Comprehensive Evaluation of Nutritional Qualities of Chinese Cabbage (Brassica rapa ssp. pekinensis) Varieties Based on Multivariate Statistical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Preparation and Determination of Nutritional Constituents
2.3. Multivariate Statistical Analysis
3. Results
3.1. Difference Analysis of Nutritional Constituents in 35 Varieties of Chinese Cabbage
3.1.1. Component Content Analysis
3.1.2. Diversity Analysis
3.1.3. Correlation Analysis
3.1.4. Principal Component Analysis
3.2. Comprehensive Evaluation of Nutritional Constituents of 35 Chinese Cabbage Varieties Based on PCA
3.2.1. Membership Function Analysis and Comprehensive Score
3.2.2. Cluster Analysis of Nutrient Composition of Different Chinese Cabbage Varieties
3.2.3. Screening of Evaluation Indexes for Nutritional Composition of Chinese Cabbage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Number | Materials Name | Pedigrees | Number | Materials Name | Pedigrees |
|---|---|---|---|---|---|
| 1 | 1911462 | TianFu75 (NongBoDa) | 19 | 1911679 | BaoHongXin 24 |
| 2 | 1911606 | HongHaiEr (XianZhengDa) | 20 | 1911504 | heatwave 539f1-2 |
| 3 | ZB61 | F1 | 21 | 1911556 | JuLongKangReXianFeng |
| 4 | 1616030 | XY4A | 22 | LS70 | F1 |
| 5 | 1911507 | ZhenBao50 | 23 | 1911847 | TaQing07 |
| 6 | JHWW | F1 | 24 | 1911421 | XiaoBaoJian |
| 7 | 1911095 | 127WaWaCai (XY) | 25 | 1911754 | YuTianBaoJian × TaQing |
| 8 | 1911013 | MiNiHuang (South Korea longjing) | 26 | 1911757 | YuTianBaoJian × TaQing |
| 9 | 1911014 | MiNiHuang (South Korea longjing) | 27 | 1915053 | CMSJinQiu × XiaoBaoJian |
| 10 | 1915154 | CR–LiMin | 28 | 1915054 | CMSJinQiu × XiaoBaoJian |
| 11 | 1915157 | CR–ZhongLianJinBao | 29 | 1915320 | ShunYi30 |
| 12 | 1915169 | GaoShanWaWaCai | 30 | 1911792 | 234LAangFang |
| 13 | 1911156 | JinJiang45 | 31 | 1911801 | BP058 |
| 14 | 1840414 | F1 | 32 | ZB76 | F1 (BP058 × 234) |
| 15 | A04749 | F1 | 33 | 1911830 | JinLv75 |
| 16 | 1640250 | F1 | 34 | 1914040 | DongLiKuaiCai |
| 17 | JH308 | F1 | 35 | 1914078 | FuHuaKuaiCai |
| 18 | 1911676 | BaoHongXin24 |
| Components | Determination Methods |
|---|---|
| vitamin C | 2,6-dichloroindophenol titration (GB 5009.86-2016) |
| crude protein | Combustion Nitrogen Analysis (GB 5009.5-2016) |
| crude fiber | Acid—alkali washing method (GB 5009.10-2003) |
| glucose, fructose | Ion chromatography |
| malic acid, citric acid, oxalic acid | Ion chromatography (NYT2277-2012) |
| mineral elements | ICP-OES (GB 5009.268) |
| amino acid | amino acid analyzer (GB/T 30987-2020) |
- 1.
- Vitamin C:
- Test solution preparation: Weigh 100 g of the edible portion of the sample and place it in a pulverizer. Add 100 g of metaphosphoric acid solution and quickly pound it until homogenous. Accurately weigh 10–40 g of homogenized sample (to an accuracy of 0.01 g) into a beaker, transfer to a 100 mL volumetric flask, and dilute to the mark with a metaphosphoric acid solution. Shake well and filter.
- Titration: Transfer 10 mL of the filtrate to a 50 mL conical flask with precision, and titrate it with calibrated 2,6-dichloroindophenol solution until the solution remains pink for 15 s without any fading. It is critical to perform a blank test simultaneously.
- Calculation of results:
- x—the content of L(+) ascorbic acid in the sample, in mg/100 g;
- V—volume of 2,6 dichloroindophenol solution consumed in the titration of the sample, in milliliters (mL);
- V0—volume of 2,6 dichloroindophenol solution consumed in the titration blank, in milliliters (mL).
- T—titration of 2,6 dichloroindophenol solution, expressed as milligrams of ascorbic acid per milliliter of 2,6 dihloroindophenol solution (mg/mL).
- A—dilution factor;
- m—mass of the sample, in grams (g).
- 2.
- Crude protein
- Weigh 0.1 g~1.0 g fully mixed sample (accurate to 0.0001 g) according to the instructions of the instrument, wrap it with tin foil and place it on the sample tray. After entering the combustion reactor (900 °C~1200 °C), the sample is fully burned in high-purity oxygen (≥99.99%). The product (NOx) in the combustion furnace is transported by the carrier gas carbon dioxide or helium to the reduction furnace (800 °C), and its content is measured after reduction to produce nitrogen.
- Calculation of results:
- x—the protein content in the sample, expressed in grams per hundred grams (g/100 g);
- C—the content of nitrogen in the sample, expressed in grams per hundred grams (g/100 g);
- F—coefficient of conversion of nitrogen to protein.
- 3.
- Crude fiber
- Weigh 20 g~30 g of mashed specimen, transfer to 500 L-shaped flask, add 200 mL of boiling 1.25% sulphuric acid, heat to bring to a slight boil, keep the volume constant, maintained for 30 min, and shake the conical flask every 5 min, in order to fully mix the substances in the flask.
- The conical flask was removed and immediately filtered using linen cloth and washed with boiling water until the washings were not acidic.
- Then, use 200 mL of boiling 1.25% potassium hydroxide solution to wash the residue on the linen into the original conical flask and heat for 30 min, remove the conical flask, immediately filter using linen, wash with boiling water for 2~3 times, transfer to a dry and weighed G2 pendant crucible or pendant funnel of the same type, extract the filter, wash with hot water, and then pump dry. The crucible is washed once more with ethanol and ether. Dry the crucible and contents in an oven at 105 °C and weigh, repeating the operation until a constant amount is obtained.
- If the sample contains more insoluble impurities, the sample can be moved to the asbestos crucible, dried and weighed, and then moved to the 550 °C high-temperature furnace ashing; all the carbon-containing material ashing was placed in the desiccator, cooled to room temperature and weighed, and the amount of loss of crude fiber content was calculated.
- Calculation of results:
- x—the content of coarse fibers in the sample;
- G—the mass of the residue (or mass lost by a high-temperature furnace), expressed in grams (g);
- m—the mass of the sample in grams (g).
- 4.
- Glucose and fructose
- Homogenate 5.00 g was extracted using 80% ethanol at a constant volume of 50 mL for 30 min by ultrasound or shaker oscillator. After centrifugation at 3000 r/min for 10 min, take 1 mL of the supernatant and put it into a 100 mL volumeter bottle, dilute it with water to the scale, and shake well. Finally, the diluent was directly injected into 0.22 μm aqueous filtration membrane and C18 solid phase extraction column for analysis.
- Column parameters: anion exchange sugar protection column CarboPac PA 10 (50 mm × 4 mm); anion exchange sugar analysis column CarboPac PA 10 (250 mm × 4 mm). NaOH gradient leaching, flow rate: 0.80 mL/min. Sample size: 10 µL. Column temperature: 30 °C. Amperometric detector: Gold working electrode, Ag/AgCl reference electrode mode, and sugar standard four-potential waveform pulse amperometric detection.
- The sample treatment solution and the standard working solution were separately injected into the ion chromatograph for separation and detection. The sample solution was qualitatively determined using the retention time of the standard solution peak and quantitatively determined using the area of the standard solution peak.
- Calculation of results:
- x—the content of fructose and glucose in the sample, expressed in grams per hundred grams or grams per hundred milliliters (g/100 g or g/100 mL);
- C—the concentration of fructose and glucose in the sample solution calculated from the standard curve, in mg/L;
- C0—the concentration of fructose and glucose in the blank calculated from the standard curve, in milligrams per liter (mg/L);
- V—the volume of the constant volume, in milliliters (mL);
- m—the weight of the sample, expressed in grams (g) or milliliters (mL);
- f—dilution ratio;
- 10—conversion factor;
- 1000—conversion factor.
- Malic acid, citric acid and oxalic acid
- Sample preparation: The edible part is extracted according to the provisions of GB/T 8855; after it is reduced, it is chopped, thoroughly mixed and crushed in the food processor to produce the test sample.
- Withdraw: Weigh 5 g (accurate to 0.001 g) sample in a 100 mL beaker, add 80 mL water, put it into an ultrasonic meter, followed by ultrasonic treatment for 30 min, transfer it to a 100 m volumetric bottle and maintain constant volume with water, fully mixed; 0.22 μm of the water phase filter membrane was ready to be measured.
- Instrument: Column: High volume anion exchange column, such as AS19, or other columns with comparable performance; column temperature: 30 °C; the sample size was 25 μL; mobile phase: potassium hydroxide solution was used as eluent at a flow rate of 1.0 mL/min.
- Standard curve drawing: The standard curve was drawn using the mass concentration of the standard series solution as the horizontal coordinate and the peak area as the vertical coordinate.
- Test solution determination: The retention time was used for qualitative analysis, and the peak area of the test solution and the standard working solution was compared for quantitative analysis.
- Result calculation:
- x—the content of Malic acid, citric acid and oxalic acid in the sample, expressed in in milligrams per kilogram (mg/kg);
- ρ—The mass concentration of components to be measured in the test solution was obtained by linear regression equation, and the unit was mg/L.
- V—Constant volume unit, in milliliters (mL).
- m—Sample mass, in grams (g).
- 6.
- Mineral elements
- Sample preparation: homogenize the edible part of the sample.
- Sample digestion: Accurately weigh 0.5 g~5 g (accurate to 0.001 g) or accurately remove 2.00 mL~10.0 mL of the sample into glass or Teflon containers. In the solution vessel, the samples containing ethanol or carbon dioxide are first heated on the electric heating plate at low temperature to remove ethanol or carbon dioxide, and 10 mL of nitric acid–perchloric acid (10 + 1) mixed solution is added and then digested on the electric heating plate or graphite digestion device. If the digestion solution turns brown and black during digestion, a small amount of mixed acid can be added appropriately until white smoke is emitted, and the digestion solution is colorless, transparent or slightly yellow and cold. Next, add 25 mL or 50 mL of water, mix well and set aside; perform a blank test at the same time.
