Effect of Nitric Oxide on Browning of Stem Tip Explants of Malus sieversii
Abstract
:1. Introduction
2. Results
2.1. Effects of NO Treatment on Shoot Tip Browning Phenotype and Browning Index during Tissue Culture
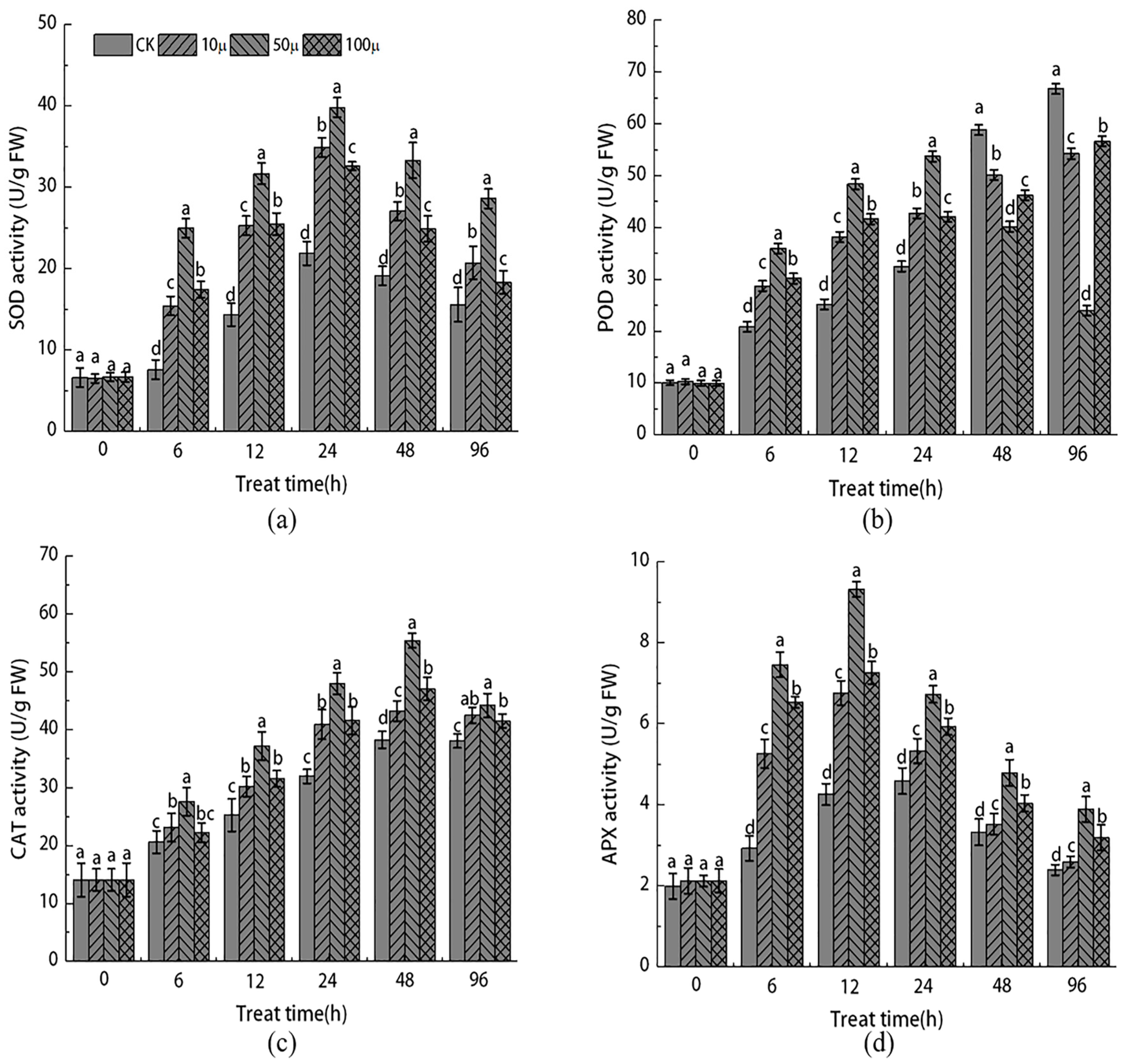
2.2. Effect of NO Treatment on Enzymatic Antioxidant System of Explants
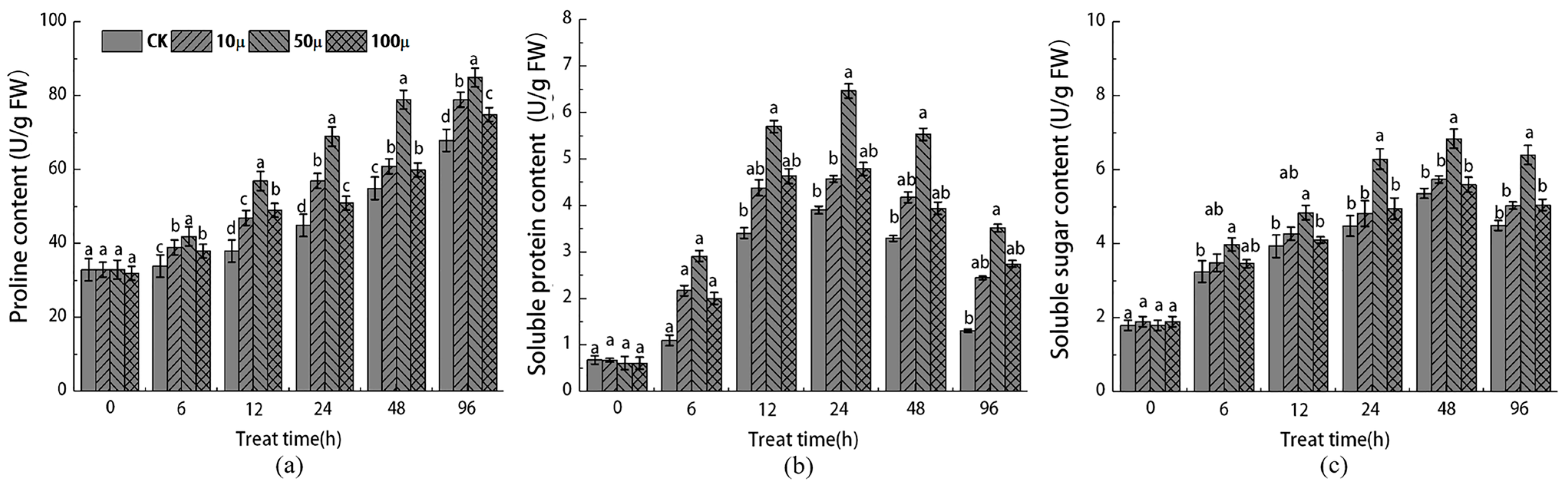
2.3. Effects of NO Treatment on Non-Enzymatic Antioxidants in Explants
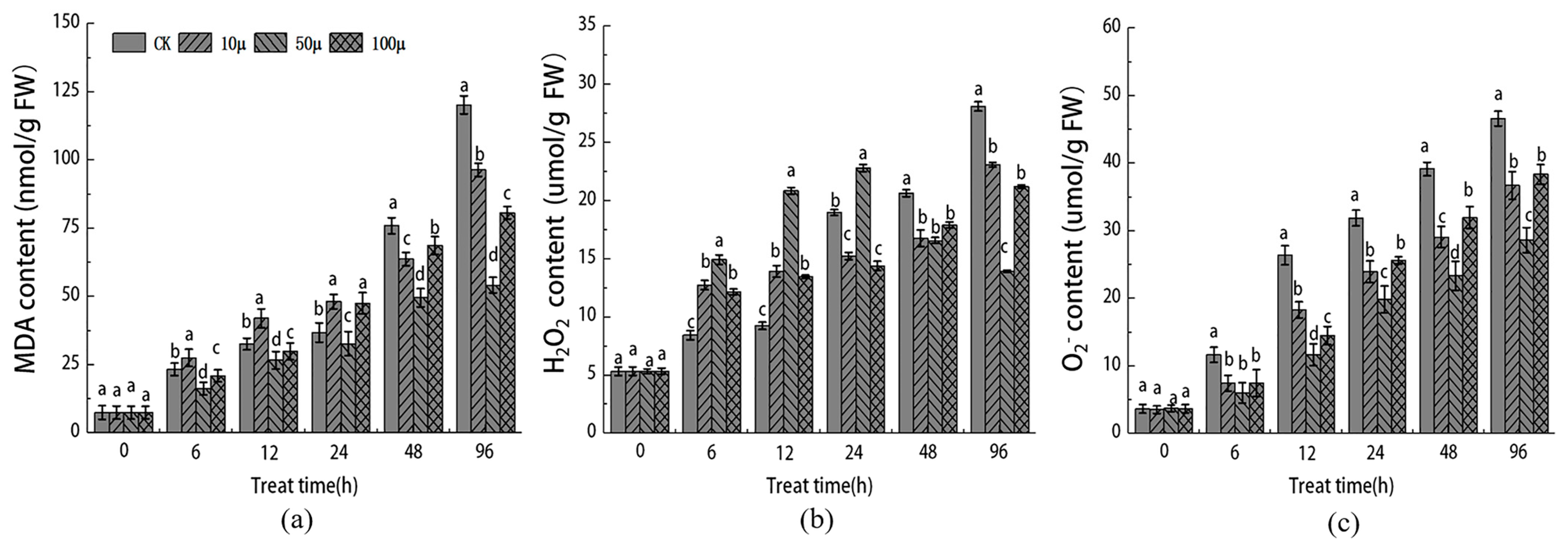
2.4. Effect of SNP on Markers of Oxidative Stress in Explants
2.5. Effects of SNP Treatment on the Activities of PAL and PPO and the Contents of Total Phenols and Flavonoids in Stem Tip Explants of Malus sieversii
3. Discussion
4. Materials and Methods
4.1. Test Materials and Their Treatment
4.2. Determination of Browning Index
4.3. Determination of the Activity of Antioxidant Enzymes
4.4. Determination of Non-Enzymatic Antioxidants
4.5. Determination of Markers of Oxidative Stress
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ha, Y.H.; Oh, S.H.; Lee, S.R. Genetic Admixture in the Population of Wild Apple (Malus sieversii) from the Tien Shan Mountains, Kazakhstan. Genes 2021, 12, 104. [Google Scholar] [CrossRef]
- Jashenko, R.; Tanabekova, G.; Lu, Z. Assessment of Biological and Ecological Characteristics of Sievers Apple Tree Pests in Trans-Ili Alatau, Kazakhstan. Sustainability 2023, 15, 11303. [Google Scholar] [CrossRef]
- Li, X.L.; Ding, Z.J.; Miao, H.Y.; Bao, J.B.; Tian, X.M. Complete chloroplast genome studies of different apple varieties indicated the origin of modern cultivated apples from Malus sieversii and Malus sylvestris. PeerJ 2022, 10, e13107. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Artlip, T.; Liu, J.; Ma, J.; Dardick, C. Fox Hunting in Wild Apples: Searching for Novel Genes in Malus sieversii. Int. J. Mol. Sci. 2020, 21, 9516. [Google Scholar] [CrossRef]
- Jashenko, R.; Tanabekova, G. Insects that damage the wild populations of Malus sieversii in Kazakhstan. IOP Conf. Ser. Earth Environ. Sci. 2019, 298, 012018. [Google Scholar] [CrossRef]
- Bakhtaulova, A.; Shadenova, E.; Kanagatov, Z.; Bukenova, E.; Oksikbaev, B.; Akmullayeva, A. Restoration of wild fruit forests of Sievers apple (Malus sieversii) by micropropagation. J. Biotechnol. 2018, 280, S83. [Google Scholar] [CrossRef]
