J. Sci. Food Agric. 2020, 100, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Moreno, C.; Roselló, S.; Beltrán, J.; Cebolla-Cornejo, J.; Moreno, M.M. Breeding tomato flavor: Modeling consumer preferences of tomato landraces. Sci. Hortic. 2023, 308, 111597. [Google Scholar] [CrossRef]
- Negri, V. Landraces in central Italy: Where and why they are conserved and perspectives for their on-farm conservation. Genet. Resour. Crop Evol. 2003, 50, 871–885. [Google Scholar] [CrossRef]
- Caramante, M.; Rouphael, Y.; Corrado, G. Genetic diversity among and within tomato (Solanum lycopersicum L.) landraces grown in Southern Italy. Genet. Resour. Crop Evol. 2023, 1–10. [Google Scholar] [CrossRef]
- Landi, S.; Punzo, P.; Nurcato, R.; Albrizio, R.; Sanseverino, W.; Cigliano, R.A.; Giorio, P.; Fratianni, F.; Batelli, G.; Esposito, S.; et al. Transcriptomic landscape of tomato traditional long shelf-life landraces under low water regimes. Plant Physiol. Biochem. 2023, 201, 107877. [Google Scholar] [CrossRef] [PubMed]
- Donoso, A.; Salazar, E. Yield components and development in indeterminate tomato landraces: An agromorphological approach to promoting their utilization. Agronomy 2023, 13, 434. [Google Scholar] [CrossRef]
- Egea, I.; Estrada, Y.; Faura, C.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Salt-tolerant alternative crops as sources of quality food to mitigate the negative impact of salinity on agricultural production. Front. Plant Sci. 2023, 14, 1092885. [Google Scholar] [CrossRef]
- Arias, R.; Lee, T.C.; Logendra, L.; Janes, H. Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Levin, I.; De Vos, C.R.; Tadmor, Y.; Bovy, A.; Lieberman, M.; Oren-Shamir, M.; Segev, O.; Kolotilin, I.; Keller, M.; Ovadia, R.; et al. High pigment tomato mutants—More than just lycopene (a review). Isr. J. Plant Sci. 2006, 54, 179–190. [Google Scholar] [CrossRef]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free. Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, M.L.; De Prisco, R.; Nicolaus, B.; Pari, P.; Moriello, V.S.; Strazzullo, G.; Iorio, E.L.; Servillo, L.; Balestrieri, C. Lycopene in association with α-tocopherol or tomato lipophilic extracts enhances acyl-platelet-activating factor biosynthesis in endothelial cells during oxidative stress. Free. Radic. Biol. Med. 2004, 36, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.; Shiwakoti, S.; Ko, J.Y.; Dhakal, B.; Park, S.H.; Choi, I.J.; Kim, H.J.; Oak, M.H. Oxidative stress in calcific aortic valve stenosis: Protective role of natural antioxidants. Antioxidants 2022, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Banks, S.W.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in a control and an NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiol. 1996, 112, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E content and composition in tomato fruits: Beneficial roles and bio-fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in plants: Novel concepts, new perspectives, and outstanding issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Daood, H.; Azam, M.; Siddiqui, M.W.; R’him, T.; Durante, M.; Lenucci, M.S.; et al. Effect of Individual and Selected Combined Treatments with Saline Solutions and Spent Engine Oil on the Processing Attributes and Functional Quality of Tomato (Solanum lycopersicon L.) Fruit: In Memory of Professor Leila Ben Jaballah Radhouane (1958–2021). Front. Nutr. 2022, 9, 844162. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Bonilla-Barrientos, O.; Lobato-Ortiz, R.; García-Zavala, J.J.; Cruz-Izquierdo, S.; Reyes-Lopez, D.; Hernandez-Leal, E.; Hernandez-Bautista, A. Agronimic and morphological diversity of local kidney and bell pepper-shaped tomatoes from Puebla and Oaxaca, México. Rev. Fitotec. Mex. 2014, 37, 129–139. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant activity and bioactive compound changes duringfruit ripening of high-lycopene tomato cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Phytochemical composition and antioxidant activity of high-lycopene tomato (Solanum lycopersicum L.) cultivars grown in Southern Italy. Sci. Hortic. 2011, 127, 255–261. [Google Scholar] [CrossRef]
- Ladewig, P.; Trejo-TéLlez, L.I.; Servin-Juarez, R.; Contreras-Oliva, A.; Gomez-Merino, F.C. Growth, yield and fruit quality of Mexican tomato landraces in response to salt stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12005. [Google Scholar] [CrossRef]
- Tagiakas, R.I.; Avdikos, I.D.; Goula, A.; Koutis, K.; Nianiou-Obeidat, I.; Mavromatis, A.G. Characterization and evaluation of Greek tomato landraces for productivity and fruit quality traits related to sustainable low-input farming systems. Front. Plant Sci. 2022, 13, 994530. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, A.; Riahi, A.; Ujj, A.; Ramos-Diaz, F.; Marjanović, J.; Hdider, C. Comparative Nutrient and Antioxidant Profile of High Lycopene Variety with hp Genes and Ordinary Variety of Tomato under Organic Conditions. Agronomy 2023, 13, 649. [Google Scholar] [CrossRef]
- Tihanyi, A.; Csambalik, L. Motivations of small-scale producers and gardeners towards tomato landrace utilization. Rev. Agric. Rural. Dev. 2022, 11, 176–180. [Google Scholar] [CrossRef]
- Hdider, C. Processing tomato variety trials in 1999. Tomato News 2000, 12, 30–33. [Google Scholar]
- Riahi, A.; Hdider, C.; Sanaa, M.; Tarchoun, N.; Ben Kheder, M.; Guezal, I. Effect of conventional and organic production systems on the yield and quality of field tomato cultivars grown in Tunisia. J. Sci. Food Agric. 2009, 89, 2275–2282. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Hachicha, M.; Rejeb, S.; Rejeb, M.N.; Hanchi, B.; Misle, E. Effects of saline water on tomato under subsurface drip irrigation: Nutritional and foliar aspects. J. Soil. Sci. Plant Nutr. 2011, 11, 69–86. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Hachicha, M.; Misle, E.; Fidalgo, F.; Teixeira, J. Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J. Saudi Soc. Agric. Sci. 2018, 17, 17–23. [Google Scholar] [CrossRef]
- Elbaz, M.; Timoumi, M.; Hanson, P. Behavior of new entries and developed tomato hybrids carrying Ty-2 gene. 01-Behavior of new entries and developed tomato hybrids carrying Ty-2. Tunis. J. Plant Prot. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Daood, H.G.; Bencze, G.; Palotas, G.; Pék, Z.; Sidikov, A.; Helyes, L. HPLC analysis of carotenoids from tomatoes using cross-linked C18 column and MS detection. J. Chromatogr. Sci. 2014, 52, 985–991. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Abushita, A.A.; Hebshi, E.A.; Daood, H.G.; Biacs, P.A. Determination of antioxidant vitamins in tomatoes. Food Chem. 1997, 60, 207–212. [Google Scholar] [CrossRef]
- Duah, S.A.; e Souza, C.S.; Daood, H.G.; Pék, Z.; Neményi, A.; Helyes, L. Content and response to γ -irradiation before over-ripening of capsaicinoid, carotenoid, and tocopherol in new hybrids of spice chili peppers. LWT Food Sci. Technol. 2021, 147, 111555. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Hdider, C. La culture de la tomate de saison en Tunisie: Développements récents et perspectives. Ann. De L’inrat 2012, 85, 3–23. [Google Scholar]