- Instrument operation reference conditions: Observation method: vertical observation—if the instrument has a two-way observation method, high-concentration elements, such as potassium, sodium, calcium, magnesium and other elements, should be observed vertically, and the rest should be observed horizontally.
- Power: 1150 W; plasma gas flow rate: 15 L/min; auxiliary gas flow rate: 0.5 L/min; atomizing gas flow rate: 0.65 L/min; analysis pump speed: 50 r/min.
- Production of standard curves: The standard series of working solutions was injected into the inductively coupled plasma emission spectrometer, and the intensity signal response value of the analytical spectral line of the element to be measured was determined. The concentration of the element to be measured was the horizontal coordinate, the intensity response value of the analytical spectral line was the longitudinal coordinate, and the standard curve was drawn.
- Determination of sample solution: The blank solution and the sample solution were injected into the inductively coupled plasma emission spectrometer to measure the signal response of the analysis spectral line intensity of the element to be measured, and the concentration of the element to be measured in the digestion solution was obtained according to the standard curve.
- Result calculation:
- x—the content of elements to be measured in the sample, expressed in milligrams per kilogram or milligrams per liter (mg/kg or mg/L).
- ρ—mass concentration of the element to be measured in the sample solution, in mg/L.
- ρ0—Mass concentration of the element to be measured in the blank solution of the sample, in milligrams per liter (mg/L).
- V—constant volume of the digestive fluid of the sample, in milliliters (mL).
- f—dilution ratio of the sample.
- m—sample weighed by mass or removed volume in grams or milliliters (g or mL).
| Element Symbol | The Wave Length of Analytical Line (nm) | LOD1 (mg/kg) | LOD2 (mg/L) | LOQ1 (mg/kg) | LOQ2 (mg/L) |
|---|---|---|---|---|---|
| Ca | 315.8/317. | 5 | 2 | 20 | 5 |
| Cu | 324.75 | 0.2 | 0.05 | 0.5 | 0.2 |
| Fe | 239.5/259.9 | 1 | 0.3 | 3 | 1 |
| K | 766.49 | 7 | 3 | 30 | 7 |
| Mg | 279.079 | 5 | 2 | 20 | 5 |
| Mn | 257.6/259. | 0.1 | 0.03 | 0.3 | 0.1 |
| P | 213.6 | 1 | 0.3 | 3 | 1 |
| Zn | 206.2/213. | 0.5 | 0.2 | 2 | 0.5 |
- 7.
- Amino acid
- The samples were prepared according to 6.1 and 6.2 in GB/T8303-2013 and passed through a 40-mesh sieve. Mix well and put into a clean container as a sample.
- Test procedure:
- Standard solution composition: Preparation of mixed amino acid standard reserve solution. Weigh an appropriate amount of each amino acid standard (slightly to 0.01 mg) and dissolve in water to prepare a mixed solution. The concentrations of theanine and other amino acids in the mixed standard solution were 5.00 μmol/mL and 1.25 μmol/mL, respectively. The shelf life of the frozen solution is 1 month.
- Mixed amino acid standard working liquid preparation: Accurately absorb 2 mL of the standard reserve liquid of mixed amino acids into a 5 mL volumetric bottle, dilute the volume with water to the scale, and mix well to obtain the first standard solution. The first standard solution is diluted step by step with water to produce a total of 7 different concentrations of the series of mixed standard working solutions. The concentrations of theanine and other basic acids in the mixed standard working solution of 7 concentrations were 2000.00 nmol/mL and 500.00 nmol/mL, 1000.00 nmol/mL and 250.00 nmol/mL, respectively. A total of 500.00 nmol/mL and 125.00 nmol/mL, 250.00 nmol/mL and 62.50 nmol/mL,125.00 nmol/mL and 31.25 mol/mL, 62.50 mol/mL and 15.63 nmol/mL, 31.25 nmol/mL and 7.81 nmol/mL, were ready for use.
- The mobile phase and the post-column derivatization reaction solution were prepared.
- Configure the mobile phase B1, B2, B3, B4, B5 and B6, and configure the reaction solution R1, R2 and R3.
- Sample extraction: Accurately weigh the sample, which is about 20 g (accurate to 0.0001 g), add 200 mL boiling water into a 250 mL cone, heat it in a 95 °C water bath, mix it well every 5 min, extract it for 10 min, and then filter it while it is hot. After the filtrate is cooled to room temperature, fill it with water to 250 mL, mix it well and take an appropriate amount of sample solution. After filtration through a 0.45 μm water phase filter membrane, it is ready to be measured.
- Measurement: Column: sulfonic acid type cation exchange column, 3 μm, 4.6 mm × 60 mm, or equivalent performance column. Instrument separation system operation reference conditions: The flow rate of the mobile phase was 0.35 mL/min, and the sample volume was 20 μL. Amino acid reaction detection system operation reference conditions were as follows:
- The temperature of the reaction column was 135 °C, the flow rate of the reaction liquid was 0.30 mL/min, and the detection wavelengths of the detector were 570 nm and 440 nm.
- Draw a standard working curve: The automatic amino acid analyzer was started and the working parameters were set. After the baseline was stabilized, a series of mixed amino acid standard working solutions of different concentrations were absorbed and injected into the automatic amino acid analyzer for determination, and the peak areas of different amino acids were obtained, respectively. The standard working curve was established using the peak area of each amino acid as the vertical coordinate and the concentration as the horizontal coordinate. The peak order of each amino acid was identified by retention time.
- Sample determination: The chromatographic peak area of each amino acid in the sample was obtained using the amino acid analyzer, and the content was calculated from the standard working curve. Using water as a blank sample, the amino acid background values in the blank sample were calculated under the same conditions. The net amino acid content in each sample was obtained by deducting the amino acid background value in the blank sample.
- Result calculation:
- W1—The content of each amino acid component in a sample, expressed in milligrams per 100 g (mg/100 g);
- C—The amino acid concentration calculated from the standard working curve in the sample solution, in units of nanomolar per milliliter (nmol/mL);