- Warakagoda, P.S.; Subasinghe, S.; Gunasekare, M. In vitro clonal propagation of Coscinium fenestratum (Gertn.) Colebr. (Weniwel) through nodal explants. J. Natl. Sci. Found. Sri Lanka 2017, 45, 133. [Google Scholar] [CrossRef]
- Zhang, Y.; Bozorov, T.A.; Li, D.X.; Zhou, P.; Zhang, D.Y. An efficient in vitro regeneration system from different wild apple (Malus sieversii) explants. Plant Methods 2020, 16, 56. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Jeong, B.R. Callus induction and browning suppression in tree peony Paeonia ostii ‘Fengdan’. Hortic. Environ. Biotechnol. 2020, 61, 591–600. [Google Scholar] [CrossRef]
- Dhavala, A.; Rathore, T.S. Micropropagation of Embelia ribes Burm f. through proliferation of adult plant axillary shoots. In Vitro Cellular & Developmental Biology. Vitr. Cell. Dev. Biol.-Plant 2010, 46, 180–191. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; Lu, R.; Niu, B.; Pasapula, V.; Hou, P. Changes in morphology and biochemical indices in browning callus derived from Jatropha curcas hypocotyls. Plant Cell Tissue Organ Cult. 2009, 98, 11–17. [Google Scholar] [CrossRef]
- Wu, G.; Wei, X.; Wang, X.; Wei, Y. Changes in biochemistry and histochemical characteristics during somatic embryogenesis in Ormosia henryi Prain. Plant Cell Tissue Organ Cult. 2021, 144, 505–517. [Google Scholar] [CrossRef]
- Su, Y.; Wei, M.; Guo, Q.S.; Huang, J.M.; Zhao, K.; Huang, J.B. Investigating the relationships between callus browning in Isatis indigotica Fortune, total phenol content, and PPO and POD activities. Plant Cell Tissue Organ Cult. 2023, 155, 175–182. [Google Scholar] [CrossRef]
- Kaewubon, P.; Hutadilok-Towatana, N.; Teixeira da Silva, J.A.; Meesawat, U. Ultrastructural and biochemical alterations during browning of pigeon orchid (Dendrobium crumenatum Swartz) callus. Plant Cell Tissue Organ Cult. 2015, 121, 53–69. [Google Scholar] [CrossRef]
- Samsampour, D.; Sadeghi, F.; Asadi, M.; Ebrahimzadeh, A. Effect of nitric oxide (NO) on the induction of callus and antioxidant capacity of Hyoscyamus niger under in vitro salt stress. Appl. Bot. Food Qual. 2018, 91, 24–32. [Google Scholar] [CrossRef]
