Abstract
As a type of abiotic stress, drought limits plant growth and productivity. The increased demand for the valuable essential oil extracted from geranium (Pelargonium graveolens L.) is mainly regulated by plant growth, which is adversely affected by drought. Melatonin (MT) has been used to enhance plant growth under various abiotic stresses, although its impact on overcoming drought stress in aromatic plants, including geranium, has not yet been investigated. In the current investigation, MT at 100 µM was applied at 100% (well-watered) or 50% (drought stress) field capacity to verify the role of MT in geranium under drought stress. Drought stress markedly reduced growth parameters, herb yield, and total chlorophyll content; however, MT alleviated these effects. The herb yield of the stressed plants was reduced by 59.91% compared to the unstressed plants, while this reduction was only 14.38% when MT was applied. In contrast, drought enhanced the essential oil percentage in geranium leaves. Despite the reduction in oil yield caused by drought, MT application mitigated this reduction and improved both oil yield and oil components. Moreover, the MT treatment enhanced the accumulation of total phenols, glutathione, and proline and improved the activity of ascorbate peroxidase, catalase, and glutathione reductase, resulting in the alleviation of drought-induced oxidative damage. Consequently, MT reduced both hydrogen peroxide and malondialdehyde accumulation by 71.11 and 48.30%, respectively, under drought, thereby maintaining the cellular structures’ integrity. Overall, this is the first report that reveals the ability of MT application to improve geranium oil yield and resistance to drought by enhancing the antioxidant defense system. The results enrich awareness regarding the potential benefits of the external application of MT and its roles that can help researchers to improve aromatic plants’ performance and productivity under drought stress.
1. Introduction
Limited water resources are caused by global warming, which eventually affects the agricultural sector via drought [1]. Drought, as a form of abiotic stress, adversely affects the growth and development of various species and therefore lowers their productivity [2,3]. Drought stress greatly impacts the morphological, physiological, and biochemical processes of horticultural plants. Reduced root and shoot growth, impaired membrane integrity, imbalanced mineral accumulation, and damaged photosynthetic apparatuses have been identified as the most deleterious effects faced by crop plants under drought stress [4]. Drought stress also triggers oxidative stress due to the overproduction of reactive oxygen species (ROS), which attack cellular membrane lipids, proteins, and nucleic acids [5]. Plants have evolved various mechanisms to cope with the adverse effects of drought stress. Among these mechanisms, the accumulation of compatible solutes reduces the cellular osmotic potential to retain continuous water absorption and maintain cell turgidity [6]. In addition, plants combat drought-stress-induced oxidative damage by enhancing their enzymatic and non-enzymatic antioxidants, which scavenge ROS and attenuate their hazardous effects [1,6].
Plants’ responses to drought stress to combat its adverse effects seem to be insufficient for completely mitigating them. Therefore, different approaches are necessary to alleviate these detrimental effects, and exogenous applications of various natural and unnatural compounds supplied in different protocols are often adopted to improve drought stress tolerance [4,5]. In medicinal and aromatic plants, different treatments have been applied to enhance drought stress tolerance, such as silicon [3], ketoconazole [7], calcium chloride [8], and mycorrhizal inoculation [9]. Interestingly, the synthesis of secondary metabolites by aromatic plants is considered to be a functional stabilization of such plants induced to cope with abiotic stress conditions via a signaling pathway [10]. In support of this idea, the accumulation of secondary metabolites has been observed to be markedly increased in several aromatic plants, including black cumin [11], Salvia species [12], Lamiaceae [13], and Citrus [14], in response to stress conditions. However, it is essential to use effective eco-friendly applications for the sustainable cultivation of medicinal and aromatic plants to enhance tolerance to drought stress [2]. Among them, exogenous treatment using bio-stimulators or phytohormones is considered a useful and powerful method of improving crop adaptability and protection against stressful conditions [15,16]. One of the bio-stimulators that has been shown to play crucial roles in plant growth, development, and response to abiotic stresses is Melatonin (MT); it is a multifunctional hormone [17].
MT, a tryptophan derivative, is an important indoleamine plant hormone that is commonly produced in several species [18]. MT is a potential bio-stimulator phytohormone that helps to regulate several functions in horticultural crops, especially under abiotic stresses, mainly via improvements in osmolyte production and membrane stability [19]. MT plays an important role in numerous biological processes, including germination, growth regulation, flowering, photosynthesis, senescence, the maintenance of postharvest quality, and abiotic stress tolerance [20,21,22]. MT is also an antioxidant and a powerful free radical scavenger, boosting plants’ performance against oxidative injury [23]. Therefore, MT ameliorates abiotic stress by enhancing its antioxidant machinery (both enzymatic and non-enzymatic) that directly eliminates ROS [24,25]. As such, MT has been demonstrated to ameliorate the adverse effects of abiotic stress in several horticultural species, such as Citrus [14], cucumber [26], apple [27], Moldavian balm [28], rapeseed [29], and tomato [30]. Noticeably, the available information concerning MT’s functions in enhancing drought stress resistance is mainly from field crops or other horticultural plants, but MT’s role in promoting drought stress tolerance in aromatic plants is rarely investigated. For example, MT treatment ameliorated the adverse impact of drought on lemon verbena by protecting photosynthetic pigments, accumulating proline and soluble sugars, increasing total phenolic compounds, and preventing oxidative damage, resulting in enhanced growth and essential oil content [31]. Although MT has been applied in agriculture to enhance plant growth and development, the impact of exogenous application of MT on the secondary metabolites of important horticultural crops, including pelargonium, has been poorly studied. MT has been reported to have an auxin-like (IAA) activity and, therefore, is thought to be able to promote the synthesis of essential oil [12].
Geranium (Pelargonium graveolens L. Herit), belonging to the Geraniaceae family, is a crucial perennial aromatic plant that is usually cultivated for essential oil production [32]. The valuable geranium essential oil is mainly used in the cosmetics, perfumery, and flavor industries [33]. Due to the various oxygenated monoterpenes that are present in geranium essential oil, such as linalool, geraniol, citronellol, and geranyl formate [34,35], it has high antioxidant activity and antibacterial and antifungal properties. Therefore, it is generally used in the treatment of hemorrhoids, inflammation, heavy menstrual flows, dysentery, and even cancer [36,37]. Despite the fact that several countries produce geranium, essential oil production for this plant is lower than the industry requirement [38]. Although geranium essential oil and its several uses are in globally high demand, drought stress is negatively affecting the development of this commercial aromatic plant [9]. Although MT application in agriculture for enhancing plant growth and development has been frequently examined, MT’s impact on the accumulation of essential oils in aromatic species such as geranium has been poorly studied. To the best of our knowledge, this is the first work to evaluate the impact of this promising biofriendly molecule on the geranium essential oil profile under drought stress. This study was undertaken to evaluate the effect of the exogenous application of MT on drought stress tolerance in geranium plants and its underlying mechanisms.
2. Materials and Methods
2.1. Experimental Site and Plant Materials
A pot experiment was conducted at the open field of the Faculty of Agriculture, Shebin Elkom, Menoufia University, Egypt (30°33′24.8″ N 31°00′51.3″ E), from February to July in 2021 and 2022. The average day and night temperatures during the study were 30 and 17 ± 1 °C, while day and night RH were 34 and 67 ± 5% with an average photoperiod of 13 h. Geranium (Pelargonium graveolens L. (Herit) seedlings (one-year-old) were transplanted on the 1st of March into 30 cm pots filled with clay soil. The physical and chemical features of this soil are presented in Table 1.

Table 1.
The results of the physical and chemical analysis of the soil used.
2.2. Treatments and Experimental Design
In this research, 64 homogenous plants were divided into 4 equal groups. The first group was subjected to field capacity (FC) application at 100% (control), while the second one was subjected to FC application at 100% and foliar-sprayed with MT at 100 µM. The third group was exposed to FC application at 50% (stressed plants), and the last group was subjected to FC application at 50% and foliar-sprayed with melatonin (MT) at 100 µM. Both groups that were not treated with MT were distilled-water-sprayed at the same time as the MT application. Each treatment had three replicates, and each replicate consisted of 4 pots (12 plants per treatment). A completely randomized design (CRD) was applied to arrange the treatments. MT was attained from Sigma-Aldrich (St. Louis, MO, USA). Ethanol was first used to dissolve MT, and then a concentration of 100 µM was prepared. Tween-20 at 0.1% (v/v) was applied as a surfactant for MT foliar application. Geranium seedlings were simultaneously exposed to drought stress and MT one month after transplanting. Melatonin was applied using a manual pump two times weekly for 3 months. A weighting procedure was used to control the drought stress treatment [39]. For soil dry weight determination, 4 kg of soil was placed into an electric oven at 103 °C for 48 h. The pots were then filled with this oven-dried soil. Next, the pots were watered completely to enable soil saturation. The FC percentage was calculated using the following equation:
FC (%) = [(Wet soil weight − Dry soil weight)/Dry soil weight] × 100
The water amount stored under FC conditions was estimated after deducting the soil dry weight and the weight of the pots. Consequently, the 100% FC for the control and 50% FC for drought stress were determined.
2.3. Growth and Herb Yield Determination
Twelve weeks after the treatments, the growth and herb yield characteristics were evaluated by measuring plant height (cm), main branch number/plant, and herb fresh weight (g). Samples for the subsequent analysis were also collected since some of the samples were rapidly frozen in liquid nitrogen and kept at −80 °C, while other samples were oven-dried at 60 °C for further investigation.
2.4. Assessment of Chlorophyll Pigment
To extract chlorophyll from geranium leaf samples (0.2 g), acetone (80%) solvent was used as described by Metzner et al. [40]. The extract was then centrifuged for 10 min at 15,000× g and evaluated using a spectrophotometer (ST150SA Model 7205, Cole-Parmer Ltd. Stone, Staffs, UK) at 663 and 645 nm. Chlorophyll a and b concentrations were calculated using the equations developed by Lichtenthaler [41]
where A663 and A647 are the absorbance at wavelengths of 663 and 647 nm, respectively.
Chl a = 12.25.A663 − 2.79.A647
Chl b = 21.50.A647 − 5.10.A663
Total Chl = Chl a + Chl b
The total chlorophyll content was calculated by combining both values, and it is expressed as mg g−1 FW.
2.5. Essential Oil Determination
Fresh weight samples (50 g) of the geranium herb were extracted from cuttings after 24 h, and the essential oil percentage was determined using hydro distillation units in a Clevenger apparatus over the course of three hours [42] via the following formula:
Essential oil percentage = (volume of oil in the graduated tube/sample fresh weight) × 100
The collected essential oil was dried using sodium sulfate (anhydrous) and stored in dark conditions at −4 °C until GC-MS analysis.
2.6. Essential Oil Composition
GC-MS analysis of essential oil was performed using a Varian GC (CP-3800) and MS (Saturn 2200) equipped with a column (with a film thickness of 0.25 µm and ID VF-5ms 30 × 0.25 mm). To determine the GC-MS parameters, a system of electron ionization with an energy of 70 eV was applied. Helium was used as a carrier gas at a 1 mL per minute flow rate. The temperature program was as follows: The MS transfer line temperatures of the injector and detector were set at 140 and 300 °C, respectively. The initial temperature of the column was 40 °C, increasing in steps of 2 °C min−1. This was increased to 280 °C in steps of 5 °C min−1. Finally, the temperature was held at 280 °C for 6 min. An essential oil sample of 1 μL (with a spilled ratio of 1:30) was manually injected. The essential oil components of geranium were identified by linking the components’ mass spectra and retention times with standards and the NIST library of the GC-MS system.