- Ck—The amino acid concentration calculated from the standard working curve in the blank sample solution, expressed in nanomolar per milliliter (nmol/mL);
- V—Total sample volume, in milliliters (mL);
- M—The molar mass of an amino acid, expressed in grams per mole (g/mol);
- m—Sample mass, expressed in grams (g);
- m1—The dry matter rate of the sample was determined by GB/T8303.
| Materials Number | DW % | VC mg/kg | CP % | CF % | Glc g/kg | Flu g/kg | MA g/kg | CA g/kg | OA g/kg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.53 ± 0.00 | 230.37 ± 3.19 | 1.35 ± 0.02 | 0.55 ± 0.03 | 11.05 ± 0.08 | 8.53 ± 0.03 | 0.93 ± 0.04 | 0.09 ± 0.01 | 0.18 ± 0.03 |
| 2 | 5.73 ± 0.00 | 281.32 ± 37.36 | 1.36 ± 0.02 | 0.52 ± 0.00 | 11.61 ± 0.66 | 9.20 ± 0.87 | 0.90 ± 0.13 | 0.11 ± 0.01 | 0.26 ± 0.05 |
| 3 | 4.86 ± 0.00 | 186.49 ± 16.09 | 1.10 ± 0.04 | 0.45 ± 0.01 | 11.31 ± 0.62 | 8.54 ± 0.51 | 1.04 ± 0.12 | 0.14 ± 0.02 | 0.19 ± 0.03 |
| 4 | 6.45 ± 0.00 | 351.77 ± 32.32 | 1.21 ± 0.03 | 0.59 ± 0.01 | 14.83 ± 0.89 | 9.44 ± 1.15 | 0.75 ± 0.49 | 0.14 ± 0.02 | 0.29 ± 0.02 |
| 5 | 5.24 ± 0.00 | 182.35 ± 11.20 | 1.42 ± 0.03 | 0.45 ± 0.03 | 11.20 ± 0.48 | 7.72 ± 0.50 | 0.82 ± 0.16 | 0.13 ± 0.02 | 0.13 ± 0.04 |
| 6 | 5.11 ± 0.00 | 206.20 ± 12.39 | 1.06 ± 0.05 | 0.46 ± 0.01 | 13.35 ± 0.85 | 15.30 ± 1.07 | 0.71 ± 0.04 | 0.08 ± 0.02 | 0.11 ± 0.02 |
| 7 | 5.09 ± 0.01 | 211.11 ± 34.29 | 1.48 ± 0.11 | 0.44 ± 0.02 | 11.30 ± 3.35 | 9.33 ± 2.87 | 0.54 ± 0.15 | 0.09 ± 0.08 | 0.12 ± 0.04 |
| 8 | 5.26 ± 0.00 | 158.16 ± 8.55 | 1.46 ± 0.01 | 0.47 ± 0.03 | 14.66 ± 1.25 | 9.92 ± 1.82 | 0.49 ± 0.07 | 0.06 ± 0.02 | 0.11 ± 0.01 |
| 9 | 5.21 ± 0.00 | 196.27 ± 35.13 | 1.48 ± 0.10 | 0.45 ± 0.04 | 14.68 ± 0.95 | 9.41 ± 1.15 | 0.75 ± 0.22 | 0.06 ± 0.02 | 0.13 ± 0.05 |
| 10 | 5.05 ± 0.00 | 228.45 ± 10.61 | 1.15 ± 0.00 | 0.49 ± 0.03 | 18.35 ± 1.59 | 12.12 ± 2.09 | 0.45 ± 0.02 | 0.04 ± 0.00 | 0.06 ± 0.01 |
| 11 | 4.34 ± 0.01 | 189.42 ± 9.32 | 1.13 ± 0.14 | 0.44 ± 0.01 | 13.68 ± 0.34 | 12.78 ± 0.98 | 0.76 ± 0.14 | 0.06 ± 0.01 | 0.11 ± 0.04 |
| 12 | 5.17 ± 0.00 | 204.03 ± 10.09 | 1.24 ± 0.21 | 0.44 ± 0.01 | 15.65 ± 4.77 | 13.88 ± 2.73 | 0.84 ± 0.20 | 0.11 ± 0.06 | 0.19 ± 0.06 |
| 13 | 4.90 ± 0.00 | 173.68 ± 10.61 | 1.45 ± 0.04 | 0.42 ± 0.03 | 12.14 ± 0.78 | 12.82 ± 0.87 | 0.95 ± 0.14 | 0.07 ± 0.01 | 0.26 ± 0.03 |
| 14 | 5.26 ± 0.00 | 219.55 ± 8.56 | 1.25 ± 0.01 | 0.49 ± 0.02 | 13.97 ± 4.10 | 10.84 ± 3.78 | 0.54 ± 0.25 | 0.06 ± 0.02 | 0.12 ± 0.04 |
| 15 | 5.14 ± 0.00 | 222.29 ± 7.54 | 1.38 ± 0.22 | 0.45 ± 0.03 | 12.98 ± 0.78 | 11.05 ± 1.18 | 0.89 ± 0.10 | 0.08 ± 0.01 | 0.20 ± 0.07 |
| 16 | 5.44 ± 0.00 | 204.26 ± 11.00 | 1.25 ± 0.03 | 0.43 ± 0.00 | 15.67 ± 0.77 | 17.52 ± 0.47 | 0.89 ± 0.10 | 0.09 ± 0.04 | 0.17 ± 0.04 |
| 17 | 4.67 ± 0.00 | 181.61 ± 11.62 | 1.06 ± 0.04 | 0.47 ± 0.02 | 10.09 ± 1.09 | 8.53 ± 0.86 | 0.75 ± 0.04 | 0.09 ± 0.01 | 0.11 ± 0.01 |
| 18 | 6.95 ± 0.00 | 331.07 ± 13.01 | 1.45 ± 0.12 | 0.64 ± 0.04 | 16.04 ± 3.81 | 18.23 ± 3.85 | 1.03 ± 0.89 | 0.13 ± 0.03 | 0.35 ± 0.20 |
| 19 | 7.52 ± 0.00 | 344.58 ± 35.18 | 1.66 ± 0.05 | 0.70 ± 0.07 | 15.83 ± 0.66 | 17.41 ± 0.35 | 2.36 ± 0.31 | 0.18 ± 0.02 | 0.68 ± 0.13 |
| 20 | 5.73 ± 0.01 | 243.97 ± 14.04 | 1.26 ± 0.05 | 0.52 ± 0.03 | 19.00 ± 3.61 | 14.72 ± 3.56 | 0.63 ± 0.23 | 0.05 ± 0.02 | 0.12 ± 0.07 |
| 21 | 4.60 ± 0.00 | 201.98 ± 18.11 | 1.10 ± 0.05 | 0.42 ± 0.01 | 13.06 ± 1.53 | 12.36 ± 1.65 | 0.41 ± 0.06 | 0.04 ± 0.01 | 0.07 ± 0.01 |
| 22 | 6.00 ± 0.00 | 182.83 ± 0.00 | 1.34 ± 0.06 | 0.50 ± 0.01 | 14.54 ± 1.10 | 13.08 ± 1.05 | 0.91 ± 0.11 | 0.10 ± 0.02 | 0.10 ± 0.02 |
| 23 | 5.52 ± 0.00 | 230.37 ± 13.18 | 1.24 ± 0.06 | 0.48 ± 0.03 | 12.60 ± 1.08 | 11.31 ± 0.89 | 1.42 ± 0.06 | 0.19 ± 0.01 | 0.12 ± 0.01 |
| 24 | 5.89 ± 0.00 | 252.07 ± 28.53 | 1.53 ± 0.09 | 0.55 ± 0.02 | 12.22 ± 1.31 | 10.01 ± 1.47 | 0.85 ± 0.05 | 0.13 ± 0.01 | 0.22 ± 0.01 |
| 25 | 6.13 ± 0.00 | 321.90 ± 34.77 | 1.15 ± 0.01 | 0.62 ± 0.06 | 14.99 ± 1.00 | 15.06 ± 1.37 | 0.81 ± 0.59 | 0.11 ± 0.02 | 0.22 ± 0.05 |
| 26 | 7.19 ± 0.01 | 353.98 ± 74.43 | 1.32 ± 0.22 | 0.68 ± 0.06 | 21.14 ± 1.38 | 17.58 ± 1.12 | 0.22 ± 0.15 | 0.06 ± 0.03 | 0.14 ± 0.10 |
| 27 | 6.06 ± 0.00 | 288.68 ± 29.36 | 1.24 ± 0.07 | 0.65 ± 0.09 | 14.50 ± 2.04 | 14.80 ± 2.23 | 0.63 ± 0.44 | 0.12 ± 0.04 | 0.20 ± 0.06 |
| 28 | 6.29 ± 0.00 | 268.06 ± 9.09 | 1.10 ± 0.02 | 0.61 ± 0.08 | 17.94 ± 0.96 | 18.99 ± 1.52 | 0.52 ± 0.36 | 0.13 ± 0.04 | 0.14 ± 0.07 |
| 29 | 6.35 ± 0.00 | 245.15 ± 8.65 | 1.18 ± 0.04 | 0.64 ± 0.06 | 16.43 ± 0.46 | 17.45 ± 0.08 | 0.57 ± 0.04 | 0.08 ± 0.01 | 0.11 ± 0.01 |
| 30 | 9.03 ± 0.00 | 536.32 ± 94.77 | 1.52 ± 0.01 | 0.80 ± 0.05 | 21.12 ± 2.36 | 28.26 ± 2.68 | 1.10 ± 0.03 | 0.13 ± 0.04 | 1.18 ± 0.34 |
| 31 | 6.39 ± 0.00 | 214.53 ± 44.55 | 1.66 ± 0.06 | 0.62 ± 0.03 | 17.29 ± 2.72 | 13.69 ± 2.13 | 0.91 ± 0.37 | 0.06 ± 0.02 | 0.27 ± 0.10 |
| 32 | 5.72 ± 0.00 | 207.91 ± 17.19 | 1.28 ± 0.11 | 0.54 ± 0.01 | 15.07 ± 0.70 | 19.48 ± 0.90 | 0.26 ± 0.01 | 0.05 ± 0.02 | 0.22 ± 0.04 |
| 33 | 6.70 ± 0.00 | 343.25 ± 23.28 | 1.69 ± 0.17 | 0.60 ± 0.03 | 14.79 ± 1.51 | 17.23 ± 2.02 | 0.60 ± 0.50 | 0.07 ± 0.03 | 0.41 ± 0.28 |
| 34 | 5.97 ± 0.00 | 378.03 ± 6.87 | 1.17 ± 0.01 | 0.50 ± 0.01 | 12.44 ± 0.55 | 12.05 ± 0.64 | 2.43 ± 0.24 | 0.19 ± 0.03 | 0.43 ± 0.05 |
| 35 | 5.28 ± 0.00 | 352.83 ± 7.94 | 1.16 ± 0.02 | 0.44 ± 0.01 | 12.58 ± 0.69 | 10.35 ± 0.62 | 0.77 ± 0.06 | 0.09 ± 0.02 | 0.16 ± 0.06 |
| Materials Number | Ca mg/kg | K mg/kg | Mg mg/kg | P mg/kg | Cu mg/kg | Fe mg/kg | Mn mg/kg | Zn mg/kg |
|---|---|---|---|---|---|---|---|---|
| 1 | 677.71 ± 32.37 | 2166.25 ± 103.88 | 146.00 ± 6.15 | 422.29 ± 27.92 | 0.53 ± 0.00 | 6.67 ± 1.76 | 1.39 ± 0.08 | 2.70 ± 0.11 |
| 2 | 666.83 ± 111.07 | 2025.56 ± 155.54 | 127.77 ± 11.95 | 412.41 ± 10.02 | 0.50 ± 0.06 | 8.19 ± 3.33 | 1.39 ± 0.24 | 2.06 ± 0.13 |
| 3 | 525.02 ± 30.87 | 1803.97 ± 57.14 | 106.64 ± 6.64 | 327.34 ± 12.36 | 0.43 ± 0.01 | 5.72 ± 0.70 | 1.19 ± 0.03 | 1.78 ± 0.12 |
| 4 | 637.08 ± 42.84 | 2134.11 ± 145.82 | 134.36 ± 6.34 | 386.96 ± 28.23 | 0.58 ± 0.04 | 9.77 ± 1.46 | 1.44 ± 0.08 | 2.23 ± 0.22 |
| 5 | 537.10 ± 62.93 | 2028.48 ± 21.38 | 116.38 ± 4.68 | 375.43 ± 11.27 | 0.50 ± 0.02 | 8.30 ± 1.90 | 1.84 ± 0.10 | 2.47 ± 0.14 |