- Girija, A.; Devakumar, S.P.J.L.; Vijayanathan, M. Nitric oxide as a bioactive molecule in the regulation of chalcone synthase during jasmonic acid mediated defense signaling in ginger. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 128, 715–721. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.; Shi, R.; Fan, Q.; An, L. Salinity-induced Physiological Modification in the Callus from Halophyte Nitraria tangutorum Bobr. J. Plant Growth Regul. 2010, 29, 465–476. [Google Scholar] [CrossRef]
- Ramalingam, S.; Sivakumar, H.P.; Sundararajan, S.; Rajendran, V.; Kumariah, M. Growth modulation by nitric oxide donor sodium nitroprusside in in vitro plant tissue cultures—A review. Biologia 2022, 77, 1699–1711. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.T.; Zhou, Q.; Yang, Y.C.; Qi, Y.H.; Wang, Q.N.; Zhang, L.J. Preliminary analysis on the mechanism of STEM Browning in tissue culture of Juglans mandshurica. Mol. Plant Breed. 2021, 19, 8239–8244. [Google Scholar] [CrossRef]
- Liu, C.P.; Yang, L.; Shen, H.L. Proteomic Analysis of Immature Fraxinus mandshurica Cotyledon Tissues during Somatic Embryogenesis: Effects of Explant Browning on Somatic Embryogenesis. Int. J. Mol. Sci. 2015, 6, 13692–13713. [Google Scholar] [CrossRef]
- Intarasit, S.S.K. Transient production of H2O2 and NO induced by ascorbic acid coincides with promotion of antioxidant enzyme activity and reduction of pericarp browning of harvested longan fruit. Sci. Hortic. 2021, 277, 109784. [Google Scholar] [CrossRef]
- Xu, J.; Yin, H.; Wang, W.; Mi, Q.; Liu, X. Effects of sodium nitroprusside on callus induction and shoot regeneration in micropropagated Dioscorea opposita. Plant Growth Regul. 2009, 59, 279–285. [Google Scholar] [CrossRef]
- Han, X.; Yang, H.; Duan, K.; Zhang, X.; Zhao, H.; You, S. Sodium nitroprusside promotes multiplication and regeneration of Malus hupehensis in vitro plantlets. Plant Cell Tissue Organ Cult. 2009, 96, 29–34. [Google Scholar] [CrossRef]
- Pandey, S.; Sundararajan, S.; Ramalingam, S.; Pant, B. Effects of sodium nitroprusside and growth regulators on callus, multiple shoot induction and tissue browning in commercially important Valeriana jatamansi Jones. Plant Cell Tissue Organ Cult. 2020, 142, 653–660. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, Y.J.; Liu, G.Y.; Xu, S.Z.; Dai, J.L.; Li, W.J.; Dong, H.Z. Nitric oxide increases the biomass and lint yield of field-grown cotton under temporary waterlogging through physiological and molecular regulation. Field Crops Res. 2021, 261, 107989. [Google Scholar] [CrossRef]