2.7. Determination of H2O2
The method used by Patterson et al. [43] was followed to measure H2O2 production in geranium leaf samples. A leaf extract was prepared by homogenizing a 0.5 g sample in chilled acetone (6 mL of 100%) and centrifuging it at 12,000× g for 10 min at 4 °C. A volume of 1 mL taken from the extract was then added to NH4OH (0.2 mL) and Ti (SO4)2 (0.1 mL of 5%) and centrifuged at 3000× g for 10 min. After that, 4 mL of 2M from H2SO4 was used to dissolve the pellet. Finally, the absorbance was spectrophotometrically (Cole-Parmer Ltd. ST150SA, Model 7205Stone, Staffs, UK) investigated at 412 nm. To calibrate the absorbance, a standard curve was generated using several known H2O2 concentrations, and the production of H2O2 was recorded in µmol g−1 FW.
2.8. Assessment of Lipid Peroxidation
Malondialdehyde (MDA) content in fresh leaf samples was measured to evaluate lipid peroxidation, as described by Hodges et al. [44]. Each 0.2 g sample was homogenized in 2 mL of 0.1% trichloroacetic acid and centrifuged for 15 min at 14,000× g. Next, in 3 mL of 0.5% thiobarbituric acid and 5% trichloroacetic acid, 2 mL of the supernatant was mixed in a water bath at 95 °C for 30 min. The reaction was ended thereafter via cooling on ice, and mixture centrifugation was then performed for 15 min at 5000× g. Finally, the absorbance of the supernatant was assessed at 450, 532, and 600 nm using 1,1,3,3-tetraethoxy propane as a standard. The content of MDA in μmol mL−1 was calculated as follows:
MDA = 6.45 × (A532 − A600) − 0.56 × A450
Here, A refers to the absorbance at the exact wavelength.
2.9. Membrane Permeability Measurement
Membrane permeability was determined using Yan et al.’s method [45]. Fresh leaf samples were weighed in glass beakers with reverse osmosis water. The beakers containing samples were kept for three hours at 30 ± 1 °C, and solution conductivity was measured. After that, the samples were boiled for two minutes, and the conductivity was evaluated again after cooling at room temperature. Electrolyte leakage (EC, %) was calculated as shown below
where C1 and C2 are the conductivities recorded before and after the sample was boiled.
EC = (C1/C2) × 100
2.10. Determination of Glutathione (GSH)
The GSH level in geranium leaves was assessed using the spectrophotometric method reported by Anderson [46] with slight modification [47]. A pure GSH sample was used as a standard for the calibration curve following linear regression analysis.
2.11. Proline Determination
The method developed by Bates et al. [48] was followed to estimate the proline content in geranium leaves. Frozen samples (0.5 g) of leaf tissue were homogenized in sulfosalicylic acid (10 mL of 3%) at 4 °C. Next, Whatman No. 2 filter paper was used to filter the extract. A mixture of acid-ninhydrin and glacial acetic acid (2 mL each) was added to 2 mL of the resultant filtrate and incubated for one hour at 100 °C, and then the mixture was put on ice to stop the reaction. A total of a mL of toluene was then used to extract the mixture; finally, the solution absorbance was monitored at 520 nm. Pure proline was used to create a calibration curve, and the level of proline was measured as μmol g−1 FW.
2.12. Determination of Total Phenol Content
Each powdered sample (1 g) was stirred together with methanol (50 mL of 80%) for two days at room temperature. After removing the methanol, the resulting extract was maintained at 4 °C to determine the total phenol content using the method developed by McDonald et al. [49]. A volume of 0.5 mL of diluted extract (1:10 g mL−1) or Gallic acid (GA, a standard phenolic compound) was added to 5 mL of diluted Folin–Ciocalteu reagent (1:10) and 4 mL of sodium carbonate (1 M). Finally, the absorbance was investigated at 765 nm, and total phenol content was estimated as mg GAE g−1 DW.
2.13. Determination of Antioxidant Enzyme Activities