| 6 | 572.19 ± 17.71 | 1792.12 ± 159.96 | 123.46 ± 3.27 | 359.87 ± 11.84 | 0.46 ± 0.03 | 7.97 ± 2.44 | 1.28 ± 0.05 | 1.90 ± 0.13 |
| 7 | 530.38 ± 73.33 | 1957.07 ± 296.86 | 116.94 ± 9.97 | 352.58 ± 31.62 | 0.59 ± 0.25 | 4.95 ± 0.73 | 1.26 ± 0.15 | 2.10 ± 0.16 |
| 8 | 503.39 ± 67.39 | 1944.17 ± 113.53 | 106.83 ± 3.48 | 387.64 ± 23.65 | 0.41 ± 0.00 | 5.44 ± 0.98 | 0.94 ± 0.04 | 2.28 ± 0.29 |
| 9 | 570.22 ± 77.11 | 1959.03 ± 151.69 | 119.42 ± 16.62 | 420.38 ± 19.97 | 0.43 ± 0.03 | 6.14 ± 1.20 | 0.99 ± 0.21 | 2.65 ± 0.14 |
| 10 | 521.82 ± 23.22 | 1524.09 ± 202.22 | 96.37 ± 2.82 | 343.90 ± 6.17 | 0.40 ± 0.01 | 4.46 ± 0.24 | 1.16 ± 0.07 | 1.48 ± 0.11 |
| 11 | 536.48 ± 47.00 | 1420.33 ± 137.58 | 87.71 ± 6.40 | 333.49 ± 20.30 | 0.37 ± 0.04 | 3.82 ± 0.69 | 0.85 ± 0.17 | 1.20 ± 0.16 |
| 12 | 543.00 ± 73.70 | 2195.39 ± 64.11 | 120.07 ± 17.71 | 390.77 ± 40.80 | 0.25 ± 0.06 | 4.80 ± 0.84 | 0.98 ± 0.14 | 2.05 ± 0.32 |
| 13 | 777.19 ± 137.72 | 1835.02 ± 96.57 | 143.49 ± 7.81 | 408.49 ± 28.90 | 0.30 ± 0.03 | 5.41 ± 1.68 | 1.23 ± 0.26 | 2.22 ± 0.16 |
| 14 | 635.06 ± 14.37 | 1836.12 ± 90.64 | 123.41 ± 6.31 | 397.96 ± 26.80 | 0.28 ± 0.02 | 3.82 ± 0.24 | 1.01 ± 0.02 | 1.65 ± 0.07 |
| 15 | 657.54 ± 149.14 | 2161.58 ± 31.84 | 141.73 ± 23.29 | 388.14 ± 63.09 | 0.30 ± 0.06 | 5.93 ± 1.46 | 1.08 ± 0.08 | 2.21 ± 0.50 |
| 16 | 607.41 ± 18.33 | 2099.98 ± 194.02 | 125.67 ± 4.41 | 381.26 ± 28.17 | 0.34 ± 0.15 | 4.84 ± 0.46 | 0.95 ± 0.11 | 2.11 ± 0.18 |
| 17 | 497.83 ± 17.61 | 1508.75 ± 51.24 | 101.44 ± 1.68 | 360.00 ± 28.02 | 0.43 ± 0.02 | 4.76 ± 0.46 | 1.24 ± 0.13 | 1.46 ± 0.12 |
| 18 | 945.87 ± 328.79 | 1876.05 ± 224.20 | 148.13 ± 41.10 | 395.80 ± 13.15 | 0.75 ± 0.22 | 12.85 ± 4.86 | 2.17 ± 0.35 | 2.10 ± 0.43 |
| 19 | 1364.61 ± 106.71 | 2342.73 ± 109.80 | 177.31 ± 12.71 | 430.31 ± 23.46 | 1.16 ± 0.16 | 21.34 ± 7.05 | 2.66 ± 0.23 | 1.94 ± 0.06 |
| 20 | 459.54 ± 69.01 | 1994.19 ± 219.00 | 108.28 ± 3.47 | 319.01 ± 35.88 | 0.39 ± 0.04 | 5.13 ± 0.07 | 1.24 ± 0.06 | 2.02 ± 0.28 |
| 21 | 576.31 ± 55.23 | 1560.48 ± 95.11 | 97.57 ± 2.59 | 417.48 ± 17.97 | 0.40 ± 0.01 | 4.97 ± 0.47 | 0.98 ± 0.05 | 1.92 ± 0.24 |
| 22 | 426.00 ± 48.75 | 1776.67 ± 32.15 | 117.67 ± 4.04 | 424.00 ± 16.64 | 0.47 ± 0.03 | 3.89 ± 0.43 | 1.25 ± 0.10 | 2.91 ± 0.12 |
| 23 | 366.33 ± 23.03 | 1810.00 ± 75.50 | 103.67 ± 2.52 | 358.00 ± 18.19 | 0.41 ± 0.03 | 4.17 ± 0.26 | 1.34 ± 0.06 | 2.63 ± 0.14 |
| 24 | 432.67 ± 47.88 | 2006.67 ± 85.05 | 106.00 ± 3.61 | 433.33 ± 9.02 | 0.48 ± 0.05 | 4.51 ± 0.30 | 1.38 ± 0.04 | 3.42 ± 0.07 |
| 25 | 843.97 ± 130.81 | 2124.25 ± 98.19 | 133.22 ± 7.55 | 425.38 ± 29.74 | 0.65 ± 0.16 | 11.58 ± 1.76 | 1.65 ± 0.15 | 2.14 ± 0.20 |
| 26 | 782.39 ± 251.83 | 2281.98 ± 190.84 | 130.92 ± 24.90 | 412.05 ± 40.37 | 0.54 ± 0.07 | 9.00 ± 2.77 | 1.75 ± 0.28 | 2.36 ± 0.34 |
| 27 | 755.90 ± 248.64 | 1989.48 ± 102.98 | 125.97 ± 24.19 | 412.85 ± 12.92 | 1.09 ± 0.97 | 9.15 ± 1.99 | 1.61 ± 0.10 | 1.75 ± 0.15 |
| 28 | 411.58 ± 9.88 | 1880.22 ± 41.37 | 101.23 ± 6.86 | 379.64 ± 22.80 | 0.44 ± 0.05 | 5.35 ± 0.62 | 1.33 ± 0.07 | 1.96 ± 0.13 |
| 29 | 522.39 ± 53.52 | 1833.84 ± 256.16 | 92.25 ± 2.59 | 443.07 ± 42.51 | 0.43 ± 0.01 | 6.92 ± 1.53 | 1.49 ± 0.11 | 2.12 ± 0.18 |
| 30 | 941.73 ± 218.47 | 2128.14 ± 129.35 | 151.89 ± 26.20 | 429.98 ± 24.97 | 0.56 ± 0.06 | 12.15 ± 3.29 | 2.12 ± 0.52 | 2.37 ± 0.12 |
| 31 | 876.40 ± 102.55 | 2102.62 ± 128.73 | 156.41 ± 12.37 | 483.21 ± 19.27 | 0.59 ± 0.14 | 6.64 ± 0.42 | 1.34 ± 0.03 | 2.67 ± 0.28 |
| 32 | 791.51 ± 161.52 | 1837.68 ± 201.69 | 125.58 ± 4.51 | 466.02 ± 41.75 | 0.33 ± 0.03 | 5.59 ± 0.25 | 1.33 ± 0.11 | 2.30 ± 0.48 |
| 33 | 1237.70 ± 188.43 | 2314.79 ± 81.55 | 180.46 ± 16.94 | 502.14 ± 32.98 | 0.40 ± 0.02 | 13.68 ± 7.78 | 1.21 ± 0.11 | 2.52 ± 1.26 |
| 54 | 1190.57 ± 94.93 | 2306.85 ± 106.74 | 141.69 ± 10.46 | 329.06 ± 26.69 | 0.70 ± 0.48 | 11.16 ± 0.73 | 1.24 ± 0.15 | 1.86 ± 0.10 |
| 55 | 1027.28 ± 56.25 | 1768.00 ± 38.65 | 140.40 ± 6.69 | 313.94 ± 15.25 | 0.48 ± 0.13 | 9.32 ± 0.46 | 1.02 ± 0.11 | 1.39 ± 0.28 |
| Materials Name | Asp mg/kg | Thr mg/kg | Ser mg/kg | Asn mg/kg | Glu mg/kg | Gln mg/kg |
|---|---|---|---|---|---|---|
| 1 | 112.47 ± 7.73 | 52.30 ± 10.67 | 157.97 ± 22.08 | 277.83 ± 44.90 | 268.27 ± 24.73 | 614.30 ± 319.12 |
| 2 | 95.47 ± 6.44 | 58.87 ± 1.34 | 129.63 ± 4.20 | 300.23 ± 16.61 | 304.17 ± 40.91 | 802.23 ± 351.67 |
| 3 | 44.60 ± 6.42 | 44.40 ± 2.31 | 78.27 ± 1.81 | 213.97 ± 1.57 | 128.47 ± 15.46 | 560.37 ± 274.27 |
| 4 | 87.27 ± 10.40 | 49.13 ± 8.24 | 83.83 ± 9.50 | 151.27 ± 12.65 | 137.33 ± 9.91 | 423.83 ± 194.77 |
| 5 | 73.33 ± 9.87 | 87.20 ± 19.00 | 178.57 ± 36.59 | 447.13 ± 85.91 | 209.57 ± 28.52 | 1576.80 ± 855.89 |
| 6 | 90.60 ± 4.53 | 59.50 ± 0.95 | 86.90 ± 2.46 | 295.60 ± 15.52 | 289.67 ± 11.60 | 741.57 ± 208.07 |
| 7 | 109.93 ± 4.57 | 147.17 ± 10.74 | 151.60 ± 19.28 | 1005.20 ± 161.82 | 402.20 ± 97.04 | 1461.17 ± 248.25 |
| 8 | 85.63 ± 3.86 | 116.40 ± 20.75 | 139.60 ± 21.01 | 517.67 ± 42.47 | 358.97 ± 59.61 | 2100.13 ± 972.27 |
| 9 | 95.50 ± 18.93 | 115.80 ± 22.88 | 141.03 ± 19.56 | 544.53 ± 115.34 | 278.53 ± 94.63 | 2439.67 ± 600.22 |
| 10 | 72.90 ± 11.39 | 72.20 ± 11.95 | 140.87 ± 10.29 | 317.17 ± 46.59 | 150.57 ± 19.77 | 1489.13 ± 246.59 |
| 11 | 69.13 ± 1.87 | 63.27 ± 4.39 | 116.93 ± 23.65 | 290.30 ± 24.55 | 320.27 ± 43.50 | 1136.87 ± 174.68 |
| 12 | 100.57 ± 20.13 | 86.13 ± 52.27 | 117.33 ± 22.09 | 503.53 ± 455.87 | 381.77 ± 58.50 | 1180.00 ± 110.33 |
| 13 | 89.97 ± 2.18 | 106.13 ± 8.35 | 139.13 ± 7.86 | 515.33 ± 36.29 | 434.10 ± 20.80 | 1656.60 ± 267.49 |
| 14 | 79.83 ± 7.05 | 55.97 ± 1.65 | 131.20 ± 16.53 | 318.93 ± 31.37 | 249.83 ± 10.05 | 1272.73 ± 211.27 |
| 15 | 90.00 ± 7.03 | 100.10 ± 33.20 | 128.80 ± 28.18 | 604.33 ± 394.23 | 332.03 ± 24.23 | 1448.73 ± 754.08 |
| 16 | 83.87 ± 8.33 | 68.70 ± 9.08 | 99.10 ± 7.84 | 314.23 ± 30.06 | 291.13 ± 15.79 | 1134.40 ± 187.84 |
| 17 | 61.17 ± 9.77 | 43.30 ± 12.16 | 96.57 ± 17.00 | 240.13 ± 50.78 | 207.30 ± 10.85 | 612.60 ± 320.16 |
| 18 | 83.93 ± 16.78 | 67.67 ± 10.84 | 215.03 ± 29.24 | 294.67 ± 49.12 | 291.03 ± 17.50 | 862.97 ± 204.12 |
| 19 | 94.53 ± 15.67 | 65.57 ± 4.65 | 182.60 ± 24.56 | 242.53 ± 16.40 | 250.50 ± 19.22 | 790.53 ± 101.86 |
| 20 | 132.10 ± 19.77 | 140.97 ± 15.51 | 146.60 ± 8.34 | 404.63 ± 57.05 | 417.10 ± 61.02 | 1258.53 ± 286.36 |
| 21 | 85.37 ± 4.11 | 71.33 ± 13.17 | 179.57 ± 24.52 | 336.50 ± 68.37 | 123.70 ± 6.22 | 949.53 ± 205.80 |