- Maslennikova, D.R.; Lastochkina, O.V.; Shakirova, F.M. Exogenous Sodium Nitroprusside Improves Salt Stress Tolerance of Wheat (Triticum aestivum L.) via Regulating the Components of Ascorbate-Glutathione Cycle, Chlorophyll Content and Stabilization of Cell Membranes State. Russ. J Plant Physiol. 2022, 69, 130. [Google Scholar] [CrossRef]
- Rahim, W.; Khan, M.; Al, A.T.; Pande, A.; Methela, N.J.; Ali, S.; Imran, M.; Lee, D.S.; Lee, G.M.; Mun, B.G. Exogenously Applied Sodium Nitroprusside Mitigates Lead Toxicity in Rice by Regulating Antioxidants and Metal Stress-Related Transcripts. Int. J. Mol. Sci. 2022, 23, 9729. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Baluska, F. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Esringu, A.; Aksakal, O.; Tabay, D.; Kara, A.A. Effects of sodium nitroprusside (SNP) pretreatment on UV-B stress tolerance in lettuce (Lactuca sativa L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Mengxi, L.I.; Die, H.U.; Pan, X.; Fei, Y. Effects of exogenous NO on antioxidant system of Taxus plants under simulated acid rain stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12052. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, C.; Wu, F.; Cheng, J. Effect of nitric oxide on browning and lignification of peeled bamboo shoots. Postharvest Biol. Technol. 2010, 57, 72–76. [Google Scholar] [CrossRef]
- Hesami, M.; Tohidfar, M.; Alizadeh, M.; Daneshvar, M.H. Effects of sodium nitroprusside on callus browning of Ficus religiosa: An important medicinal plant. J. For. Res. 2020, 31, 789–796. [Google Scholar] [CrossRef]
- Dwivedi, P.; Choudhury, S.R. Nitric oxide as a signaling molecule in plants. Int. J. Agric. Environ. Biotechnol. 2012, 5, 303–308. [Google Scholar]
- Yin, J.Y.; Bai, S.F.X.; Wu, F.H.; Lu, G.Q.; Yang, H.Q. Effect of nitric oxide on the activity of phenylalanine ammonia-lyase and antioxidative response in sweetpotato root in relation to wound-healing. Postharvest Biol. Technol. 2012, 74, 125–131. [Google Scholar] [CrossRef]
- Leng, P.; Su, S.; Wei, F.; Yu, F.; Duan, Y. Correlation between browning, total phenolic content, polyphenol oxidase and several antioxidation enzymes during pistachio tissue culture. Acta Hortic. 2009, 829, 127–131. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J. Increase of polyphenol oxidase and decrease of polyarnines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.). Plant Sci. 2004, 167, 621–628. [Google Scholar] [CrossRef]