First, we homogenized a 0.5 g leaf sample in 5 mL sodium phosphate buffer (50 mM and pH 7.5) and 1 mM of PMSF (phenylmethylsulfonyl fluoride). Second, centrifugation of the extract was performed at 12,000× g for 20 min at 4 °C, and the resultant supernatant was used for enzyme assays. Catalase (CAT, EC 1.11.1.6) activity was measured following the method used by Chandlee and Scandalios [50]. Extract samples (0.04 mL each) were homogenized with potassium phosphate (50 mM) buffer (pH 7.0) and 2.6 mL of H2O2 (15 mM). Then, the obtained H2O2 decomposition level was estimated by measuring the reduction in absorbance at 240 nm, and CAT activity was expressed as U mg−1 protein, where 1 U equals a decrease of 1 mM of H2O2 per minute.
Ascorbate peroxidase activity (AXP, EC 1.11.1.11) was assayed using the protocol employed by Nakano and Asada [51]. Fresh leaf samples (0.1 g) were ground in 0.2 mL of the extraction buffer, which consisted of Triton X-100 (1%), polyvinylpyrrolidone 1% [PVP], EDTA (3.0 mM), and Na-phosphate (0.1 M, pH 7.0), and the mixture was then centrifuged for 20 min at 10,000× g. A buffer reaction consisting of 0.5 mM of ascorbate, 0.1 mM of EDTA, 0.1 mM of H2O2, and 0.05 mL enzyme extract was created, and the reaction was executed for 5 min at 25 °C. The activity of APX was assessed by measuring the absorbance at 290 nm using a Pharmacia LKB-Novaspec II spectrophotometer. The activity of APX was calculated using the coefficient of absorbance (2.8 mM−1 cm−1), where one APX enzyme unit can decompose 1.0 µmol of ascorbate in one minute.
Glutathione reductase enzyme (GR, EC 1.6.4.2) activity was determined following the procedure used by Foyer and Halliwell [52] and the modification made by Rao [53]. Leaf samples (0.5 g) were extracted in 2.0 mL of buffer consisting of 1.0% Triton X-100, 3.0 mM EDTA (0.1% PVP), and 1 M of Na-phosphate (pH 7). The obtained mixture was then centrifuged for 10 min at 10,000× g, and the resulting supernatant was investigated at 340 nm to determine GR activity, following NADPH glutathione-dependent oxidation. The reaction mixture was composed of 0.2 NADPH, 0.05 mL of enzyme extract, and 0.5 mM of glutathione disulfide and was left to settle for 5 min at 25 °C. To overcome glutathione disulfide oxidation, correction was performed without NADPH. Finally, the activity of GR was measured using the absorbance coefficient, namely, 6.2 mM−1 cm−1, since one GR unit can decompose 1.0 µmol per minute of NADPH.
2.14. Statistical Analysis
A combined analysis was performed on the results for both years, and the results were pooled. The ANOVA test was performed using the SPSS program (13.3 versions), and the separation of means was conducted using the Duncan multiple range test [54] with significance set at p ≤ 0.05.
3. Results
3.1. Growth Characteristics and Herb Yield
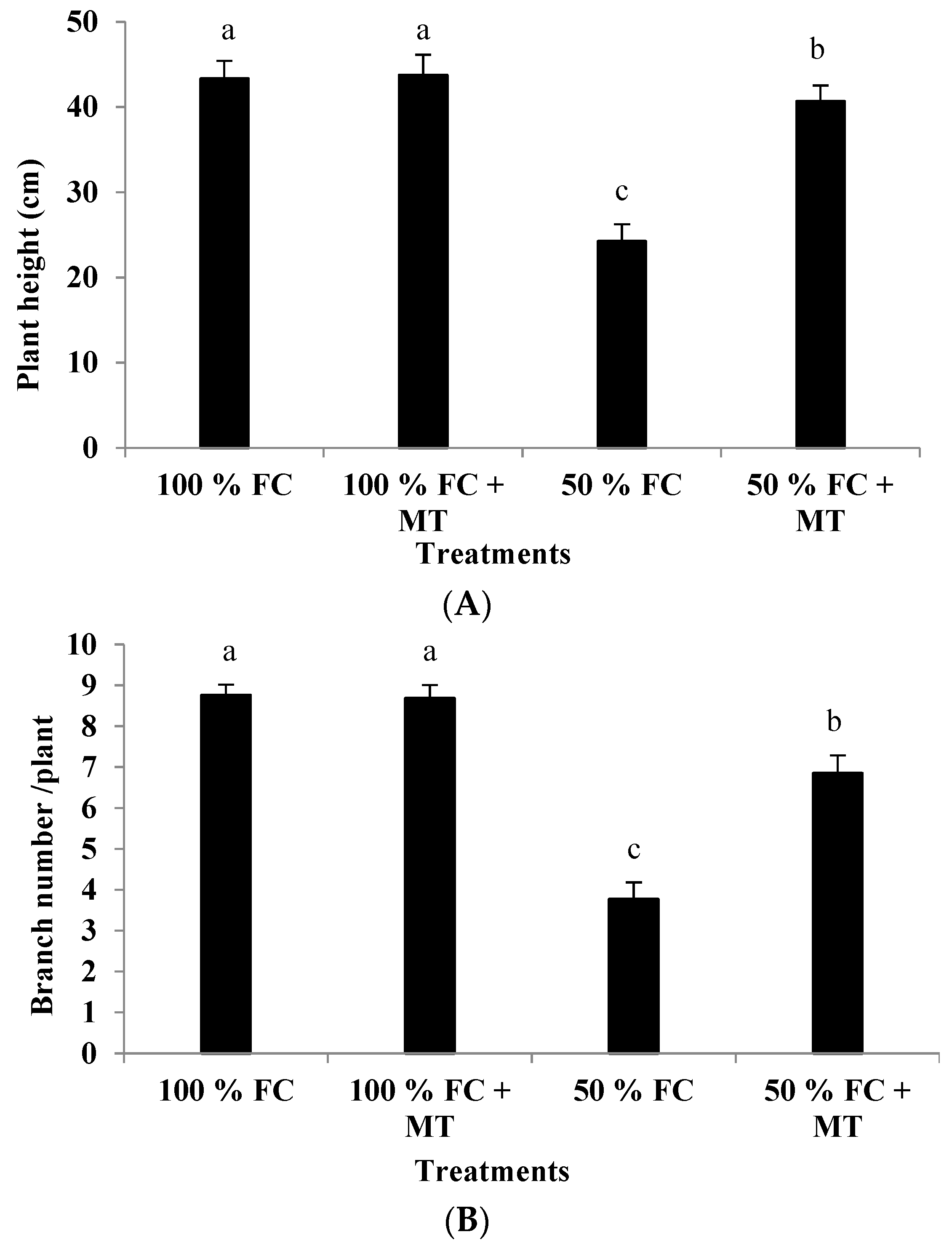
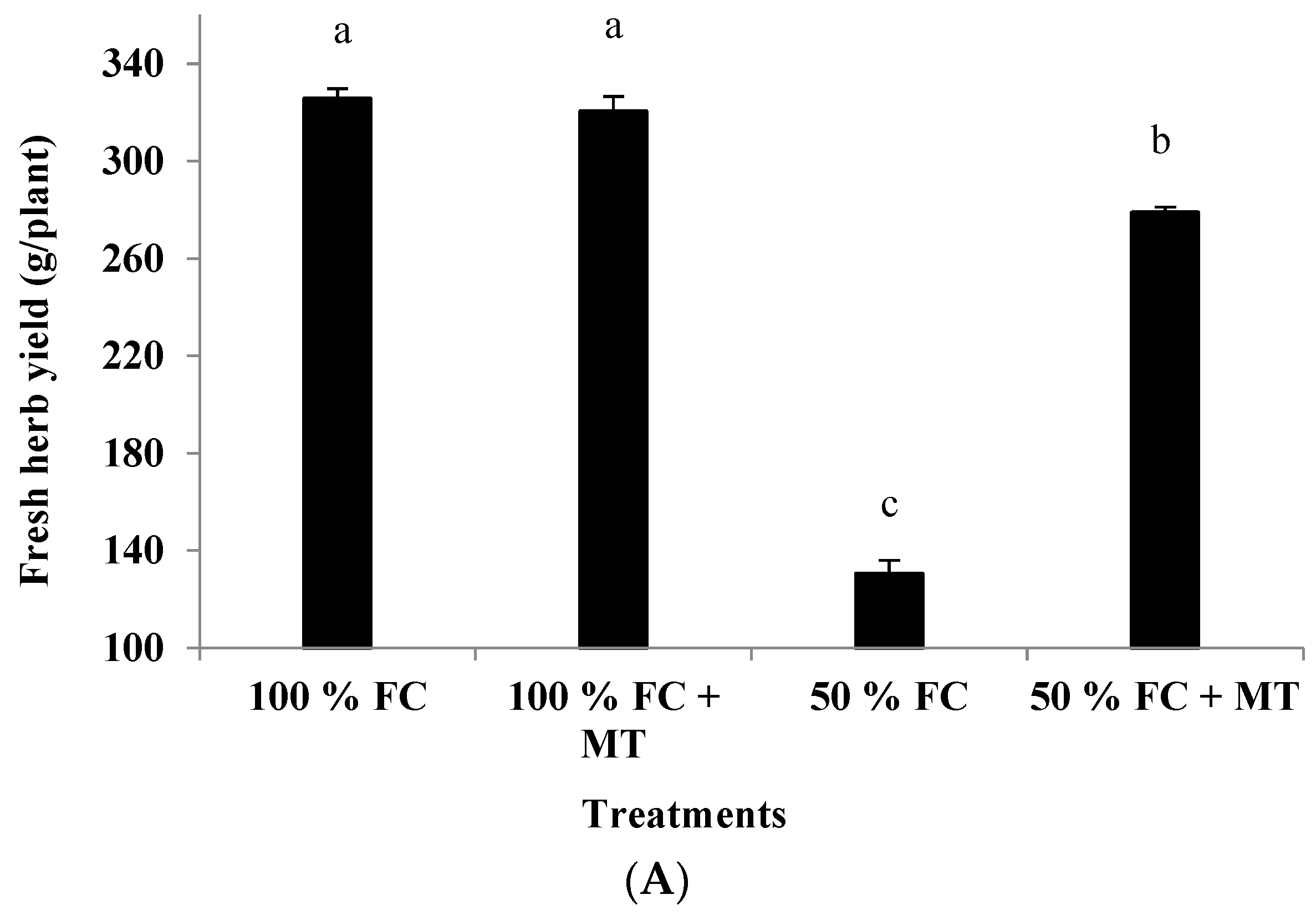
Drought stress (50% FC) significantly reduced the plant height and branch number of the geranium subjects (Figure 1), resulting in a herb yield reduction (Figure 2A) in comparison with the unstressed plants. Contrarily, MT application markedly increased growth and yield relative to the unstressed plants. Plant height was reduced by 44.01% in comparison with the unstressed plants, while this reduction was only 6.14% when MT was applied. Also, relative to the unstressed plants (100% FC), the herb yield of the stressed plants was reduced by 59.91%, while this reduction was only 14.38% when MT was applied. Notably, MT treatment had no effect when it was applied to the unstressed plants (100% FC).
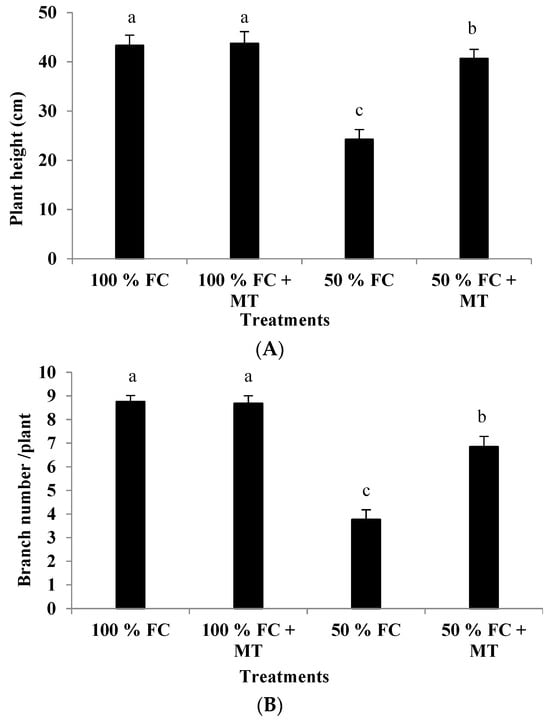
Figure 1.
Impact of MT treatment on plant height (A) and branch number (B) of drought-stressed Pelargonium graveolens L. Her. Plants were exposed to 50 or 100% field capacity (FC) MT application and foliar-sprayed with 100 µM of melatonin (MT). Results are means ± SE of two seasons (n = 6). Different letters represent statistically significant differences between the treatments (p ≤ 0.05).
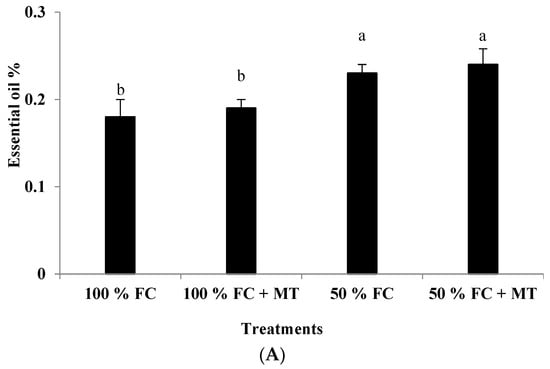
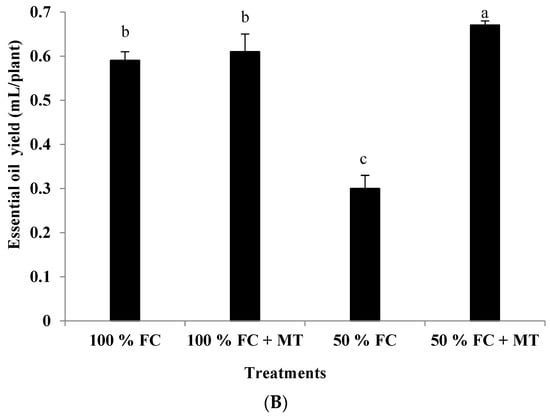
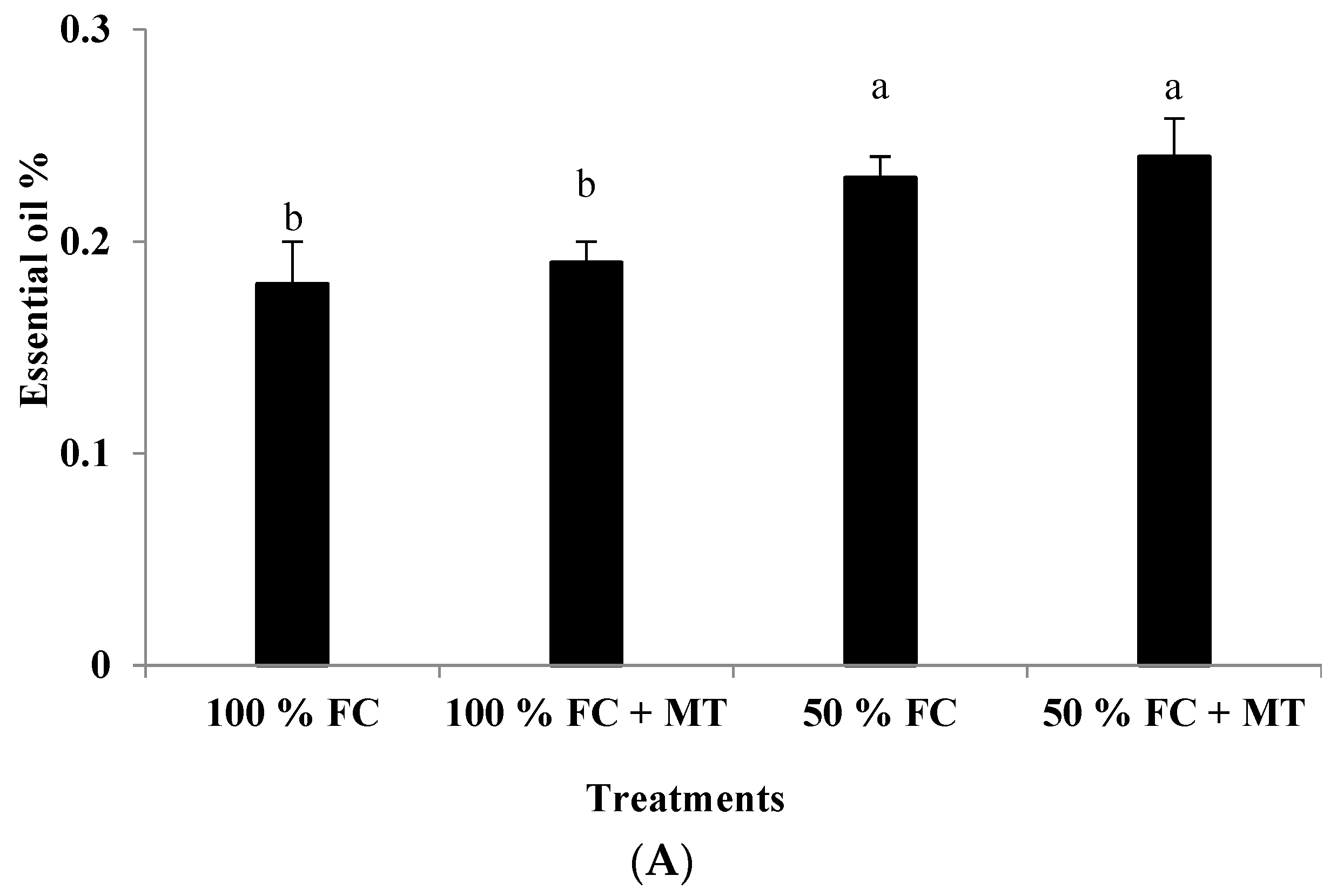
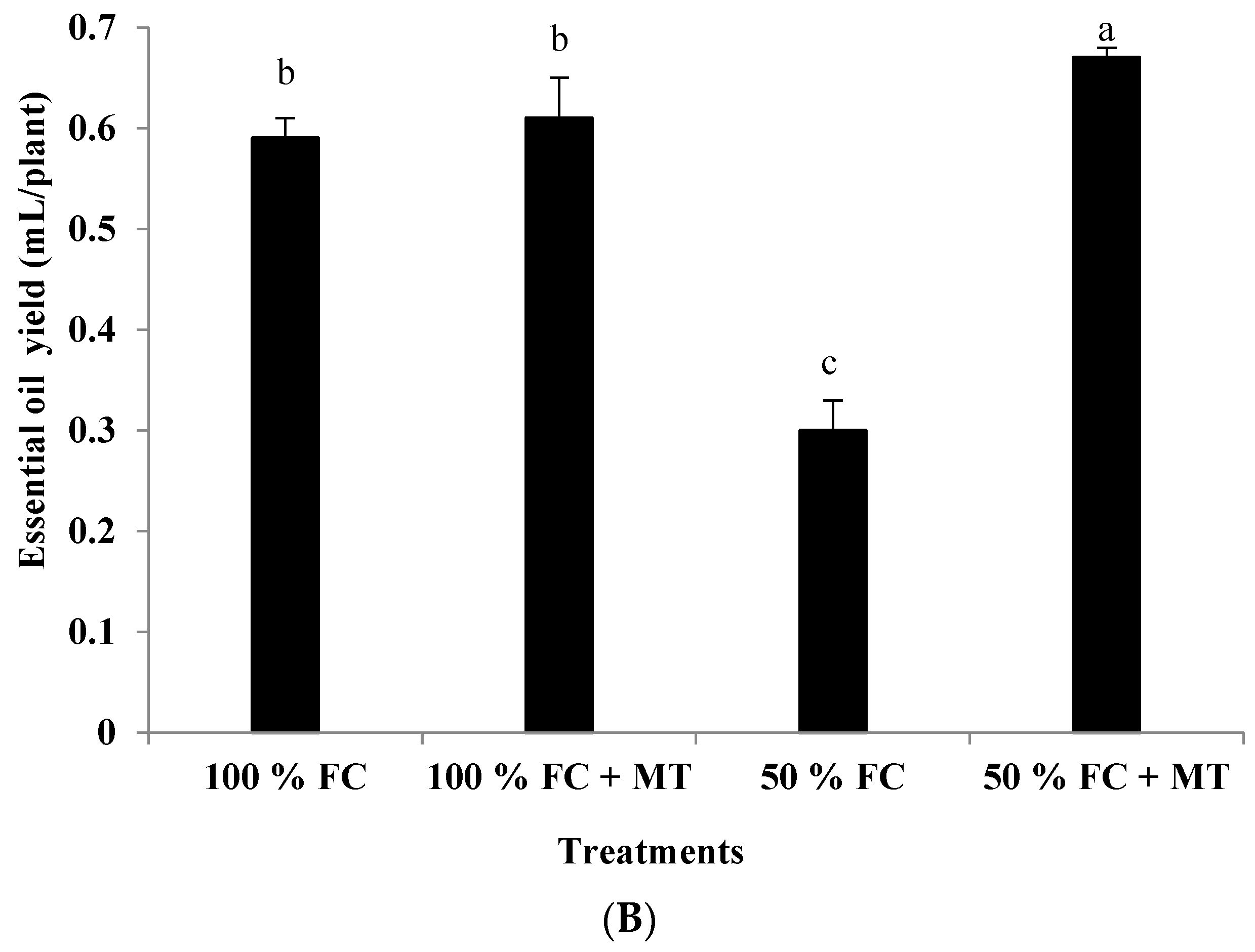
Figure 2.