- Caiazzo, R.; Ricci, S.; Cantarella, C.; Urciuolo, G.; Cimmino, C.; Parisi, M.; Mennella, G.; D’Agostino, N. Combining transcriptomics and metabolomics to investigate ripening and post-harvest fruit withering in the cherry-like tomato landrace “pomodorino del Piennolo del Vesuvio”. In Proceedings of the 11th Solanaceae Conference, Bahia, Brazil, 2–6 November 2014. [Google Scholar]
- Hdider, C.; Guezel, I.; Arfaoui, K. Agronomic and qualitative evaluation of processing tomato cultivars in Tunisia. Acta Hortic. (ISHS) 2007, 758, 281–286. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Vitti, A.; Francia, E. Agronomic Comparisons of Heirloom and Modern Processing Tomato Genotypes Cultivated in Organic and Conventional Farming Systems. Agronomy 2021, 11, 349. [Google Scholar] [CrossRef]
- Tripodi, P.; Pepe, R.; Francese, G.; Rosaria, M.; Onofaro Sanajà, V.; Di Cesare, C.; Festa, G.; D’Alessandro, A.; Mennella, G. Biochemical Characterisation and Genetic Structure Provide Insight into the Diversity of the Mediterranean Tomato Ancient Varieties ‘San Marzano’ and ‘Re Fiascone’: New Resources for Breeding. Agronomy 2022, 12, 18. [Google Scholar] [CrossRef]
- Athinodorou, F.; Foukas, P.; Tsaniklidis, G.; Kotsiras, A.; Chrysargyris, A.; Delis, C.; Kyratzis, A.C.; Tzortzakis, N.; Nikoloudakis, N. Morphological Diversity, Genetic Characterization, and Phytochemical Assessment of the Cypriot Tomato Germplasm. Plants 2021, 10, 1698. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.M.; Yang, R.; Wu, J.; Chen, J.; Ledesma, D.; Tsou, S.C.S. Variation for antioxidant activity and antioxidants in tomato. J. Am. Soc. Hortic. Sci 2004, 129(5), 704–711. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The antioxidant profile evaluation of some tomato Landraces with soil salinity tolerance correlated with high nutraceuticaland functional value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Parisi, M.; Lo Scalzo, R.; Migliori, C.A. postharvest quality evolution in long shelf-life “Vesuviano” tomato landrace. Sustainability 2021, 13, 11885. [Google Scholar] [CrossRef]
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730. [Google Scholar] [CrossRef]
- Liu, Y.; Roof, S.; Ye, Z.; Barry, C.; Van Tuinen, A.; Vrebalov, J.; Bowler, C.; Giovannoni, J. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc. Natl. Acad. Sci. USA 2004, 101, 9897–9902. [Google Scholar] [CrossRef]
- Bino, R.J.; Ric de Vos, C.H.; Lieberman, M.; Hall, R.D.; Bovy, A.; Jonker, H.H.; Tikunov, Y.; Lommen, A.; Moco, S.; Levin, I. The light-hyperresponsive high pigment-2dg mutation of tomato: Alterations in the fruit metabolome. New Phytol. 2005, 166, 427–438. [Google Scholar] [CrossRef]
- Kolotilin, I.; Koltai, H.; Tadmor, Y.; Bar-Or, C.; Reuveni, M.; Meir, A.; Nahon, S.; Shlomo, H.; Chen, L.; Levin, I. Transcriptional profiling of high pigment-2dg tomato mutant links early fruit plastid biogenesis with its overproduction of phytonutrients. Plant Physiol. 2007, 145, 389–401. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kamimura, S. Inheritance of vitamin C content and its relation to other characters in crosses between hp and og varieties of tomatoes. In Proceedings of the 9th Meeting of the EUCARPIA Tomato Workshop, Wageningen, The Netherlands, 22–24 May 1984; pp. 8–13. [Google Scholar]