- Zhao, S.G.; Wang, H.X.; Liu, K.; Li, L.Q.; Yang, J.B.; An, X.X.; Zhang, Z.H. The role of JrPPOs in the browning of walnut explants. BMC Plant Biol. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Pan, H.; Zhang, J.; Wang, Q.; Que, Q.X.; Pan, R.; Lai, G.T. Light Quality Modulates Growth, Triggers Differential Accumulation of Phenolic Compounds, and Changes the Total Antioxidant Capacity in the Red Callus of Vitis davidii. J. Agric. Food Chem. 2022, 70, 13264–13278. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.B. Ultraviolet-B-induced changes on phenolic compounds, antioxidant capacity and HPLC profile of in vitro-grown plant materials in Echium orientale L. Ind. Crops Prod. 2020, 153, 112584. [Google Scholar] [CrossRef]
- Huque, R.; Wills, R.B.H.; Pristijono, P.; Golding, J.B. Effect of nitric oxide (NO) and associated control treatments on the metabolism of fresh-cut apple slices in relation to development of surface browning. Postharvest Biol. Technol. 2013, 78, 16–23. [Google Scholar] [CrossRef]
- Tan, D.M. Establishment of tissue culture system of stem tip of Malus sieversii. J. Weifang Univ. 2009, 9, 2. [Google Scholar]
- Liu, B.; Peng, L.X. Establishment of tissue culture system of Malus sieversii. J. Tianjin Agric. Univ. 2011, 18, 3. [Google Scholar] [CrossRef]
- Shi, X.; Yang, L.; Yan, G.; Du, G. Medium pH between 5.5 and 7.5 has Minimal Effects on Tissue Culture of Apple. J. HortScience 2017, 52, 475–478. [Google Scholar] [CrossRef]
- Kal, Ü.; Dal, Y.; Kayak, N.; Yavuz, D.; Türkmen, Ö.; Seymen, M. Application of nitrogen for mitigating the adverse effects of flooding stress in lettuce. J. Plant Nutr. 2023, 46, 4664–4678. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Zhai, J.L.; Yan, J.P.; Li, K.Z.; Xu, H.N. Physiological and transcriptomic responses of antioxidant system and nitrogen metabolism in tomato seedlings treated with nitrogen starvation and re-supply. J. Hortic. Sci. Biotechnol. 2023, 98, 57–71. [Google Scholar] [CrossRef]