Impact of melatonin (MT) treatment on essential oil percentage (A) and oil yield (B) of drought-stressed Pelargonium graveolens L. Her. Plants were exposed to 50 or 100% field capacity (FC) MT application and foliar-sprayed with 100 µM of melatonin (MT). Results are means ± SE of two seasons (n = 6). Letters represent statistical differences of treatments determined at p ≤ 0.05.
3.2. Essential Oil Content
Essential oil accumulation in geranium leaves markedly elevated under drought stress (50% FC) compared with that of the control (100% FC). A slight and insignificant increase in essential oil percentage due to MT application was noticed for both the stressed and non-stressed plants. On a percentage basis, MT enhanced the oil percentage in the stressed plants by 33% compared to that of the well-watered plants (Figure 2A). In contrast, the essential oil yield was decreased in response to drought stress in comparison with the non-stressed plants, whereas MT foliar spraying considerably enhanced oil yields for the drought-stressed plants by 123% relative to the plants that were subjected to drought stress (Figure 2B).
3.3. Essential Oil Composition
The essential oil composition obtained in the current work indicated that the main oil components were citronellol, geraniol, linalool, citronellyl formate, and geranyl formate. Among them, citronellol accounted for more than 26% of the essential oil (Table 2). Generally, drought stress treatment enhanced the number of essential oil components compared with that for the well-watered plants, in which this number was further elevated via MT foliar spraying—even more so when applied under stress.

Table 2.
Impact of melatonin (MT) treatment on essential oil composition of drought-stressed Pelargonium graveolens L. Her. Plants were exposed to 50 or 100% (field capacity) FC and foliar-sprayed with 100 µM MT.
3.4. Chlorophyll Content
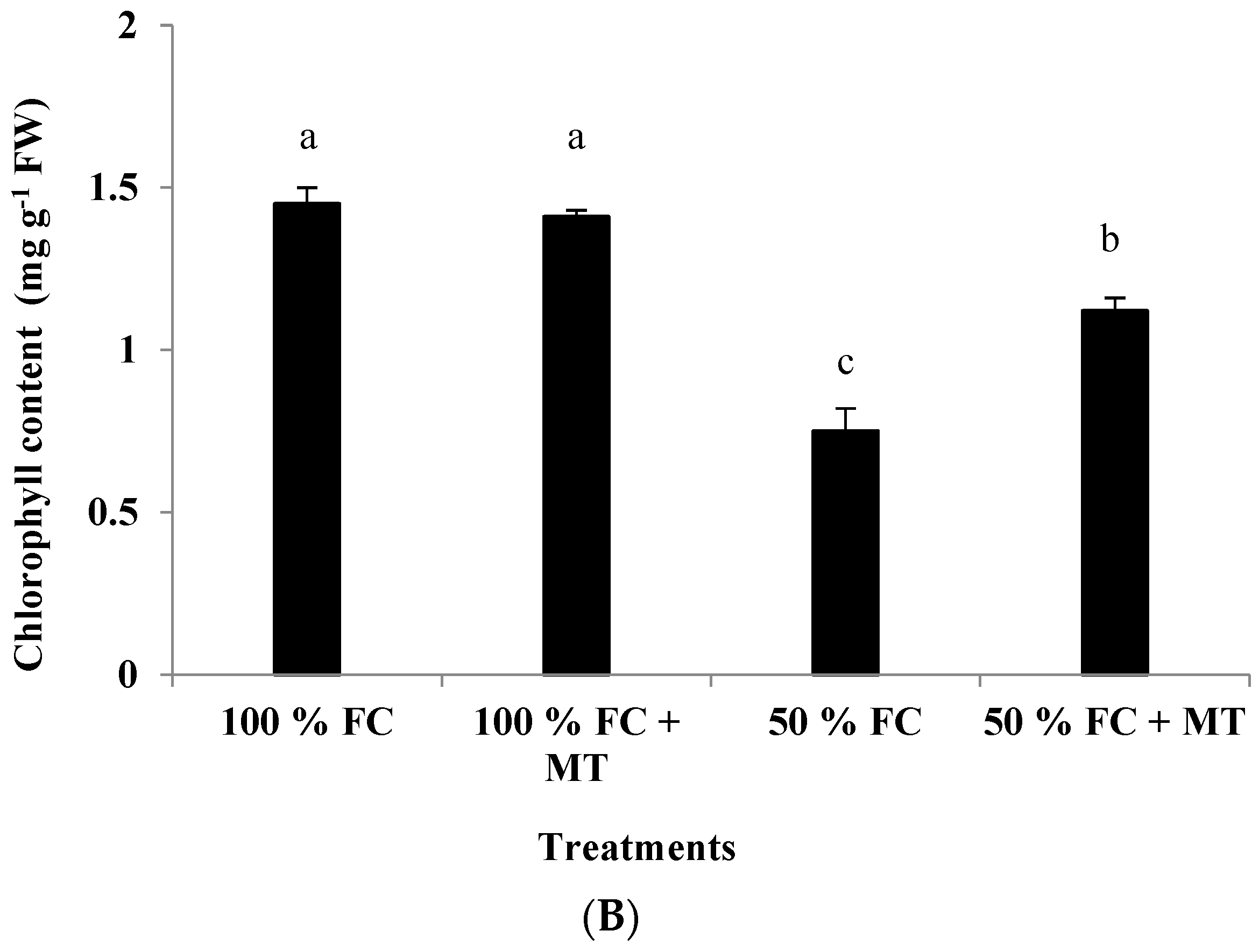
The leaf chlorophyll content was adversely affected by drought stress, whereas MT application significantly improved the chlorophyll content in the 50%-FC-stressed plants (Figure 3B). The chlorophyll content in the plants exposed to 50% FC application was reduced by 48.28%, while it was reduced by only 22.76% when MT was applied. Under 100% FC conditions, MT application had no effect on the chlorophyll content (Figure 3B).
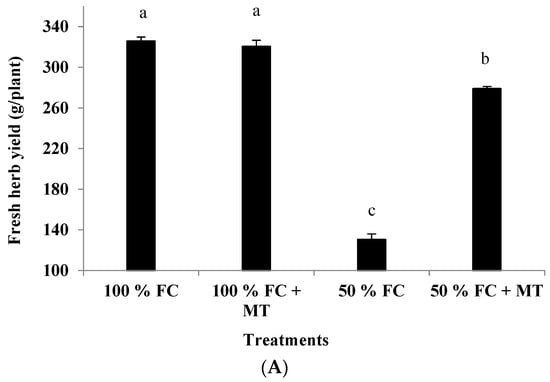
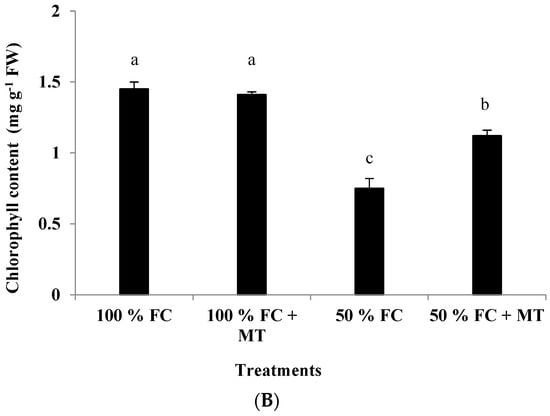
Figure 3.
Impact of MT treatment on herb yield (A) and chlorophyll content (B) of drought-stressed Pelargonium graveolens L. Her. Plants were exposed to 50 or 100% of field capacity (FC) and foliar-sprayed with 100 µM of melatonin (MT). Results are means ± SE of two seasons (n = 6). Different letters represent statistically significant differences between the treatments at p ≤ 0.05.
