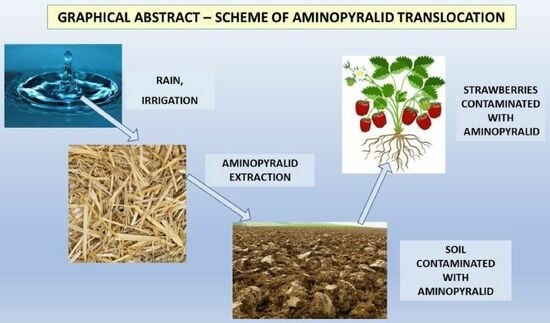
Translocation of Aminopyralid from Straw Mulch to Plants in Perennial Strawberry Plantations: Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strawberry Plant Growth Experiments
2.1.1. Plant Material
2.1.2. Pot Experiments Investigating the Effect of Aminopyralid on Strawberry Flowering and Crop
2.1.3. Analysis of Commercial-Farm, Strawberry Production Showing Translocation of Aminopyralid
2.2. Determination of Aminopyralid
2.2.1. Sample Preparation
2.2.2. Analytical Conditions
2.2.3. Data Processing
2.2.4. Chemicals and Reagents
3. Results
3.1. Assessment of Aminopyralid in Straw Mulch
3.2. Effect of Aminopyralid on Strawberry Flowering and Fruit Crop
3.3. Translocation of Aminopyralid from Straw Mulch to Strawberry Plant Tissues
4. Discussion
4.1. Development and Validation of the Extraction Method
4.2. Effect of AP on Flowering and Fruit Crop
4.3. Translocation of Aminopyralid from Straw Mulch to Strawberry Plants and Fruits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Z.; Ting, W.; Haixia, W.; Min, W.; Xiangping, M.; Siwei, M.; Rui, Z. Effects of straw mulch on soil water and winter wheat production in dryland farming. Sci. Rep. 2015, 5, 10725. [Google Scholar] [CrossRef] [PubMed]
- Král, M.; Dvořák, P.; Capouchová, I. The effect of straw mulch and compost application on the soil losses in potatoes cultivation. Plant Soil Environ. 2020, 66, 446–452. [Google Scholar] [CrossRef]
- Gannett, M.; Pritts, M.P.; Lehmann, J. Soil amendments affect soil health indicators and crop yield in perennial strawberry. Horttechnology 2019, 29, 179–188. [Google Scholar] [CrossRef]
- Commercial Strawberry Production on the Prairies, Alberta Agriculture and Forestry, Horticulture, Agdex 232/20-1, Government of Alberta. Available online: https://www.agric.gov.ab.ca/app08/ppsropintheweb?PubID=100035 (accessed on 11 September 2023).
- Borner, H. Liberation of organic substances from higher plants and their role in the soil sickness problem. Bot. Rev. 1960, 26, 393–424. [Google Scholar] [CrossRef]
- Opoku, G.; Vyn, T.J.; Vorone, R.P. Wheat straw placement effects on total phenolic compounds in soil and corn seedling growth. Can. J. Plant Sci. 1997, 77, 301–305. [Google Scholar] [CrossRef]
- Summary of Aminopyralid Toxicity and Fate for Application to Sensitive Areas of Rights-of-Way, Massachusetts Department of Environmental Protection Office of Research and Standards and Massachusetts Department of Agricultural Resources [Section 4(1)(E) of 333CMR 11.00 Rights-of-Way Management Regulations]. 2011. Available online: https://www.mass.gov/doc/aminopyralid/download (accessed on 11 September 2023).
- US-EPA: Aminopyralid: Draft Ecological Risk Assessment for Registration Review, Washington, DC, USA, 2020. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2013-0749-0048 (accessed on 30 August 2021).
- Pfleeger, T.; Blakeley-Smith, M.; Henty Lee, E.; King, G.; Plocher, M.; Olszyk, D. Effects of single and multiple applications of glyphosate or aminopyralid on simple constructed plant communities. Environ. Toxicol. Chem. 2014, 33, 2368–2378. [Google Scholar] [CrossRef]
- Fast, B.J.; Ferrell, J.A.; MacDonald, G.E.; Sellers, B.A.; MacRae, A.W.; Krutz, L.J.; Kline, W.N. Aminopyralid soil residues affect rotational vegetable crops in Florida. Pest. Manag. Sci. 2011, 67, 825–830. [Google Scholar] [CrossRef]
- Bukun, B.; Gaines, T.A.; Nissen, S.J.; Westra, P.; Brunk, G.; Shaner, D.L.; Sleugh, B.B.; Peterson, V.F. Aminopyralid and clopyralid absorption and translocation in Canada thistle (Cirsium arvense). Weed Sci. 2009, 57, 10–15. [Google Scholar] [CrossRef]
- Description of Chemical Generic Name: 2-Pyridine Carboxylic Acid, 4-Amino-3,6-dichloro, Common Name: Aminopyralid, Trade Names: Aminopyralid Technical Milestone™ EPA Chemical Code: 005100. 2005. pp. 1–56. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-005100_10-Aug-05.pdf (accessed on 11 September 2023).