| 22 | 28.63 ± 10.84 | 122.87 ± 29.61 | 183.63 ± 49.00 | 385.87 ± 80.52 | 113.30 ± 17.61 | 2406.47 ± 547.15 |
| 23 | 35.50 ± 1.49 | 127.03 ± 47.08 | 181.57 ± 35.96 | 435.63 ± 105.84 | 88.17 ± 25.08 | 2480.57 ± 497.30 |
| 24 | 90.53 ± 22.45 | 165.43 ± 33.83 | 264.73 ± 37.07 | 520.97 ± 74.28 | 231.53 ± 16.65 | 2702.27 ± 529.12 |
| 25 | 79.00 ± 8.25 | 40.30 ± 5.66 | 63.43 ± 14.09 | 94.33 ± 41.17 | 81.43 ± 29.41 | 249.97 ± 172.92 |
| 26 | 109.40 ± 7.45 | 66.53 ± 8.95 | 110.43 ± 16.43 | 165.97 ± 43.81 | 174.70 ± 36.70 | 555.70 ± 182.63 |
| 27 | 94.97 ± 12.49 | 51.13 ± 2.21 | 120.33 ± 20.65 | 186.07 ± 35.10 | 175.33 ± 50.75 | 512.23 ± 157.55 |
| 28 | 63.60 ± 13.81 | 51.70 ± 4.50 | 149.63 ± 13.84 | 193.03 ± 13.11 | 294.97 ± 16.66 | 513.97 ± 56.09 |
| 29 | 75.07 ± 12.76 | 52.40 ± 4.40 | 148.10 ± 10.35 | 214.77 ± 14.93 | 252.60 ± 20.13 | 577.33 ± 111.00 |
| 30 | 132.53 ± 14.06 | 72.10 ± 6.71 | 145.17 ± 32.32 | 143.60 ± 26.52 | 188.57 ± 20.57 | 501.97 ± 114.65 |
| 31 | 119.63 ± 8.27 | 79.50 ± 9.04 | 142.20 ± 11.26 | 350.30 ± 60.77 | 251.83 ± 11.05 | 1675.67 ± 313.64 |
| 32 | 67.70 ± 7.84 | 52.00 ± 10.77 | 100.73 ± 14.70 | 260.57 ± 50.15 | 209.27 ± 25.65 | 1282.50 ± 472.54 |
| 33 | 141.03 ± 18.08 | 72.40 ± 7.69 | 252.63 ± 25.10 | 135.07 ± 73.63 | 143.27 ± 14.23 | 638.53 ± 308.68 |
| 34 | 105.07 ± 1.63 | 32.77 ± 0.59 | 87.83 ± 1.65 | 85.77 ± 4.86 | 49.13 ± 1.15 | 314.73 ± 13.32 |
| 35 | 112.17 ± 4.86 | 43.70 ± 1.90 | 73.23 ± 3.06 | 60.40 ± 8.08 | 55.47 ± 8.33 | 213.53 ± 5.91 |
| Materials Name | Pro mg/kg | Gly mg/kg | Ala mg/kg | Val mg/kg | Cys mg/kg | Met |
| 1 | 31.87 ± 6.18 | 22.67 ± 2.52 | 163.33 ± 26.08 | 63.40 ± 16.96 | 8.63 ± 1.80 | mg/kg |
| 2 | 33.90 ± 1.39 | 35.73 ± 2.15 | 285.87 ± 11.63 | 76.40 ± 11.44 | 21.17 ± 3.10 | 10.97 ± 2.15 |
| 3 | 19.87 ± 1.25 | 28.30 ± 2.72 | 137.57 ± 12.40 | 53.23 ± 8.35 | 13.77 ± 2.57 | 15.27 ± 2.45 |
| 4 | 58.90 ± 19.51 | 23.70 ± 2.21 | 155.37 ± 15.30 | 54.57 ± 11.46 | 31.87 ± 1.14 | 9.90 ± 2.46 |
| 5 | 23.00 ± 3.27 | 50.43 ± 8.96 | 272.93 ± 59.59 | 88.27 ± 25.18 | 18.40 ± 1.87 | 10.63 ± 1.86 |
| 6 | 40.33 ± 6.93 | 26.27 ± 0.67 | 124.17 ± 3.82 | 67.77 ± 6.39 | 17.07 ± 2.54 | 20.13 ± 1.47 |
| 7 | 57.30 ± 38.29 | 63.40 ± 5.77 | 410.97 ± 28.07 | 115.17 ± 7.23 | 38.03 ± 13.64 | 11.23 ± 5.33 |
| 8 | 47.87 ± 7.45 | 67.73 ± 14.05 | 546.67 ± 138.05 | 98.57 ± 4.06 | 31.87 ± 6.87 | 15.33 ± 7.98 |
| 9 | 50.77 ± 11.39 | 63.73 ± 9.53 | 510.73 ± 83.99 | 91.13 ± 14.23 | 32.53 ± 5.56 | 18.60 ± 5.96 |
| 10 | 88.70 ± 14.16 | 34.10 ± 2.38 | 351.17 ± 34.10 | 72.30 ± 18.89 | 11.57 ± 3.10 | 20.67 ± 2.97 |
| 11 | 52.03 ± 14.82 | 30.07 ± 4.04 | 160.30 ± 28.95 | 72.77 ± 9.71 | 14.20 ± 2.23 | 15.53 ± 1.85 |
| 12 | 66.13 ± 29.12 | 47.87 ± 4.53 | 392.93 ± 20.80 | 85.10 ± 7.36 | 28.10 ± 2.36 | 15.93 ± 0.71 |
| 13 | 68.83 ± 13.87 | 47.60 ± 2.17 | 379.57 ± 35.07 | 102.30 ± 17.71 | 37.67 ± 9.25 | 17.70 ± 1.65 |
| 14 | 94.33 ± 5.89 | 29.87 ± 1.93 | 246.53 ± 16.51 | 68.30 ± 16.49 | 15.03 ± 2.86 | 18.67 ± 2.06 |
| 15 | 76.73 ± 14.72 | 49.70 ± 12.70 | 407.03 ± 43.30 | 102.77 ± 39.18 | 31.47 ± 12.96 | 16.07 ± 1.59 |
| 16 | 53.07 ± 1.61 | 44.70 ± 2.95 | 290.50 ± 36.68 | 77.77 ± 20.53 | 22.83 ± 3.69 | 20.83 ± 5.25 |
| 17 | 20.97 ± 2.33 | 25.73 ± 4.32 | 167.83 ± 37.02 | 56.33 ± 16.26 | 10.00 ± 1.22 | 16.03 ± 2.29 |
| 18 | 74.10 ± 8.53 | 27.33 ± 1.81 | 199.33 ± 15.51 | 70.13 ± 8.09 | 17.50 ± 2.87 | 11.57 ± 0.95 |
| 19 | 107.77 ± 14.87 | 23.20 ± 2.44 | 144.80 ± 17.97 | 73.83 ± 6.13 | 11.17 ± 1.10 | 13.90 ± 3.59 |
| 20 | 56.60 ± 18.36 | 30.90 ± 7.54 | 220.20 ± 26.20 | 89.97 ± 11.04 | 13.97 ± 3.52 | 20.67 ± 5.14 |
| 21 | 43.23 ± 2.60 | 29.00 ± 3.10 | 177.10 ± 27.27 | 71.60 ± 16.46 | 13.67 ± 3.09 | 19.40 ± 5.60 |
| 22 | 59.97 ± 17.68 | 57.37 ± 9.76 | 278.63 ± 68.74 | 86.30 ± 14.86 | 37.60 ± 4.57 | 17.93 ± 2.50 |
| 23 | 47.83 ± 6.07 | 55.07 ± 8.20 | 185.87 ± 39.44 | 94.10 ± 18.27 | 36.87 ± 7.85 | 14.27 ± 2.58 |
| 24 | 193.23 ± 42.06 | 62.67 ± 9.60 | 471.53 ± 86.63 | 152.97 ± 28.42 | 43.47 ± 9.07 | 16.53 ± 2.41 |
| 25 | 68.20 ± 10.08 | 20.27 ± 6.85 | 112.33 ± 32.81 | 47.47 ± 0.78 | 17.77 ± 6.82 | 21.47 ± 1.70 |
| 26 | 158.50 ± 75.40 | 26.17 ± 5.25 | 161.50 ± 31.28 | 69.43 ± 21.68 | 19.43 ± 4.65 | 13.20 ± 4.76 |
| 27 | 87.37 ± 10.95 | 24.10 ± 6.55 | 155.93 ± 39.14 | 61.37 ± 5.36 | 16.37 ± 6.60 | 17.20 ± 3.11 |
| 28 | 66.33 ± 11.15 | 37.60 ± 7.62 | 244.70 ± 26.85 | 68.17 ± 15.10 | 32.50 ± 8.34 | 14.23 ± 5.66 |
| 29 | 79.87 ± 2.50 | 33.37 ± 2.63 | 192.73 ± 3.45 | 72.53 ± 18.66 | 24.30 ± 4.18 | 15.97 ± 4.05 |
| 30 | 266.67 ± 35.51 | 25.43 ± 6.21 | 319.93 ± 89.40 | 93.00 ± 2.26 | 11.72 ± 1.88 | 16.47 ± 4.30 |
| 31 | 144.07 ± 24.17 | 26.37 ± 3.64 | 254.53 ± 32.16 | 91.07 ± 7.58 | 14.13 ± 2.27 | 29.10 ± 0.46 |
| 32 | 122.10 ± 51.69 | 30.73 ± 7.43 | 242.30 ± 58.57 | 78.73 ± 25.48 | 13.11 ± 3.82 | 23.47 ± 2.30 |
| 33 | 136.23 ± 42.32 | 24.67 ± 3.98 | 228.33 ± 50.16 | 82.83 ± 18.56 | 10.43 ± 3.00 | 18.77 ± 2.97 |
| 34 | 94.43 ± 1.19 | 13.70 ± 0.17 | 91.97 ± 1.79 | 38.43 ± 2.26 | 2.92 ± 0.16 | 30.17 ± 4.35 |
| 35 | 48.27 ± 0.67 | 22.83 ± 1.72 | 112.93 ± 5.28 | 40.70 ± 4.16 | 6.81 ± 1.26 | 18.17 ± 0.46 |
| Materials Name | Ile mg/kg | Leu mg/kg | Tyr mg/kg | Phe mg/kg | γ-Aba mg/kg | His mg/kg |
| 1 | 32.37 ± 6.77 | 36.60 ± 3.26 | 22.63 ± 3.65 | 29.50 ± 5.35 | 46.43 ± 2.48 | 23.57 ± 4.01 |
| 2 | 37.37 ± 1.19 | 47.83 ± 4.94 | 23.57 ± 1.56 | 32.83 ± 5.89 | 51.83 ± 5.05 | 26.17 ± 2.90 |
| 3 | 25.63 ± 1.66 | 27.37 ± 2.45 | 13.83 ± 1.47 | 17.73 ± 2.76 | 108.73 ± 12.37 | 16.07 ± 0.86 |
| 4 | 24.20 ± 3.82 | 30.90 ± 3.47 | 14.27 ± 1.31 | 18.93 ± 3.60 | 206.83 ± 21.25 | 12.13 ± 1.65 |
| 5 | 51.23 ± 10.12 | 39.60 ± 4.06 | 18.37 ± 2.08 | 25.00 ± 4.79 | 170.20 ± 25.24 | 39.60 ± 7.65 |
| 6 | 34.13 ± 2.61 | 39.47 ± 6.77 | 25.27 ± 3.95 | 32.00 ± 3.47 | 55.20 ± 9.44 | 25.67 ± 1.15 |
| 7 | 69.77 ± 6.94 | 49.13 ± 9.46 | 26.43 ± 5.35 | 43.73 ± 1.72 | 188.57 ± 87.05 | 60.57 ± 9.00 |
| 8 | 78.80 ± 14.35 | 53.30 ± 7.99 | 27.67 ± 4.98 | 45.40 ± 9.37 | 200.13 ± 24.35 | 46.43 ± 3.56 |
| 9 | 72.73 ± 15.42 | 55.90 ± 9.77 | 28.07 ± 4.32 | 48.57 ± 12.27 | 205.80 ± 34.57 | 45.13 ± 9.96 |
| 10 | 41.00 ± 4.92 | 40.60 ± 3.97 | 25.50 ± 2.34 | 26.60 ± 4.16 | 199.40 ± 11.51 | 29.77 ± 4.35 |
| 11 | 43.63 ± 1.47 | 48.60 ± 0.52 | 25.07 ± 1.55 | 32.60 ± 2.29 | 22.07 ± 1.70 | 26.37 ± 0.47 |