3.5. H2O2 Production
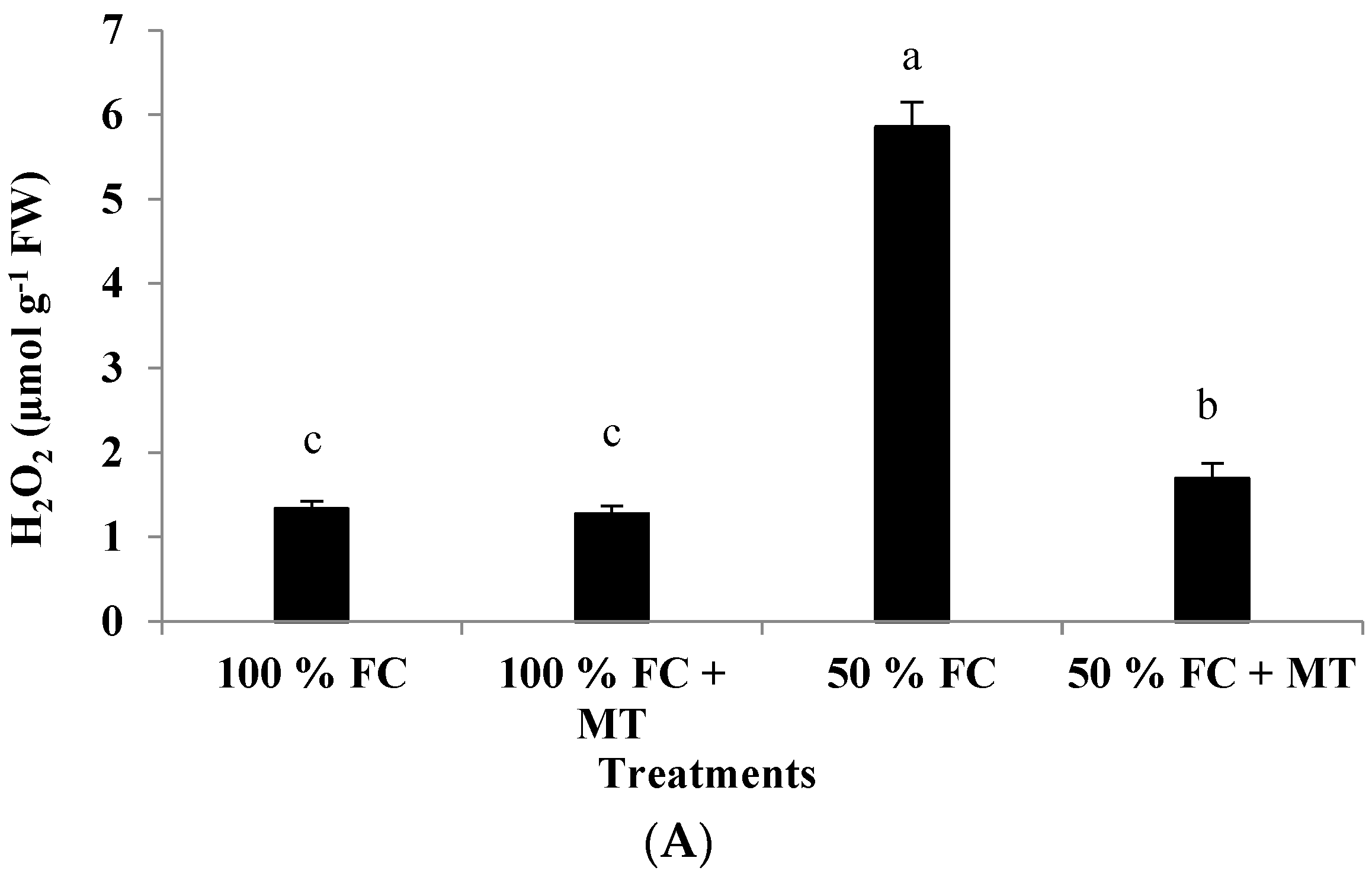
The hydrogen peroxide content in the drought-stressed plants was markedly increased (4.40-fold) compared with that of the well-watered plants (Figure 4A). However, MT foliar spray significantly reduced H2O2 content relative to the 50%-FC-stressed plants, for which a 1.27-fold increase was recorded in comparison to plants exposed to 100% FC. The well-watered plants treated with MT presented insignificant H2O2 production levels compared with those that did not receive MT application (Figure 4A).
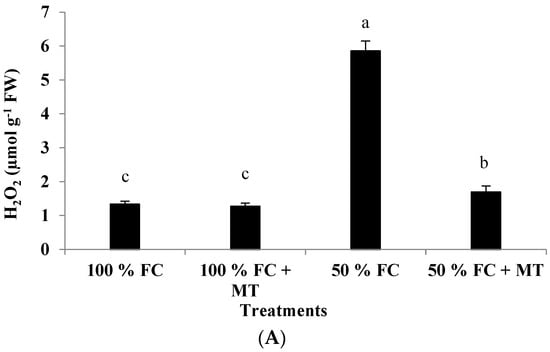
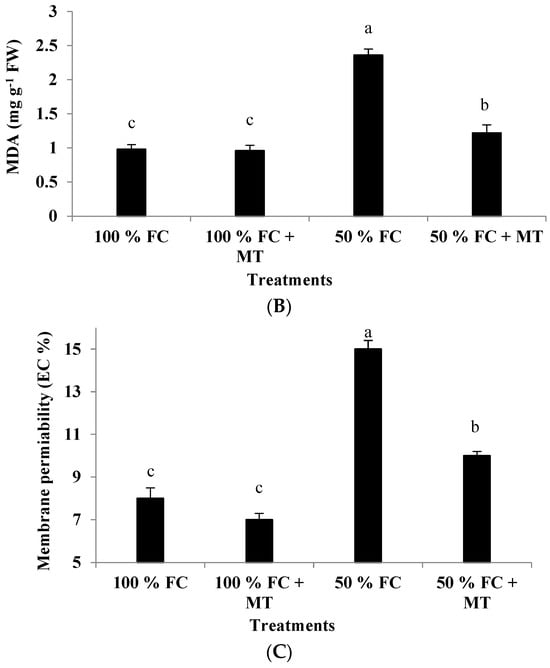
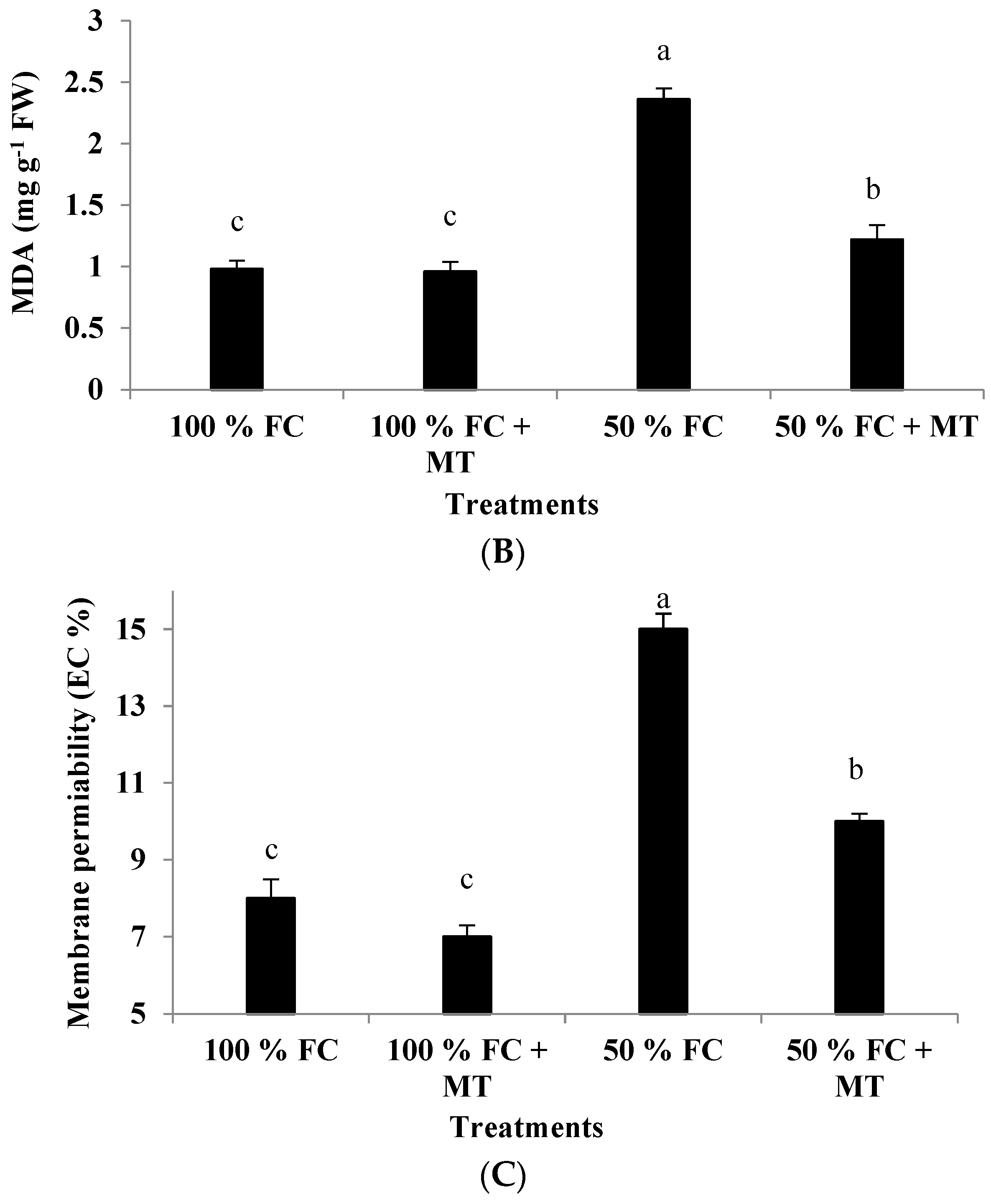
Figure 4.
Impact of MT treatment on H2O2 production (A), malondialdehyde (MDA) content (B), and membrane permeability (C) of drought-stressed Pelargonium graveolens L. Her. Plants were exposed to 50 or 100% field capacity (FC) and subjected to foliar spraying with 100 µM of melatonin (MT). Results are means ± SE of two seasons (n = 6). Different letters represent statistically significant differences between the treatments (determined at p ≤ 0.05).
3.6. MDA Content
Lipid peroxidation, as detected according to MDA accumulation, was considerably elevated in the geranium-stressed plants under 50% FC conditions, while these plants had a significantly lower value of MDA when subjected to foliar spraying with MT (Figure 3B). In the stressed plants that received MT application, compared with the 100% FC treatment, MDA content was 1.24-fold higher, while it was 2.41-fold higher in the absence of MT treatment (Figure 4B).
3.7. Membrane Permeability
The drought-stressed geranium subjects showed a remarkable increase in membrane permeability, while the MT-treated plants presented significantly reduced permeability of their cell membranes (Figure 3C). Compared with the well-watered plants, the increase in membrane permeability of the stressed plants was 87.5%, but it was only 25% when the stressed plants were sprayed with MT (Figure 4C). Melatonin application under 100% FC conditions had no impact on membrane permeability.