- Roach, T.; Neuner, G.; Kranner, I.; Buchner, O. Heat Acclimation under Drought Stress Induces Antioxidant Enzyme Activity in the Alpine Plant Primula minima. Antioxidants 2023, 12, 1093. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Saradhi, P.P. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma 2016, 253, 1577–1582. [Google Scholar] [CrossRef]
- Hang, Y.; Hu, T.; Tian, Y.H.; Zhang, Y.G.; Shangguan, L.Y.; Liu, M.; Zhang, M.S. Physiological and transcriptomic responses of Pinellia ternata to continuous cropping. Ind. Crops Prod. 2023, 205, 117511. [Google Scholar] [CrossRef]
- Gong, M.; Jiang, D.Z.; Liu, R.; Tian, S.M.; Xing, H.T.; Chen, Z.D.; Li, H.L. Influence of High-Temperature and Intense Light on the Enzymatic Antioxidant System in Ginger (Zingiber officinale Roscoe) Plantlets. Metabolites 2023, 13, 992. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Han, Y.; Zou, Y.J.; Li, L.; Ma, X.X.; Zhao, Z.J.; Bao, Y.H. Effect of cinnamon extract combined with Ɛ-polylysine infusion treatment on the sensory, physicochemical and biological quality of Brassica rapa L. (Chinensis Group). Sci. Hortic. 2024, 323, 112470. [Google Scholar] [CrossRef]
- Budiawan, A.; Purwanto, A.; Puradewa, L.; Cahyani, E.D.; Purwaningsih, C.E. Wound healing activity and flavonoid contents of purslane (Portulaca grandiflora) of various varieties. RSC Adv. 2023, 13, 9871–9877. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Liu, J.; Qin, X.; Liu, Y.; Sui, M.; Zhang, Y.; Hu, Y.; Mao, Y.; Shen, X. Effect of Nitric Oxide on Browning of Stem Tip Explants of Malus sieversii. Horticulturae 2023, 9, 1246. https://doi.org/10.3390/horticulturae9111246
Yang C, Liu J, Qin X, Liu Y, Sui M, Zhang Y, Hu Y, Mao Y, Shen X. Effect of Nitric Oxide on Browning of Stem Tip Explants of Malus sieversii. Horticulturae. 2023; 9(11):1246. https://doi.org/10.3390/horticulturae9111246
Chicago/Turabian StyleYang, Chen, Jiangfei Liu, Xin Qin, Yangbo Liu, Mengyi Sui, Yawen Zhang, Yanli Hu, Yunfei Mao, and Xiang Shen. 2023. "Effect of Nitric Oxide on Browning of Stem Tip Explants of Malus sieversii" Horticulturae 9, no. 11: 1246. https://doi.org/10.3390/horticulturae9111246
APA StyleYang, C., Liu, J., Qin, X., Liu, Y., Sui, M., Zhang, Y., Hu, Y., Mao, Y., & Shen, X. (2023). Effect of Nitric Oxide on Browning of Stem Tip Explants of Malus sieversii. Horticulturae, 9(11), 1246. https://doi.org/10.3390/horticulturae9111246