| 12 | 50.90 ± 13.71 | 46.40 ± 3.25 | 23.30 ± 1.76 | 37.53 ± 10.89 | 46.20 ± 22.45 | 30.63 ± 17.38 |
| 13 | 60.00 ± 7.81 | 52.73 ± 11.85 | 25.87 ± 3.07 | 38.47 ± 1.27 | 23.57 ± 9.45 | 41.07 ± 2.76 |
| 14 | 37.90 ± 4.10 | 44.20 ± 4.67 | 23.30 ± 1.71 | 28.00 ± 1.90 | 142.80 ± 17.75 | 25.53 ± 1.44 |
| 15 | 60.40 ± 16.63 | 55.80 ± 15.49 | 27.60 ± 4.64 | 38.23 ± 7.77 | 87.77 ± 60.49 | 36.57 ± 15.30 |
| 16 | 43.33 ± 3.87 | 43.80 ± 7.33 | 20.93 ± 2.40 | 27.13 ± 4.22 | 107.80 ± 17.82 | 23.43 ± 0.81 |
| 17 | 30.20 ± 7.28 | 32.57 ± 2.27 | 18.83 ± 3.49 | 23.83 ± 6.44 | 23.40 ± 3.48 | 17.90 ± 2.26 |
| 18 | 33.33 ± 1.50 | 44.80 ± 3.22 | 23.20 ± 2.69 | 30.83 ± 3.48 | 80.90 ± 3.76 | 27.33 ± 4.46 |
| 19 | 40.43 ± 2.68 | 61.20 ± 7.71 | 29.77 ± 3.98 | 38.63 ± 3.19 | 133.73 ± 24.16 | 28.50 ± 1.92 |
| 20 | 62.77 ± 13.48 | 55.30 ± 14.25 | 31.37 ± 8.05 | 41.50 ± 12.64 | 58.50 ± 11.26 | 34.33 ± 3.35 |
| 21 | 45.00 ± 7.71 | 45.67 ± 6.72 | 27.73 ± 4.58 | 42.70 ± 12.50 | 144.60 ± 8.20 | 30.23 ± 6.69 |
| 22 | 43.57 ± 12.61 | 33.37 ± 5.30 | 27.13 ± 2.02 | 19.47 ± 2.63 | 154.03 ± 41.45 | 34.87 ± 10.04 |
| 23 | 50.63 ± 13.92 | 40.27 ± 5.29 | 31.17 ± 3.04 | 25.93 ± 3.87 | 150.93 ± 9.03 | 49.23 ± 13.88 |
| 24 | 92.70 ± 19.63 | 55.57 ± 2.73 | 36.20 ± 0.85 | 31.40 ± 2.78 | 205.77 ± 28.22 | 59.30 ± 14.42 |
| 25 | 24.83 ± 5.87 | 44.73 ± 7.16 | 21.93 ± 3.61 | 28.20 ± 3.22 | 181.07 ± 23.26 | 10.53 ± 2.31 |
| 26 | 34.57 ± 5.52 | 57.83 ± 16.61 | 26.60 ± 6.41 | 33.27 ± 9.17 | 217.87 ± 37.31 | 15.23 ± 2.27 |
| 27 | 31.47 ± 3.81 | 48.73 ± 6.20 | 25.07 ± 2.63 | 31.73 ± 1.58 | 165.10 ± 35.96 | 16.43 ± 1.76 |
| 28 | 36.13 ± 4.61 | 50.10 ± 3.56 | 25.80 ± 2.76 | 31.37 ± 3.73 | 110.00 ± 0.98 | 15.77 ± 0.64 |
| 29 | 38.73 ± 2.30 | 56.63 ± 3.30 | 29.57 ± 1.15 | 34.57 ± 2.11 | 155.90 ± 13.20 | 18.20 ± 1.31 |
| 30 | 67.57 ± 6.38 | 81.53 ± 1.45 | 44.13 ± 2.31 | 53.23 ± 10.04 | 349.93 ± 34.92 | 21.17 ± 1.53 |
| 31 | 69.00 ± 5.91 | 73.80 ± 5.73 | 39.20 ± 2.43 | 46.27 ± 7.55 | 262.40 ± 11.00 | 40.33 ± 5.51 |
| 32 | 47.50 ± 11.10 | 47.23 ± 7.35 | 22.23 ± 3.17 | 23.83 ± 5.09 | 161.70 ± 15.60 | 22.90 ± 5.21 |
| 33 | 40.67 ± 7.09 | 73.60 ± 9.93 | 32.93 ± 1.89 | 37.00 ± 3.42 | 357.50 ± 15.78 | 16.63 ± 2.59 |
| 34 | 23.63 ± 0.42 | 43.40 ± 0.46 | 18.83 ± 0.15 | 22.33 ± 4.65 | 214.63 ± 3.98 | 8.81 ± 0.09 |
| 35 | 24.93 ± 3.20 | 52.37 ± 6.01 | 23.87 ± 3.02 | 27.30 ± 4.03 | 209.60 ± 8.70 | 10.00 ± 0.46 |
| Materials Name | Trp mg/kg | Orn mg/kg | Lys mg/kg | Arg mg/kg |
| 1 | 1.71 ± 0.69 | 22.67 ± 4.51 | 48.50 ± 4.04 | 248.00 ± 45.56 |
| 2 | 2.54 ± 0.89 | 13.10 ± 1.48 | 57.07 ± 6.86 | 197.20 ± 19.66 |
| 3 | 1.68 ± 0.58 | 15.50 ± 1.21 | 34.90 ± 2.51 | 150.97 ± 8.46 |
| 4 | 1.58 ± 0.71 | 15.53 ± 1.24 | 37.67 ± 4.04 | 75.00 ± 14.31 |
| 5 | 1.98 ± 0.52 | 40.47 ± 8.81 | 46.27 ± 5.51 | 245.57 ± 34.99 |
| 6 | 3.72 ± 3.31 | 18.30 ± 0.26 | 51.87 ± 7.01 | 114.97 ± 7.11 |
| 7 | 5.64 ± 6.22 | 45.63 ± 1.23 | 66.80 ± 13.20 | 316.33 ± 58.31 |
| 8 | 6.17 ± 4.55 | 27.43 ± 3.32 | 70.60 ± 10.63 | 305.00 ± 16.36 |
| 9 | 4.74 ± 1.70 | 28.57 ± 7.06 | 72.47 ± 9.09 | 332.03 ± 80.39 |
| 10 | 2.32 ± 2.37 | 26.63 ± 2.38 | 52.17 ± 6.07 | 138.67 ± 24.69 |
| 11 | 2.26 ± 1.75 | 17.27 ± 0.49 | 66.17 ± 2.64 | 247.53 ± 2.94 |
| 12 | 2.91 ± 2.74 | 27.07 ± 11.04 | 62.20 ± 4.16 | 225.93 ± 154.65 |
| 13 | 3.36 ± 2.79 | 24.53 ± 2.02 | 68.97 ± 15.29 | 310.43 ± 10.72 |
| 14 | 2.82 ± 3.14 | 22.20 ± 1.28 | 57.93 ± 6.20 | 162.63 ± 11.65 |
| 15 | 2.72 ± 1.02 | 28.83 ± 9.62 | 73.33 ± 19.29 | 277.63 ± 126.87 |
| 16 | 2.10 ± 0.90 | 20.37 ± 0.95 | 59.07 ± 9.15 | 199.73 ± 18.61 |
| 17 | 1.54 ± 0.45 | 10.63 ± 2.87 | 42.73 ± 2.87 | 173.57 ± 41.38 |
| 18 | 4.85 ± 4.53 | 13.63 ± 1.50 | 62.67 ± 7.78 | 190.27 ± 40.20 |
| 19 | 6.37 ± 5.60 | 14.93 ± 1.36 | 78.67 ± 11.04 | 180.67 ± 14.97 |
| 20 | 5.17 ± 4.55 | 23.03 ± 3.59 | 61.23 ± 16.92 | 226.87 ± 30.12 |
| 21 | 3.83 ± 1.29 | 36.37 ± 5.97 | 43.53 ± 37.13 | 185.77 ± 34.63 |
| 22 | 4.46 ± 0.64 | 18.13 ± 7.00 | 51.07 ± 8.37 | 287.20 ± 70.54 |
| 23 | 5.61 ± 0.58 | 20.93 ± 4.90 | 57.30 ± 11.40 | 191.60 ± 55.01 |
| 24 | 5.03 ± 3.20 | 35.90 ± 6.85 | 71.77 ± 4.65 | 451.73 ± 65.35 |
| 25 | 4.90 ± 4.61 | 9.70 ± 2.46 | 57.97 ± 11.29 | 65.90 ± 19.07 |
| 26 | 4.86 ± 3.62 | 22.90 ± 4.13 | 71.43 ± 16.29 | 97.63 ± 10.89 |
| 27 | 4.07 ± 3.44 | 12.73 ± 2.00 | 65.73 ± 8.57 | 146.93 ± 31.30 |
| 28 | 2.24 ± 0.52 | 14.73 ± 0.51 | 65.60 ± 7.07 | 125.13 ± 17.74 |
| 29 | 2.37 ± 0.27 | 14.33 ± 2.25 | 74.53 ± 4.68 | 158.27 ± 35.51 |
| 30 | 7.04 ± 4.85 | 28.27 ± 3.74 | 109.47 ± 9.04 | 90.07 ± 11.84 |
| 31 | 6.01 ± 4.34 | 28.20 ± 2.50 | 102.10 ± 2.95 | 293.40 ± 23.03 |
| 32 | 2.61 ± 1.89 | 23.43 ± 4.67 | 60.07 ± 8.37 | 143.63 ± 26.06 |
| 33 | 4.63 ± 3.83 | 9.63 ± 0.95 | 100.17 ± 12.42 | 128.70 ± 19.64 |
| 34 | 3.32 ± 4.51 | 5.17 ± 0.29 | 47.77 ± 2.37 | 55.07 ± 2.84 |
| 35 | 3.37 ± 4.59 | 4.13 ± 0.43 | 64.70 ± 4.83 | 58.60 ± 4.10 |
References
- Guo, Y. Mutant resource for Chinese cabbage. Nat. Food 2022, 3, 188. [Google Scholar] [CrossRef]
- Li, H.H. Research progress of vegetable quality. North Hortic. 2006, 4, 55–56. [Google Scholar] [CrossRef]
- Gong, Z.P.; Yu, S.C.; Zhang, F.L.; Yu, Y.J.; Zhao, X.Y.; Zhang, D.S.; Wang, W.H.; Su, T.B. Evaluation of Chinese Cabbage Sensory Quality and Its Relationship with Contents of Main Nutrient Components. Agric. Sci. Technol. 2016, 17, 1592–1596. [Google Scholar] [CrossRef]
- Li, J.H.; Xu, M.; Zhang, C. Identification of comprehensive flavor quality of cabbage and its correlation and diameter analysis. J. Henan Agric. Sci. 2008, 5, 93–97. [Google Scholar]
- Qu, S.P.; Cui, C.S.; Zhang, Y.W. Comprehensive flavor quality identification of cabbage and its correlation research. In Proceedings of the 4th Youth Symposium of Chinese Horticultural Society, Harbin, China, 1 August 2000; pp. 487–492. [Google Scholar]
- Wu, C.Y.; He, Q.W.; Song, T.W.; Deng, Y.L.; Wang, C.H.; Xu, W.L. Selection of evaluation indexes in flavor quality of Chinese cabbage. J. Northwest Sci. Tech. Univ. Agric. For. (Nat. Sci. Ed.) 2012, 40, 161–168. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.X.; Wang, G.Y.; Liu, X.T. Analysis on nutrition quality of different varieties of straight cylinder Chinese cabbage. Guangdong Agric. Sci. 2013, 40, 35–37. [Google Scholar] [CrossRef]