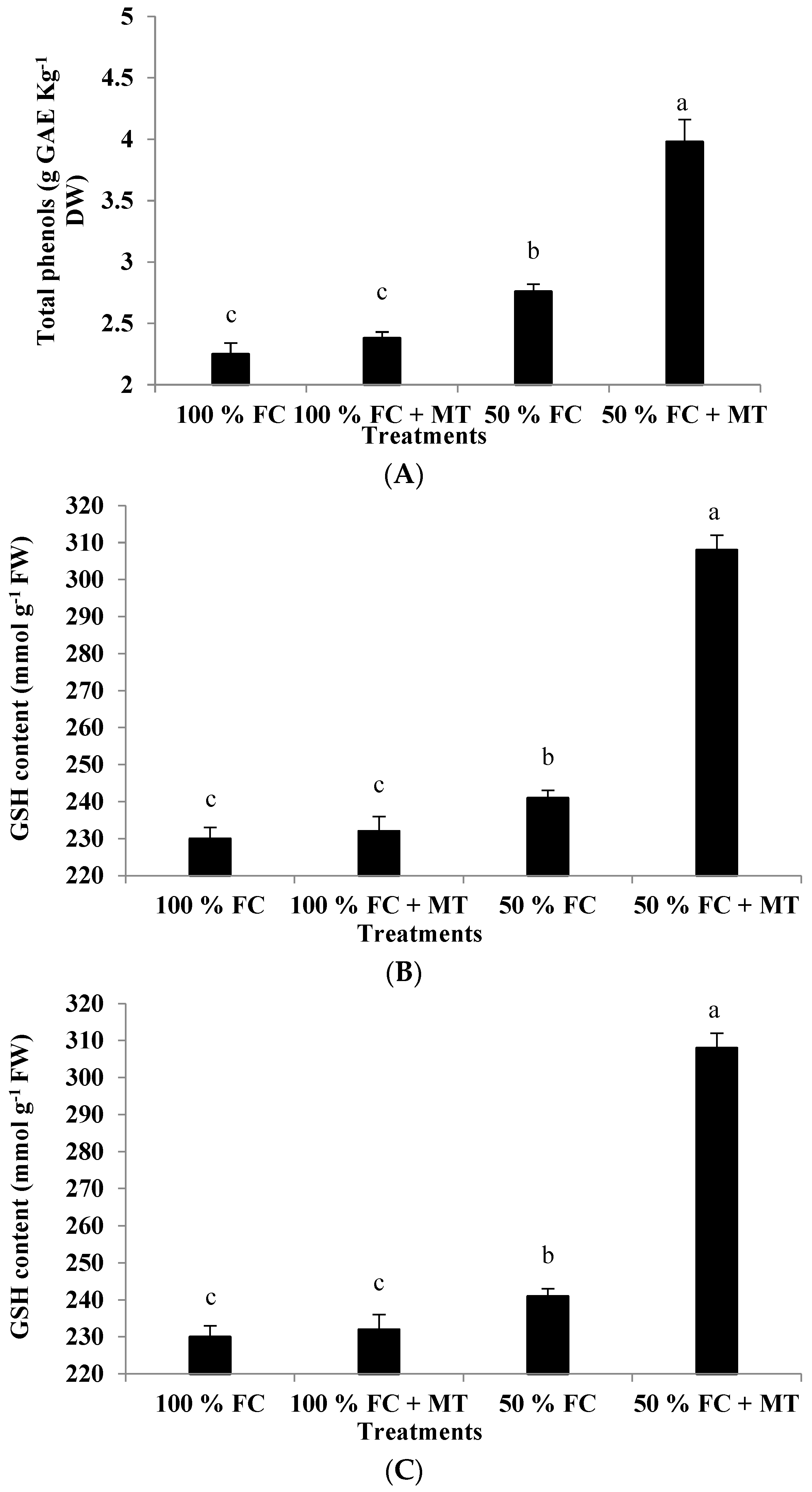
3.8. Total Phenols
A significant increase in the total phenol content was observed in the drought-stressed plants in comparison with the well-watered ones (Figure 5A). However, the geranium-stressed plants that were subjected to foliar spraying with MT accumulated significantly higher number of total phenols compared with those exposed to only 50% FC without MT treatment (Figure 5A). The plants exposed to 50% FC with or without MT application presented total phenol levels that were increased by 76.89% and 22.67%, respectively, compared with the well-watered plants. On the other hand, a slight but non-significant increase in total phenols was recorded due to MT application under 100% FC conditions (Figure 5A).
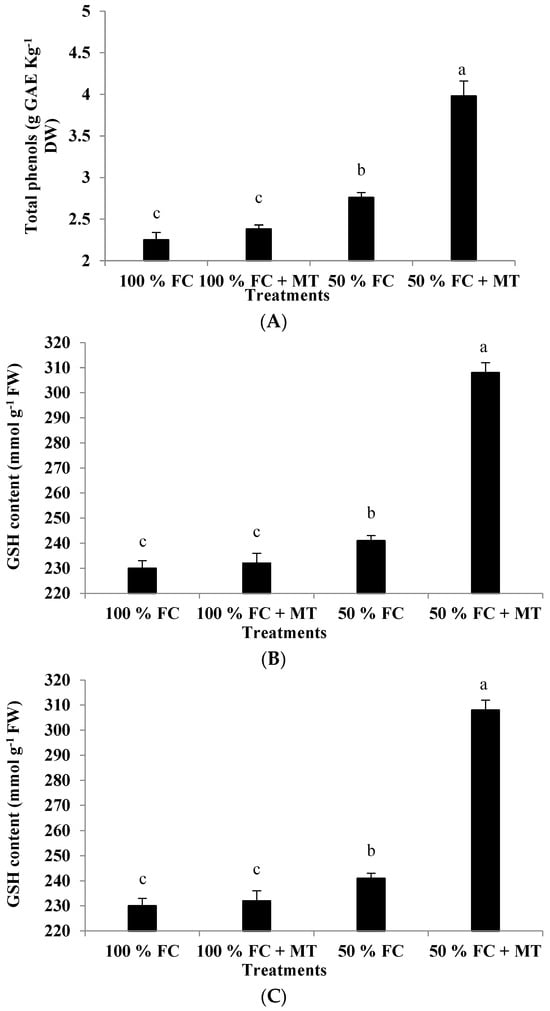
Figure 5.
Impact of MT treatment on the content of total phenols (A), glutathione (B), and proline (C) of drought-stressed Pelargonium graveolens L. Her. Plants were exposed to 50 or 100% field capacity (FC) application and subjected to foliar spraying with 100 µM of melatonin (MT). Results are means ± SE of two seasons (n = 6). Different letters represent statistically significant differences between the treatments, where significance was set at p ≤ 0.05.
3.9. Glutathione (GSH) Content
Drought stress considerably elevated the GSH content in the geranium plants relative to the well-watered plants (Figure 5B). The plants exposed to 50% FC showed a significant increase in GSH content compared with those that received 100% FC; this increase in GSH was further promoted by MT application in the drought-stressed geranium plants. Compared to the well-watered plants, the GSH content was increased by 4.78 and 33.91% in the drought-stressed plants without or with MT application, respectively (Figure 5B).
3.10. Proline Accumulation
The geranium plants exposed to 50% FC accumulated significantly higher levels of proline in comparison to the unstressed (100% FC) plants (Figure 5C). Under drought stress, the proline increase was about 1.37-fold higher than that obtained in the non-stressed geranium plants. The application of MT to the stressed plants increased proline accumulation by about 4.14-fold compared with that under the 100% FC conditions (Figure 5C). On the contrary, under non-stressful conditions, the MT application did not result in a considerable increase in proline content.
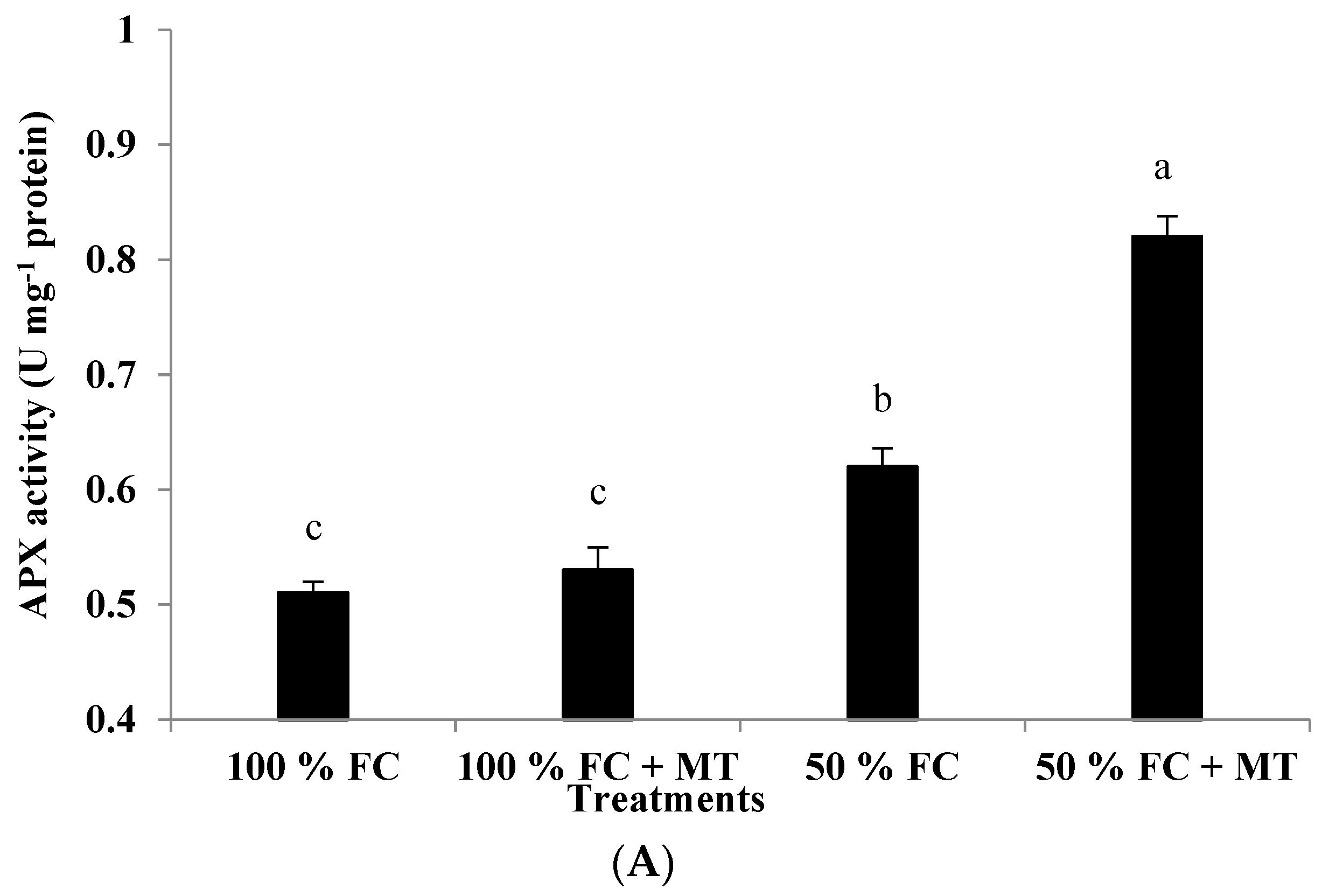
3.11. APX, CAT, and GR Enzyme Activity
The activity of APX, CAT, and GR enzymes was markedly increased under drought stress relative to the 100% FC conditions, and further increases in the three enzymes’ activity were observed following MT application (Figure 6). The stimulation of the APX, CAT, and GR activity of the drought-stressed geranium was further increased via the foliar application of MT. Compared with the well-watered plants, the APX, CAT, and GR activity was increased by 61, 128, and 171%, respectively, when the plants were exposed to 50% FC and sprayed with MT (Figure 6). On the other hand, the well-watered plants showed the lowest activity in terms of the tested enzymes.