- Could Manures, Composts or Mulch Damage Plants? Herbicides Carryover Risks, Sustainable Gardening Australia. Available online: https://www.sgaonline.org.au/could-manures-composts-or-mulch-damage-plants (accessed on 19 September 2023).
- Available online: https://www.arborchem.com/images/label-sds/qa_Dow_Milestone.pdf (accessed on 19 September 2023).
- da Silva Santos, R.T.; Della Vechia, J.F.; dos Santos, C.A.M.; Almeida, D.P.; Ferreira, M.d.C. Relationship of contact angle of spray solution on leaf surfaces with weed control. Sci. Rep. 2021, 11, 9886. [Google Scholar] [CrossRef]
- Aminopyralid Preliminary Work Plan Registration Review: Initial Docket Case Number 7267, 2014, Docket Number EPA-HQ-OPP-2013-0749, pp. 1–15. Available online: https://www.regulations.gov/docket/EPA-HQ-OPP-2013-0749 (accessed on 25 September 2023).
- Davis, J.; Johnson, S.E.; Jennings, K. Herbicide Carryover in Hay, Manure, Compost and Grass Clippings. NC State Extension Publications, 2020; pp. 1–7. Available online: https://content.ces.ncsu.edu/herbicide-carryover (accessed on 25 September 2023).
- Derr, J.; Flessner, M.; Bush, E.; Hansen, M.A. Plant Injury from Herbicide Residue; Publication PPWS-77P, Virginia Cooperative Extension, Virginia Tech; Virginia State University: Petersburg, VA, USA, 2016; pp. 1–5. Available online: www.ext.vt.edu (accessed on 25 September 2023).
- FAO; WHO. Pesticides Residues in Food 2007, Toxicological evaluations. In Proceedings of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and WHO Core Assessment Group, Geneva, Switzerland, 18–27 September 2007; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2009; pp. 3–36. [Google Scholar]
- EPA Fact Sheet for Aminopyralid, United States Office of Prevention, Pesticides Environmental Protection and Toxic Substances Agency (7501C) Pesticide Fact Sheet Name of Chemical: Aminopyralid Reason for Issuance: Conditional Registration Date Issued: 10 August 2005. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-005100_10-Aug-05.pdf (accessed on 25 September 2023).
- Mandokhail, K.; Maalik, A.; Hashmi, M.Z.; Farooq, U.; Nawaz, M.; Rehman, Z.U.; Sattar, A.; Ahmad, B. Chapter 10–Endocrine-disrupting compounds. In Environmetal Micropollutants; Hashmi, M.Z., Wang, S., Ahmed, Z., Eds.; A volume in Advances in Pollution Research; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 183–199. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Jiang, J.; Han, J.; Li, W.; Li, X.; Leung, K.M.Y.; Snyder, S.A.; Alvarez, P.J.J. Which micropollutants in water environments deserve more attention globally? Environ. Sci. Technol. 2022, 56, 13–29. [Google Scholar] [CrossRef]
- National List of Varieties Listed in the State Variety Book by 15 June 2022, Bulletin of the Central Institute for Supervising and Testing in Agriculture, Series: National Plant Variety Office, Czech Gazette for Plant Breeders Rights and National List of Plant Varieties. No XXI/3, p. 67, Central Institute for Supervising and Testing for Agriculture, Brno, Czech Republic, 2022. Available online: http://www.ukzuz.cz (accessed on 25 September 2023).
- Vissers Plant Innovators. Elsanta–Strawberry Plants–Strawberry. 2022. Available online: https://www.vissers.com/en/strawberryplants/elsanta (accessed on 24 February 2023).
- Hunnicutt, C.; MacRae, A.; Dittmar, P.; Noling, J.; Ferrell, J.; Alves, C.; Jacoby, T. Annual Strawberry Response to Clopyralid Applied During Fruiting. Weed Technol. 2013, 27, 573–579. [Google Scholar] [CrossRef]
- Sharpe, S.M.; Boyd, N.S.; Dittmar, P.J.; MacDonald, G.E.; Darnell, R.L. Clopyralid tolerance in strawberry and feasibility of early applications in Florida. Weed Sci. 2018, 66, 508–515. [Google Scholar] [CrossRef]
- Baumhover, N.J.; Larabee-Zierath, D.; Vargo, J.D.; Spak, D.R.; Netzband, D.; Dai, S.Y. A Simple Procedure for Determination of Aminocyclopyrachlor and Aminopyralid in Soil, Corn Meal, and Soy Meal using Liquid Chromatography/Tandem Mass Spectrometry. J. Regulat. Sci. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- SANTE/12682/2019, Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed-SANTE/12682/2019, 2019. Available online: https://www.accredia.it/en/documento/guidance-sante-11312-2021-analytical-quality-control-and-method-validation-procedures-for-pesticide-residues-analysis-in-food-and-feed/ (accessed on 11 September 2023).
- AffiniSep. Aminopyralid, Clopyrallid and Picloram in Cereals Extracts Using AFFINISEP SPE. Petit Couronne. Available online: www.affinisep.com (accessed on 11 September 2023).