- Xu, Y.K.; Gao, F.; Shi, L.; Sun, X.L.; Wang, F.; Xu, S.S.; Zang, Y.X. Evaluation of eight Chinese cabbage cultivars using the membership function method. J. Zhejiang AF Univ. 2018, 35, 845–852. [Google Scholar] [CrossRef]
- Caraveo-Suarez, R.O.; Garcia-Galicia, I.A.; Santellano-Estrada, E.; Carrillo-Lopez, L.M.; Huerta-Jimenez, M.; Alarcon-Rojo, A.D. Integrated multivariate analysis as a tool to evaluate effects of ultrasound on beef quality. J. Food Process Eng. 2023, 46, e14112. [Google Scholar] [CrossRef]
- Nikkhah, A.; Firouzi, S.; El Haj Assad, M.; Ghnimi, S. Application of analytic hierarchy process to develop a weighting scheme for life cycle assessment of agricultural production. Sci. Total Environ. 2019, 665, 538–545. [Google Scholar] [CrossRef]
- Wang, F.; Kang, S.; Du, T.; Li, F.; Qiu, R. Determination of comprehensive quality index for tomato and its response to different irrigation treatments. Agric. Water Manag. 2011, 98, 1228–1238. [Google Scholar] [CrossRef]
- Deng, Z.; Yin, J.; Wu, J.B.; Zhang, H.J. Comprehensive evaluation of water and fertilizer application for Lycium barbarum L. based on AHP and entropy weight method. J. Drain. Irrig. Mach. Eng. 2021, 39, 712–719. [Google Scholar]
- Liu, H.; Li, H.; Ning, H.; Zhang, X.; Li, S.; Pang, J.; Wang, G.; Sun, J. Optimizing irrigation frequency and amount to balance yield, fruit quality and water use efficiency of greenhouse tomato. Agric. Water Manag. 2019, 226, 105787. [Google Scholar] [CrossRef]
- Luo, H.; Li, F. Tomato yield, quality and water use efficiency under different drip fertigation strategies. Sci. Hortic. 2018, 235, 181–188. [Google Scholar] [CrossRef]
- Dusmatova, D.E.; Bobakulov, K.M.; Turgunov, K.K.; Mukhamatkhanova, R.F.; Uzbekov, V.V.; Gildenast, H.; Englert, U.; Sham’yanov, I.D.; Tashkhojaev, B.; Bruskov, V.P.; et al. Guaianolides fromTanacetopsis karataviensis(Kovalevsk.) Kovalevsk. Nat. Prod. Res. 2022, 36, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Rasool, G.; Guo, X.; Wang, Z.; Ali, M.U.; Chen, S.; Zhang, S.; Wu, Q.; Ullah, M.S. Coupling fertigation and buried straw layer improves fertilizer use efficiency, fruit yield, and quality of greenhouse tomato. Agric. Water Manag. 2020, 239, 106239. [Google Scholar] [CrossRef]
- Wang, X.; Duan, Q. Improved AHP-TOPSIS model for the comprehensive risk evaluation of oil and gas pipelines. Pet. Sci. 2019, 16, 1479–1492. [Google Scholar] [CrossRef]
- Fu, L.Y.; Fu, C.Z.; Xing, K.F.; Ying, W.; Yue, P.L.; Hai, Y.Z. Optimal Irrigation and Nitrogen Management Model under Drip Fertigation System Based on Spatial Analysis of Spring Maize in Sandy Soil Area in Ningxia. Trans. Chin. Soc. Agric. Mach. 2019, 50, 219–228. [Google Scholar] [CrossRef]
- Jung-Hoon, K.; Chang-Seob, S.; Seong-Sil, K.; Hyeun-Kyoo, S. Quality Assessment of Ojeok-San, a Traditional Herbal Formula, Using High-Performance Liquid Chromatography Combined with Chemometric Analysis. J. Anal. Methods Chem. 2015, 2015, 607252. [Google Scholar] [CrossRef]
- Ma, H.-L.; Qin, M.-J.; Qi, L.-W.; Wu, G.; Shu, P. Improved quality evaluation of Radix Salvia miltiorrhiza through simultaneous quantification of seven major active components by high-performance liquid chromatography and principal component analysis. Biomed. Chromatogr. 2007, 21, 931–939. [Google Scholar] [CrossRef]
- Su, J.; Zhang, F.; Yang, X.; Feng, Y.; Yang, X.; Wu, Y.; Guan, Z.; Fang, W.; Chen, F. Combining ability, heterosis, genetic distance and their intercorrelations for waterlogging tolerance traits in chrysanthemum. Euphytica 2017, 213, 42. [Google Scholar] [CrossRef]
- Ding, C.; Xu, C.; Lu, B.; Zhu, X.; Luo, X.; He, B.; Elidio, C.; Liu, Z.; Ding, Y.; Yang, J.; et al. Comprehensive Evaluation of Rice Qualities under Different Nitrogen Levels in South China. Foods 2023, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.C.; dos Santos, A.M.P.; Araujo, R.G.O.; Scarminio, I.S.; Bruns, R.E.; Ferreira, S.L.C. Principal component analysis and hierarchical cluster analysis for homogeneity evaluation during the preparation of a wheat flour laboratory reference material for inorganic analysis. Microchem. J. 2010, 95, 222–226. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Yan, H.Y.; Liu, Y.; Xu, Y.; Fang, Y.; Guo, L.P.; Liu, D.H. Analysis and evaluation of mineral elements of Chrysanthemum morifolium for medicinal and tea use of different germplasm resources. China J. Chin. Mater. Med. 2021, 46, 272–280. [Google Scholar] [CrossRef]
- Stellacci, A.M.; Castrignano, A.; Troccoli, A.; Basso, B.; Buttafuoco, G. Selecting optimal hyperspectral bands to discriminate nitrogen status in durum wheat: A comparison of statistical approaches. Environ. Monit. Assess. 2016, 188, 199. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Turner, B.J.; Dury, S.J.; Wallis, I.R.; Foley, W.J. Estimating foliage nitrogen concentration from HYMAP data using continuum removal analysis. Remote Sens. Environ. 2004, 93, 18–29. [Google Scholar] [CrossRef]
- Wang, F.M.; Huang, J.F.; Lou, Z.H. A comparison of three methods for estimating leaf area index of paddy rice from optimal hyperspectral bands. Precis. Agric. 2011, 12, 439–447. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Vicente, R.; Vergara-Diaz, O.; Fernandez-Gallego, J.A.; Kerfal, S.; Lopez, A.; Melichar, J.P.E.; Serret Molins, M.D.; Araus, J.L. Comparative UAV and Field Phenotyping to Assess Yield and Nitrogen Use Efficiency in Hybrid and Conventional Barle. Front. Plant. Sci. 2017, 8, 1733. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.P.; Alarcon, A.; Valbuena, R.I.; Galeano, C.H. Physiological Assessment of Water Stress in Potato Using Spectral Information. Front. Plant Sci. 2017, 8, 1608. [Google Scholar] [CrossRef]
- Huang, K.; Fu, D.; Jiang, Y.; Liu, H.; Shi, F.; Wen, Y.; Cai, C.; Chen, J.; Ou, L.; Yan, Q. Storability and Linear Regression Models of Pericarp Browning and Decay in Fifty Litchi (Litchi chinensis Sonn.) Cultivars at Room Temperature Storage. Foods 2023, 12, 1725. [Google Scholar] [CrossRef]
- GB5009.10-2003; National Standards for Food Safety: Determination of Protein in Food. Ministry of Health of the People’s Republic of China, China National Standardization Management Committee: Beijing, China, 2003.