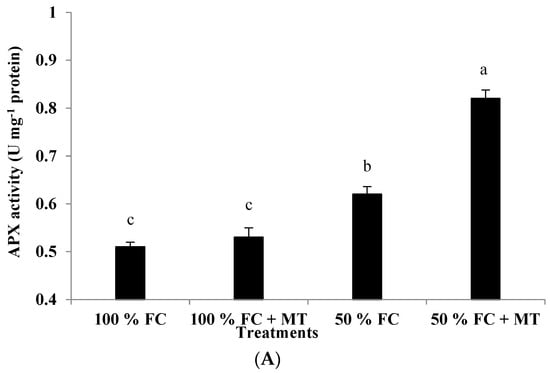
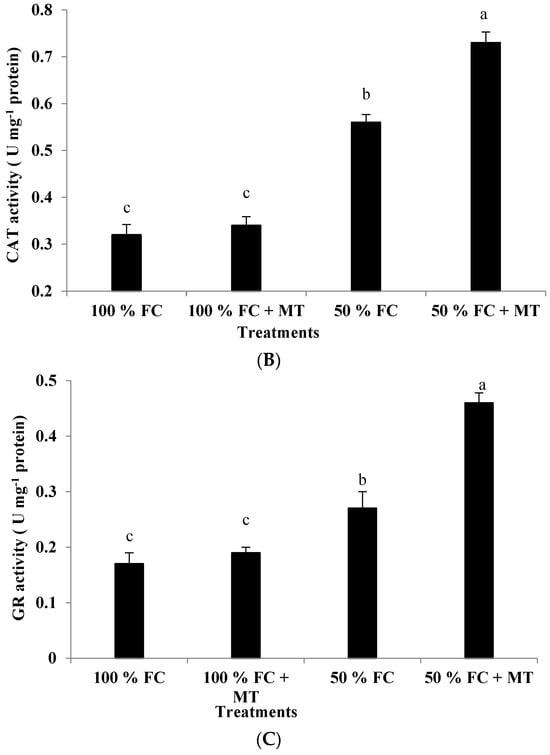
Figure 6.
Impact of MT treatment on ascorbate peroxidase (A), catalase (B), and glutathione reductase (C) enzyme activities of drought-stressed Pelargonium graveolens L. Her. Plants were exposed to 50 or 100% field capacity (FC) application and subjected to foliar spraying with 100 µM of melatonin (MT). Results are means ± SE of two seasons (n = 6). Different letters represent statistically significant differences between the treatments, with significance determined at p ≤ 0.05.
4. Discussion
Water resources are limited, especially in arid and semi-arid areas, and this situation might worsen in the future. Therefore, maximizing plant productivity per water unit is a crucial goal for scientists worldwide. The reduction in geranium growth and yield caused by drought stress observed in the current work was previously demonstrated for Ocimum [55] and lemon verbena [31]; these studies indicated adversely affected growth, leaf expansion, plant height, and biomass production via drought stress. Drought-stress-induced reductions in geranium growth and herb yield can most probably be attributed to a reduction in relative water content leading to leaf turgor reduction, hence limiting plant growth, and to a photosynthetic reduction that decreases the accumulation of dry matter [6]. Also, stomatal closure has been observed under drought stress; this limits the diffusion of CO2 into carboxylation sites and thus reduces photosynthetic rates and, in turn, plant growth and yield [56]. Further, the decrease in drought-stressed plant growth may be due to drought-induced damage to chlorophyll synthesis, which leads to photosynthesis activity reduction, which is reflected in growth and yield decline [12]. This drought-enhanced oxidative injury damages the cellular proteins, lipids, and pigments of chloroplasts under stressful conditions and may also contribute to declined growth and yield [3]. However, growth reduction in response to drought stress is presumably an adaptive mechanism for coping with the negative impacts of drought [3,6].
The decline in chlorophyll content observed in this study might have been due to drought-stimulated oxidative damage [3] and the drought-impaired grana lamella of the chloroplasts [57], which reduce photosynthesis efficiency and plant growth. In this study, MT application counterbalanced the adverse impacts of drought and enhanced the growth of geranium, and these affordances were associated with enhanced chlorophyll content. This response is suggested to be a possible strategy by which MT might augment photosynthesis efficiency and hence plant growth rate. In agreement with this idea, earlier reports have confirmed the promotional effect of MT on plant growth under drought stress [28,31,55,58]; this effect was ascribed to MT action serving as a growth enhancer that improves growth and protects plants from abiotic stresses. Two works revealed that this MT protection against drought stress is dose-dependent [59,60]. The impact of MT on preserving chlorophyll content under drought conditions is consistent with the work conducted by Sadak et al. [58], who reported maintained chlorophyll content after MT treatment in moringa under drought stress. Melatonin might preserve chlorophyll content via stimulating the synthesis of metabolites involved in chlorophyll biosynthesis and reducing the rate of chlorophyll decomposition [61].
This investigation is the first to study the effect of MT on the essential oil content in drought-stressed geranium. Golkar et al. [62] indicated that the release of secondary metabolites is induced as a defense strategy when plants are under abiotic stress, which might be the situation in our work. The high essential oil percentage produced under drought stress was also enhanced in other aromatic species in response to abiotic stresses [12]. Contrarily, drought conditions had a negative impact on the essential oil percentage of Ocimum basilicum ‘Genovese’ and Ocimum americanum, whereas no change was observed in Ocimum x africanum species [55]. Therefore, we assume that the effect of drought stress on essential oil percentage seems to be species-dependent. Interestingly, a further increase was obtained via MT application in well-watered or stressed geranium plants (more so in stressed ones (50% FC)), and this finding is consistent with the results regarding Salvia [12], Citrus [14], and lemon verbena [31]. It has been suggested that an increase in essential oil content in response to melatonin application can be due to the potential improvement of meristematic cells, the site of production of various chemical compounds that are essential for volatile oil biosynthesis [12]. The reduction in the essential oil yield, in contrast to the increase in oil percentage, observed in the stressed plants can be ascribed to a decrease in their herb yield. A similar response was observed in other species affected by water shortages [12,28]. We therefore propose that the MT-enhanced oil yield reported here may be due to MT-improved geranium growth and biomass production.
GC-MS analysis showed that the main constituents of essential oil were citronellol, geraniol, linalool, citronellyl formate, and geranyl formate; their percentages were increased in the drought-stressed plants compared with the well-watered plants (Table 2). Our results are consistent with those obtained by Bidabadi et al. [12], who reported an increase in the essential oil components of two Salvia species under drought stress. MT application significantly increased the levels of these components, even more so when applied under water deficit, and this result agrees with data relating that exogenous MT enhanced the presence of several compounds in volatile oil in Salvia [12] and bitter orange [63]. However, MT’s role in the essential oil biosynthesis of aromatic plants is poorly understood. We suggest that this promotional effect may be related to the fact that MT has an auxin-like activity [64], which has been found to promote volatile oil synthesis in several aromatic species [65,66]. It is worth mentioning that the ability of MT to increase the essential oil content in drought-stressed geranium reported in this study is novel.
Reactive oxygen species can contribute to cellular signaling only when they exist at lower levels in plant cells [67]. As a form of abiotic stress, drought perturbs cellular oxygen metabolism, leading to ROS overproduction that triggers harmful effects in several cellular organelles [68]. Herein, we report that the exposure of geranium plants to drought stress induced a considerable degree of H2O2 accumulation, which was associated with a concomitant increment in MDA, indicating drought-stress-induced oxidative damage resulting in cellular membrane lipid peroxidation. The result is in accordance with previous work on Salvia [12], lemon verbena [31], and moringa [58], showing elevated ROS production and lipid peroxidation under water deficit conditions. Lipid peroxidation promoted by drought stress results in a disturbance of membrane integrity precipitated by impaired membrane permeability. This idea is also supported by data relating that ROS has an adverse impact on membrane lipids and proteins, causing their damage [6,16]. Our study revealed that MT foliar spray caused a significant reduction in both H2O2 and MDA accumulation and restored the membrane stability (measured according to low membrane permeability) of drought-stressed geranium leaves, suggesting that MT scavenges oxidative injury via keeping a steady state of intracellular ROS levels [69]. This result is consistent with the findings in previous reports on Salvia [12] and Moringa [58], indicating that MT application detoxifies the hazardous impact of ROS under drought conditions.
Stressful-conditions-triggered oxidative stress in plants is eliminated by evolving oxygen scavenging machinery to decrease levels of ROS, which comprise antioxidant enzymes and non-enzymatic antioxidants [15,47,70]. As for the antioxidant defense system, the geranium-stressed plants generated higher levels of total phenols, GSH, and proline compared to the well-watered plants. However, it seems that the stimulation of the antioxidant system in the stressed geranium was not sufficient for coping with the overproduction of H2O2 and MDA and therefore led to the detected oxidative injury. In contrast, the application of MT enhanced both non-enzymatic and enzymatic machinery in the drought-stressed geranium: higher elevations in APX, CAT, and GR activities and total phenols, GSH, and proline content in the treated plants seemingly point to their participation in oxidative stress amelioration, which inhibits the adverse oxidative impact of drought stress. In support of this, it has been reported that MT is a potent antioxidant that plays a crucial role in mitigating oxidative stress and enhancing plant resilience [31]. Also, polyphenols have been shown to be important non-enzymatic antioxidants in the response to stress conditions [14,71]. Proline accumulation under adverse conditions has been reported to have multiple influences that help plants cope with injurious effects and enhance stress resilience [6,72]. In agreement with our results, an accumulation of proline was observed in lemon verbena along with improved tolerance to drought [31]. Additionally, the increasing GSH levels observed in the drought-stressed-geranium in this study are in accordance with findings in an investigation into Salvia [12] grown under drought stress. The functions of GSH and phenols as non-enzymatic antioxidants that participate in H2O2 and MDA reductions under MT treatment have been documented [73]. Furthermore, MT has been found to regulate the synthesis and accumulation of GSH and phenolic compounds, indicating a potential interplay between them in mitigating stress-induced damage [47]. The melatonin-enhanced phenols, GSH, and proline accumulation in drought-stressed geranium shown in this study have been reported for the first time.
The enhanced activities of APX, CAT, and GR enzymes under drought conditions observed in the current investigation were further increased when MT was applied, indicating that MT effectively provided a defense against oxidative injury under drought stress. Consistent with the current results, the induction of the release of antioxidant enzymes under conditions of water scarcity has been observed in several aromatic species [3,31]. We therefore indicate that MT application can enhance the enzymatic antioxidant system, which is able to effectively scavenge ROS, preserve membrane function, and induce resistance against water scarcity. Our interpretation is supported by previously published works on Salvia species [12] and moringa [58] reporting the role of MT in minimizing intracellular ROS, reducing their damage, and stimulating tolerance to drought imposition.
5. Conclusions
Melatonin foliar spray significantly alleviated drought-induced oxidative damage via the induction of nonenzymatic and enzymatic antioxidant mechanisms and thus enhanced geranium growth, herb yield, essential oil content, chlorophyll content, reduced H2O2 and MDA accumulation, and eventually drought stress tolerance. This report reveals the vital role of exogenous MT in enhancing drought stress tolerance in geranium due to the aforementioned responses. These findings, therefore, indicate that MT could be used as a safe and suitable alternative to other synthetic materials for attenuating the adverse impact of water scarcity and enhancing tolerance to drought in geranium and, most probably, other aromatic species.