- EURL-SRM. Workflow to Perform Quantification by Standard Addition Procedure, Sttuttgart, 2017. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=1010&LabID=200&Lang=EN (accessed on 11 September 2023).
- Bohumil, M. Czech Republic-Usage of Active Substances, Brno, 2021. Available online: https://eagri.cz/public/web/en/ukzuz/portal/plant-protection-products/usage-of-active-substances-in-cz/ (accessed on 27 January 2022).
- He, Y.; Tan, S.; Abd Ei-Aty, A.M.; Hacimüftuöǧlu, A.; She, Y. Magnetic molecularly imprinted polymers for the detection of aminopyralid in milk using dispersive solid-phase extraction. RSC Adv. 2019, 9, 29998–30006. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, S.S.; Boydston, R.A.; Kaspari, P.N.; Zhang, M.; Carr, E.; Smeenk, J.; Barnes, D.L. Aminopyralid residue impacts on potatoes and weeds. Am. J. Potato Res. 2013, 90, 239–244. [Google Scholar] [CrossRef]
- Soukupova, M.; Koudela, M. Impacts of aminopyralid on tomato seedlings. Horticulturae 2023, 9, 456. [Google Scholar] [CrossRef]
- Su, W.; Xu, H.; Hao, H.; Wu, R.; Wang, H.; Lu, C. Effect of environmental conditions on the degradation of florasulam in typical soils of northern China. J. Environ. Qual. 2017, 45, 553–558. [Google Scholar] [CrossRef]
- Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. 2,4-D Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, Oregon, 2008; Available online: http://npic.orst.edu/factsheets/archive/2,4-DTech.html (accessed on 11 September 2023).
- Pengelly, W.L.; Meins, F. Growth, auxin requirement, and indole-3-acetic acid content of cultured crown-gall and habituated tissues of tobacco. Differentiation 1984, 25, 101–105. [Google Scholar] [CrossRef]
- Ester, K.; Čurkovič-Perica, M.; Kralj, M. 2009. The phytohormone auxin induces G1 cell-cycle arrest of human tumor cells. Planta Med. 2009, 75, 1423–1426. [Google Scholar] [CrossRef]
- Barnabé, S.; Brar, S.K.; Tyagi, R.D.; Beauchesne, I.; Surampalli, R.Y. Pre-treatment and bioconversion of wastewater sludge to value-added products-Fate of endocrine disrupting compounds. Sci. Total Environ. 2009, 407, 1471–1483. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil–a critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, X.C.; Li, Q.; Jiang, S. Degradation of typical antibiotics during human feces aerobic composting under different temperatures. Environ. Sci. Pollut. Res. 2016, 23, 15076–15087. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef] [PubMed]


| Year/Straw Size (cm) | Aminopyralid (ng.g−1) | Std. Deviation | RSD% | |
|---|---|---|---|---|
| 2019 | 0.5 | 6.7867 a | 1.75 | 26% |
| 1.0 | 5.3222 a | 0.75 | 14% | |
| 1.5 | 5.2380 a | 1.48 | 28% | |
| 2020 | 0.5 | 15.5070 b | 0.91 | 6% |
| 1.0 | 16.2352 b | 0.29 | 2% | |
| 1.5 | 19.0908 c | 0.52 | 3% | |
| 2021 | 0.5 | 31.1671 d | 8.96 | 28% |
| 1.0 | 22.7486 d | 4.58 | 20% | |
| 1.5 | 22.3150 d | 1.50 | 7% | |
| Mulching | Aminopyralid (ng.g−1) | Std. Deviation | RSD% |
|---|---|---|---|
| Three layers (2019) | 1.6150 a | 0.26 | 16% |
| Two layers (2020) | 19.3325 b | 1.82 | 9% |
| One layer (2021) | 9.0566 c | 1.02 | 11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koudela, M.; Kurhan, S.; Soukupová, M.; Klouček, P.; Novotný, Č. Translocation of Aminopyralid from Straw Mulch to Plants in Perennial Strawberry Plantations: Case Study. Horticulturae 2023, 9, 1192. https://doi.org/10.3390/horticulturae9111192
Koudela M, Kurhan S, Soukupová M, Klouček P, Novotný Č. Translocation of Aminopyralid from Straw Mulch to Plants in Perennial Strawberry Plantations: Case Study. Horticulturae. 2023; 9(11):1192. https://doi.org/10.3390/horticulturae9111192
Chicago/Turabian StyleKoudela, Martin, Sebnem Kurhan, Miroslava Soukupová, Pavel Klouček, and Čeněk Novotný. 2023. "Translocation of Aminopyralid from Straw Mulch to Plants in Perennial Strawberry Plantations: Case Study" Horticulturae 9, no. 11: 1192. https://doi.org/10.3390/horticulturae9111192
APA StyleKoudela, M., Kurhan, S., Soukupová, M., Klouček, P., & Novotný, Č. (2023). Translocation of Aminopyralid from Straw Mulch to Plants in Perennial Strawberry Plantations: Case Study. Horticulturae, 9(11), 1192. https://doi.org/10.3390/horticulturae9111192