- Mustilli, A.C.; Fenzi, F.; Gliento, R.; Alfano, F.; Bowler, C. Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homologue of DEETIOLATED1. Plant Cell 1999, 11, 145–157. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Chakraborty, I.; Mishra, P.; Hazra, P. Bioactive attributes of tomatoes possessing dg, og c, and rin genes. Food Funct. 2014, 5, 936–943. [Google Scholar] [CrossRef]





| Cultivar | Fruit Shape | Marketable Yield (t ha−1) | Average Fruit Weight | Pericarp Thickness (mm) | Fruit Length (mm) | Fruit Diameter (mm) |
|---|---|---|---|---|---|---|
| HLT−F81 | Squared | 80.83 ± 2.08 a | 91.83 ± 5.50 a | 0.796 ± 0.06 c | 6.45 ± 0.21 c | 5.53 ± 0.32 bc |
| HLT−F82 | Oval | 78.26 ± 3.40 a | 82.50 ± 4.20 ab | 1.16 ± 0.055 ab | 7.14 ± 0.31 bc | 7.16 ± 0.55 a |
| BSP | Oval | 89.00 ± 5.69 a | 78.50 ± 4.84 ab | 1.21 ± 0.04 ab | 7.24 ± 0.28 bc | 5.11 ± 0.14 c |
| Justar | Squared | 82.88 ± 2.22 a | 76.81 ± 4.15 ab | 1.31 ± 0.10 a | 6.60 ± 0.30 c | 6.36 ± 0.22 ab |
| Rimone | Squared | 79.33 ± 3.73 a | 73.33 ± 3.95 | 1.04 ± 0.05 b | 7.60 ± 0.20 ab | 5.61 ± 0.17 bc |
| Rio Grande | Oval | 88.10 ± 5.09 a | 81.53 ± 4.90 ab | 0.78 ± 0.02 c | 7.57 ± 0.14 ab | 5.30 ± 0.23 c |
| Salba | Squared | 77.00 ± 4.64 a | 81.3 ± 6.74 ab | 1.22 ± 0.10 ab | 8.12 ± 0.33 a | 5.43 ± 0.19 c |
| Cultivar | Soluble Solids (°Brix) | pH | Titratable Acidity (%) | a* | b* | a*/b* |
|---|---|---|---|---|---|---|
| HLT−F81 | 6.08 ± 0.30 a | 4.44 ± 0.05 a | 0.477 ± 0.042 a | 29.83 ± 1.35 a | 19 ± 0.57 b | 1.58 ± 0.11 a |
| HLT−F82 | 5.76 ± 0.27 a | 4.29 ± 0.07 ab | 0.445 ± 0.018 ab | 26.17 ± 1.51 b | 24.66 ± 1.05 a | 1.06 ± 0.06 b |
| BSP | 5.68 ± 0.22 a | 4.24 ± 0.08 b | 0.438 ± 0.025 ab | 24.50 ± 0.957 bc | 24.03 ± 0.6 a | 1.02 ± 0.05 b |
| Justar | 4.66 ± 0.16 b | 4.35 ± 0.06 ab | 0.390 ± 0.022 b | 24.4 ± 0.428 bc | 23.0 ± 1.15 a | 1.08 ± 0.07 b |
| Rimone | 4.78 ± 0.18 b | 4.22 ± 0.03 b | 0.381 ± 0.023 b | 24.33 ± 0.760 bc | 24.36 ± 1.11 a | 1.01 ± 0.05 b |
| Rio Grande | 5.43 ± 0.16 a | 4.30 ± 0.05 ab | 0.416 ± 0.01 ab | 22.67 ± 0.558 c | 24.0 ± 1.12 a | 0.95 ± 0.055 b |
| Salba | 5.62 ± 0.23 a | 4.20 ± 0.03 b | 0.385 ± 0.022 b | 23.0 ± 1.15 bc | 23.0 ± 0.68 a | 1.00 ± 0.068 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laayouni, Y.; Tlili, I.; Henane, I.; Ali, A.B.; Égei, M.; Takács, S.; Azam, M.; Siddiqui, M.W.; Daood, H.; Pék, Z.; et al. Phytochemical Profile and Antioxidant Activity of Some Open-Field Ancient-Tomato (Solanum lycopersicum L.) Genotypes and Promising Breeding Lines. Horticulturae 2023, 9, 1180. https://doi.org/10.3390/horticulturae9111180
Laayouni Y, Tlili I, Henane I, Ali AB, Égei M, Takács S, Azam M, Siddiqui MW, Daood H, Pék Z, et al. Phytochemical Profile and Antioxidant Activity of Some Open-Field Ancient-Tomato (Solanum lycopersicum L.) Genotypes and Promising Breeding Lines. Horticulturae. 2023; 9(11):1180. https://doi.org/10.3390/horticulturae9111180
Chicago/Turabian StyleLaayouni, Yosr, Imen Tlili, Imen Henane, Ahlem Ben Ali, Márton Égei, Sándor Takács, Muhammad Azam, Mohammed Wasim Siddiqui, Hussein Daood, Zoltàn Pék, and et al. 2023. "Phytochemical Profile and Antioxidant Activity of Some Open-Field Ancient-Tomato (Solanum lycopersicum L.) Genotypes and Promising Breeding Lines" Horticulturae 9, no. 11: 1180. https://doi.org/10.3390/horticulturae9111180
APA StyleLaayouni, Y., Tlili, I., Henane, I., Ali, A. B., Égei, M., Takács, S., Azam, M., Siddiqui, M. W., Daood, H., Pék, Z., Helyes, L., R’him, T., Lenucci, M. S., & Ilahy, R. (2023). Phytochemical Profile and Antioxidant Activity of Some Open-Field Ancient-Tomato (Solanum lycopersicum L.) Genotypes and Promising Breeding Lines. Horticulturae, 9(11), 1180. https://doi.org/10.3390/horticulturae9111180