Author Contributions
Conceptualization, F.A.S.H. and R.M.M.; methodology, R.M.M.; software, M.M.M.; validation, M.M.M., M.M.F.M. and R.M.M.; formal analysis, M.M.M.; investigation, R.M.M.; resources, M.M.M.; data curation, F.A.S.H.; writing—original draft preparation, F.A.S.H.; writing—review and editing, M.M.M. and M.M.F.M.; visualization, R.M.M.; supervision, F.A.S.H.; project administration, M.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data sets supporting the results of this research are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mirajkar, S.J.; Dalvi, S.G.; Ramteke, S.D.; Suprasanna, P. Foliar application of gamma radiation processed chitosan triggered distinctive biological responses in sugarcane under water deficit stress conditions. Int. J. Biol. Macromol. 2019, 139, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Ali, E.F.; Mahfouz, S. Comparison between different fertilization sources, irrigation frequency and their combinations on the growth and yield of the coriander plant. Aust. J. Appl. Basic Sci. 2012, 6, 600–615. [Google Scholar]
- Ali, E.F.; Hassan, F.A.S. Water stress alleviation of roselle plant by silicon treatment through some physiological and biochemical responses. Annual Res. Rev. Biol. 2017, 21, 1–17. [Google Scholar] [CrossRef]
- Tabassum, S.; Ossola, A.; Marchin, R.M.; Ellsworth, D.S.; Leishman, M.R. Assessing the relationship between trait-based and horticultural classifications of plant responses to drought. Urban For. Urban Green. 2021, 61, 127109. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Attia, H.; Al-Yasi, H.; Alamer, K.; Esmat, F.; Hassan, F.; Elshazly, S.; Hessini, K. Induced anti-oxidation efficiency and others by salt stress in Rosa damascena Miller. Sci. Hortic. 2020, 274, 109681. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Induction of drought stress tolerance by ketoconazole in Catharanthus roseus is mediated by enhanced antioxidant potentials and secondary metabolite accumulation. Coll. Surf. B Biointerfaces 2007, 60, 201–206. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Water deficit stress mitigation by calcium chloride in Catharanthus roseus: Effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Coll. Surf. B Biointerfaces 2007, 60, 110–116. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Hatcher, C.R.; Ryves, D.B.; Millett, J. The function of secondary metabolites in plant carnivory. Ann. Bot. 2020, 125, 399–411. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J. Changes in essential oil yield and fatty acid contents in black cumin (Nigella sativa L.) genotypes in response to drought stress. Ind. Crops Prod. 2020, 155, 112764. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Van der Weide, J.; Sabbatini, P. Exogenous melatonin improves glutathione content, redox state and increases essential oil production in two Salvia species under drought stress. Sci. Rep. 2020, 10, 6883. [Google Scholar] [CrossRef] [PubMed]
- Kulak, M. Recurrent drought stress effects on essential oil profile of Lamiaceae plants: An approach regarding stress memory. Ind. Crops Prod. 2020, 154, 112695. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A. The effect of foliar application of melatonin on changes in secondary metabolite contents in two citrus species under drought stress conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Al-Yasi, H.; Ali, E.F.; Alamer, K.; Hessini, K.; Attia, H.; El-Shazly, S. Mitigation of salt stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 2021, 101, 157–165. [Google Scholar] [CrossRef]
- Elkarmout, A.F.; Yang, M.; Hassan, F.A. Chitosan treatment effectively alleviates the adverse effects of salinity in Moringa oleifera Lam via enhancing antioxidant system and nutrient homeostasis. Agronomy 2022, 12, 2513. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Pang, L.; Wu, Y.; Pan, Y.; Ban, Z.; Li, L.; Li, X. Insights into exogenous melatonin associated with phenylalanine metabolism in postharvest strawberry. Postharvest Biol. Technol. 2020, 168, 111244. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, J. Melatonin: Current status and future perspectives in horticultural plants. Front. Plant Sci. 2023, 14, 1140803. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.; Chen, P.; et al. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.T.; Lu, X.C.; Mitra, S.; Hussain, M.; Liu, S.; Qiuet, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Molec. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Zhou, R.; Wan, H.; Jiang, F.; Li, X.; Yu, X.; Rosenqvist, E.; Ottosen, C. The alleviation of photosynthetic damage in tomato under drought and cold stress by high CO2 and melatonin. Int. J. Mol. Sci. 2020, 21, 5587. [Google Scholar] [CrossRef]
- Hosseini, M.; Samsampour, D.; Zahedi, S.; Zamanian, K.; Rahman, M.; Mostofa, M.; Tran, L. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol. Plant. 2021, 172, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, U.B.; Ram, M.; Yadav, A.; Chanotiya, C.S. Biomass yield, essential oil yield and quality of geranium (Pelargonium graveolens L. Her.) as influenced by intercropping with garlic (Allium sativum, L.) under subtropical and temperate climate of India. Ind. Crop. Prod. 2013, 46, 234–237. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R. Biomass yield, essential oil yield and essential oil composition, of rose-scented geranium (Pelargonium species) as influenced by row spacing and inter-cropping with corn mint (Mentha arvensis L. f. piperascens Malinv. Ex Holmes). Ind. Crops Prod. 2002, 16, 133–144. [Google Scholar]
- Boukhris, M.; Hadrich, F.; Chtourou, H.; Dhouib, A.; Bouaziz, M.; Sayadi, S. Chemical composition, biological activities and DNA damage protective effect of Pelargonium graveolens L’Hér. essential oils at different phenological stages. Ind. Crops Prod. 2015, 74, 600–606. [Google Scholar] [CrossRef]
- Machalova, Z.; Sajfrtova, M.; Pavela, R.; Topiar, M. Extraction of botanical pesticides from Pelargonium graveolens using supercritical carbon dioxide. Ind. Crops Prod. 2015, 67, 310–317. [Google Scholar] [CrossRef]
- Kang, H.Y.; Na, S.S.; Kim, Y.K. Effects of oral care with essential oil on improvement in oral health status of hospice patients. J. Kor. Acad. Nurs. 2010, 40, 473–481. [Google Scholar] [CrossRef]
- Saraswathi, J.; Venkatesh, K.; Baburao, N.; Hilal, M.H.; Rani, A.R. Phytopharmacological importance of Pelargonium species. J. Med. Plant Res. 2011, 5, 2587–2598. [Google Scholar]
- Pandey, V.; Patra, D.D. Crop productivity, aroma profile and antioxidant activity in Pelargonium graveolens L’Her. under integrated supply of various organic and chemical fertilizers. Ind. Crops Prod. 2015, 67, 257–263. [Google Scholar] [CrossRef]
- Pourmeidani, A.; Jafari, A.A.; Mirza, M. Studying drought tolerance in Thymus kotschyanus accessions for cultivation in dryland farming and low efficient grassland. J. Range. Sci. 2017, 7, 331–340. [Google Scholar]
- Metzner, H.; Rau, H.; Senger, H. Unter suchungen zur synchronisier barteit einzelner pigmentan angel mutanten von chlorela. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Colowick, S.P.; Kaplan, N.O. Chlorophylls and Carotenoids Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Sedibe, M.M.; Allemann, J. Yield and quality response of rose geranium (Pelargonium graveolens L.) to sulphur and phosphorus application. S. Afr. J. Plant Soil 2012, 29, 151–156. [Google Scholar] [CrossRef]
- Patterson, B.D.; Macrae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Chem. 1984, 134, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acidreactive-substances assay for estimating lipid peroxidation in plant tissue containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Yan, B.; Dai, Q.; Liu, X.; Huang, S.; Wang, Z. Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil. 1996, 179, 261–268. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef]
- Mazrou, R.M.; Hassan, S.; Yang, M.; Hassan, F.A.S. Melatonin Preserves the Postharvest Quality of Cut Roses through Enhancing the Antioxidant System. Plants 2022, 11, 2713. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Chandlee, J.M.; Scandalios, J.G. Analysis of variants affecting the catalase developmental program in maize scutellum. Theoret. Appl. Genet. 1984, 69, 71–77. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V. Cellular detoxifying mechanisms determine the age dependent injury in tropical trees exposed to SO2. J. Plant Physiol. 1992, 140, 733–740. [Google Scholar] [CrossRef]
- Heinisch, O.; Steel, R.G.D.; Torrie, J.H. Biometrische Zeitschrift. In Principles and Procedures of Statistics. (With Special Reference to the Biological Sciences); 481 S., 15 Abb.; 81 s 6 d.; McGraw-Hill Book Company: New York, NY, USA; Toronto, ON, Canada; London, UK, 1960; Volume 4, pp. 207–208. [Google Scholar]
- Mulugeta, S.M.; Radácsi, P. Influence of Drought Stress on Growth and Essential Oil Yield of Ocimum Species. Horticulturae 2022, 8, 175. [Google Scholar] [CrossRef]
- Liu, D.; Wu, L.; Naeem, M.S.; Liu, H.; Deng, X.; Xu, L.; Zhang, F.; Zhou, W. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant. 2013, 35, 2747–2759. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Sadak, M.; Abdalla, A.; Abd Elhamid, E.; Ezzo, M. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bullet. National Res. Center 2020, 44, 1–13. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules 2018, 23, 1580. [Google Scholar] [CrossRef]
- Sadak, M.; Bakry, B. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919. [Google Scholar] [CrossRef]
- Zoufan, P.; Bavani, M.R.; Rahnama, A. Effect of exogenous melatonin on improving of chlorophyll content and photochemical efficiency of PSII in mallow plants (Malva parviflora L.) treated with cadmium. Physiol. Mole. Pathol. Plants 2023, 29, 145–157. [Google Scholar]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tiss. Org. Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Teriou, K.; Terios, L.; Koularmani, A. Effect of melatonin, salicylic acid and gibberellic acid on leaf essential oil and other secondary metabolites of bitter orange young seedlings. J. Essent. Oil Res. 2015, 27, 487–496. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Muñoz, P.; Müller, M.; Munné-Bosch, S. Biosynthesis, Metabolism and Function of Auxin, Salicylic Acid and Melatonin in Climacteric and Non-climacteric Fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.; Sato, A.; Lage, C.; Gil, R.; Azevedo, D.; Esquibel, M. Essential oil composition of Melissa officinalis L. in vitro produced under the infuence of growth regulators. J. Braz. Chem. Soc. 2005, 16, 1387–1390. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Amrani Joutei, K. Effect of gibberellic acid (GA), indole acetic acid (IAA) and benzylaminopurine (BAP) on the synthesis of essential oils and the isomerization of methyl chavicol and trans-anethole in Ocimum gratissimum L. SpringerPlus 2014, 3, 321. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Rahman, M.; Mostofa, M.G.; Keya, S.S.; Rahman, A.; Das, A.K.; Islam, R.; Abdelrahman, M.; Bhuiyan, S.; Naznin, T.; Ansary, M.; et al. Acetic acid improves drought acclimation in soybean: An integrative response of photosynthesis, osmoregulation, mineral uptake and antioxidant defense. Physiol. Plant. 2020, 172, 343–350. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.; Wang, Z.; Fang, Y.; Xi, Z.; Zhang, Z. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded moisture deficit effect on secondary metabolites, antioxidant, and inhibitory enzyme activities in leaf extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Polyphenol and flavonoid profiles and radical scavenging activity in leafy vegetable Amaranthus gangeticus. BMC Plant Biol. 2020, 20, 499. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Salama, K.H.A. Proline and abiotic stresses: Responses and adaptation. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II, Mechanisms of Adaptation and Stress Amelioration; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 357–397. [Google Scholar]
- Gan, J.; Feng, Y.; He, Z.; Li, H.; Zhang, H. Correlations between antioxidant activity and alkaloids and phenols of maca (Lepidium meyenii). J. Food Qual. 2017, 3, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).