- GB5009.5-2016; National Standards for Food Safety: Determination of Crude Fiber in Vegetable Foods. National Health and Family Planning Commission of the People’s Republic of China, National Medical Products Administration: Beijing, China, 2016.
- GB5009.268-2016; National Standards for Food Safety: Determination of Multielement in Food. National Health and Family Planning Commission of the People’s Republic of China, National Medical Products Administration: Beijing, China, 2016.
- NY/T2277-2012; Determination of Organic Acids and Anions in Fruits and Vegetables Ion Chromatography. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2012.
- GB5009.86-2016; National Standards for Food Safety: Determination of Ascorbic Acid in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB/T30987-2020; Determination of Free Amino Acids in Plants. Market Supervision Administration, China National Standardization Management Committee: Beijing, China, 2020.
- GB5009.8-2023; National Standards for Food Safety: Determination of Fructose, Glucose, Sucrose, Maltose and Lactose in Food.8. National Health and Family Planning Commission of the People’s Republic of China, Market Supervision Administration: Beijing, China, 2023.
- Song, T.Y.; Hou, X.L.; He, Q.W.; Wu, C.F.; Xu, Y.F. Evaluation of nutritional components of Chinese cabbage, bok choy and choy sum. Shandong Agric. Sci. 2007, 5, 21–22. [Google Scholar] [CrossRef]
- Yu, Z.D. Genetic Analysis of Important Nutrient Quality Characters in Chinese Cabbage and TuMV-Nib Gene Cloning and Transgenic Research. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2004. [Google Scholar] [CrossRef]
- Zhang, B.B.; Cai, Z.X.; Shen, Z.X.; Yan, J.; Ma, R.Z.; Yu, M.L. Diversity analysis of phenotypic characters in germplasm resources of ornamental peaches. Sci. Agric. Sin. 2021, 54, 2406–2418. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.G.; Jian, J.Z.; Shen, Z.Y. Study on Correlation Between Sensory Quality and Nutritional Quality of Chinese Cabbage. Acta Hortic. Sin. 1991, 18, 138–142. [Google Scholar] [CrossRef]
- Aleixandre, A.; Miguel, M. Dietary fiber and blood pressure control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kroemer, G. Dietary fibers affecting gastrointestinal immunity. Trends Immunol. 2023, 44, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Y.; Xu, W.T.; Yang, H.Y.; Li, K.R. Advances in Fructose Rsearch. West China J. Pharm. Sci. 2000, 15, 111–112. [Google Scholar] [CrossRef]
- Shi, Y.G.; Pu, D.N.; Zhou, X.W.; Zhang, Y.Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef]
- Galdón, B.R.; Rodríguez, C.T.; Rodríguez, E.R.; Romero, C.D. Organic acid contents in onion cultivars (Allium cepa L.). J. Agric. Food Chem. 2008, 56, 6512–6519. [Google Scholar] [CrossRef]
- Kalaycioglu, Z.; Kaygusuz, H.; Doker, S.; Kolayli, S.; Erim, F.B. Characterization of Turkish honeybee pollens by principal component analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT-Food Sci. Technol. 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Wang, X.J. Vegetable Breeding Science; China Agriculture Press: Beijing, China, 2001; p. 273. [Google Scholar]
- Bechara, N.; Flood, V.M.; Gunton, J.E. A Systematic Review on the Role of Vitamin C in Tissue Healing. Antioxidants 2022, 11, 1605. [Google Scholar] [CrossRef]
- Collins, N. Nutrition 411: Revisiting vitamin C and wound healing. Ostomy Wound Manag. 2013, 59, 12. [Google Scholar]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Mir-Marqués, A.; Cervera, M.L.; de la Guardia, M. Mineral analysis of human diets by spectrometry methods. Trac-Trends Anal. Chem. 2016, 82, 457–467. [Google Scholar] [CrossRef]
- Negi, J.S.; Singh, P.; Pant, G.J.N.; Rawat, M.S.M. Study on the Variations of Mineral Elements in Swertia speciosa (G. Don). Biol. Trace Elem. Res. 2010, 138, 300–306. [Google Scholar] [CrossRef]
- Mahapatra, S.; Sureja, A.K.; Bhardwaj, R.; Verma, M. Variability in antioxidant capacity and some mineral nutrients among ninety-one Indian accessions of bottle gourd. S. Afr. J. Bot. 2023, 152, 50–62. [Google Scholar] [CrossRef]
- Zheng, S.L.; Shi, Y.T.; Ye, N.X.; Zheng, Q.F.; Wang, F.Q.; Li, Y.H.; Zhang, B.; Wu, B.Q. Diversity analysis of mineral elements in 61 Camellia sinensis germplasm resources. Jiangsu Agric. Sci. 2022, 50, 143–150. [Google Scholar] [CrossRef]
- Chen, M.G.; Lin, X.E.; Li, X.G.; Liu, X.D.; Gao, H.M.; Ming, J.H.; Dai, M.J.; Zhou, Z.X. Comprehensive Evaluation of Durian Quality Based on Principal component Analysis and Cluster Analysis. Sci. Technol. Food Ind. 2023, 44, 278–286. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Yu, J.; Zhu, D.W.; Zheng, X.L.; Meng, L.Q.; Zhu, Z.W.; Shao, Y.F. Nutritional Quality Evaluation of Different Rice Varieties Based on Principal component Analysis and Cluster Analysis. China Rice 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Craine, E.B.; Murphy, K.M. Seed Composition and Amino Acid Profiles for Quinoa Grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef]
- Rajendran, S.; Silcock, P.; Bremer, P. Flavour Volatiles of Fermented Vegetable and Fruit Substrates: A Review. Molecules 2023, 28, 3236. [Google Scholar] [CrossRef]
- Schäfer, M.; Brütting, C.; Baldwin, I.T.; Kallenbach, M. High-throughput quantification of more than 100 primary- and secondary-metabolites, and phytohormones by a single solid-phase extraction based sample preparation with analysis by UHPLC–HESI–MS/MS. Plant. Methods 2016, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Willems, G.; Frerot, H.; Gennen, J.; Salis, P.; Saumitou-Laprade, P.; Verbruggen, N. Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri x Arabidopsis lyrata petraea F-2 progeny grown on cadmium-contaminated soil. New Phytol. 2010, 187, 368–379. [Google Scholar] [CrossRef] [PubMed]
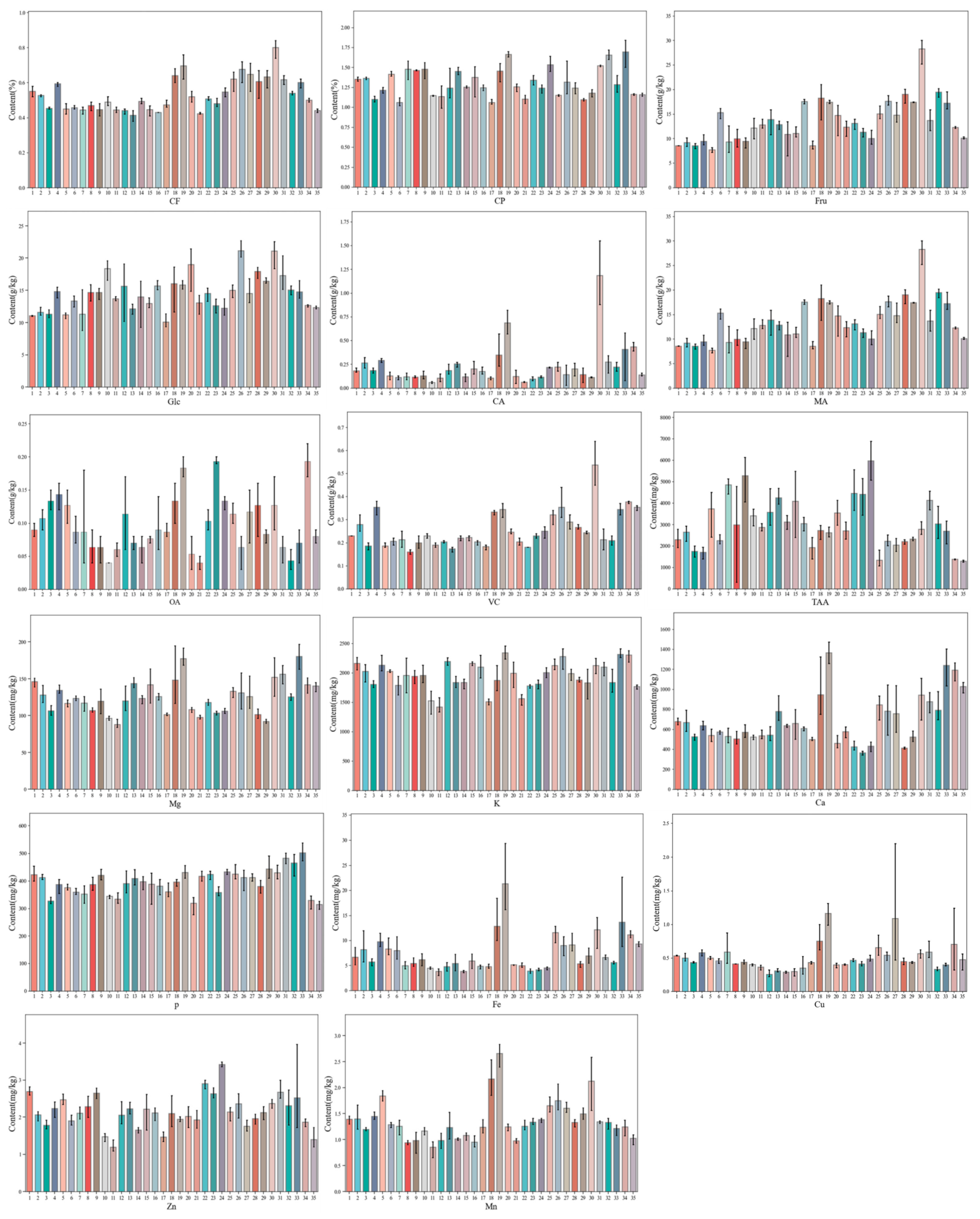
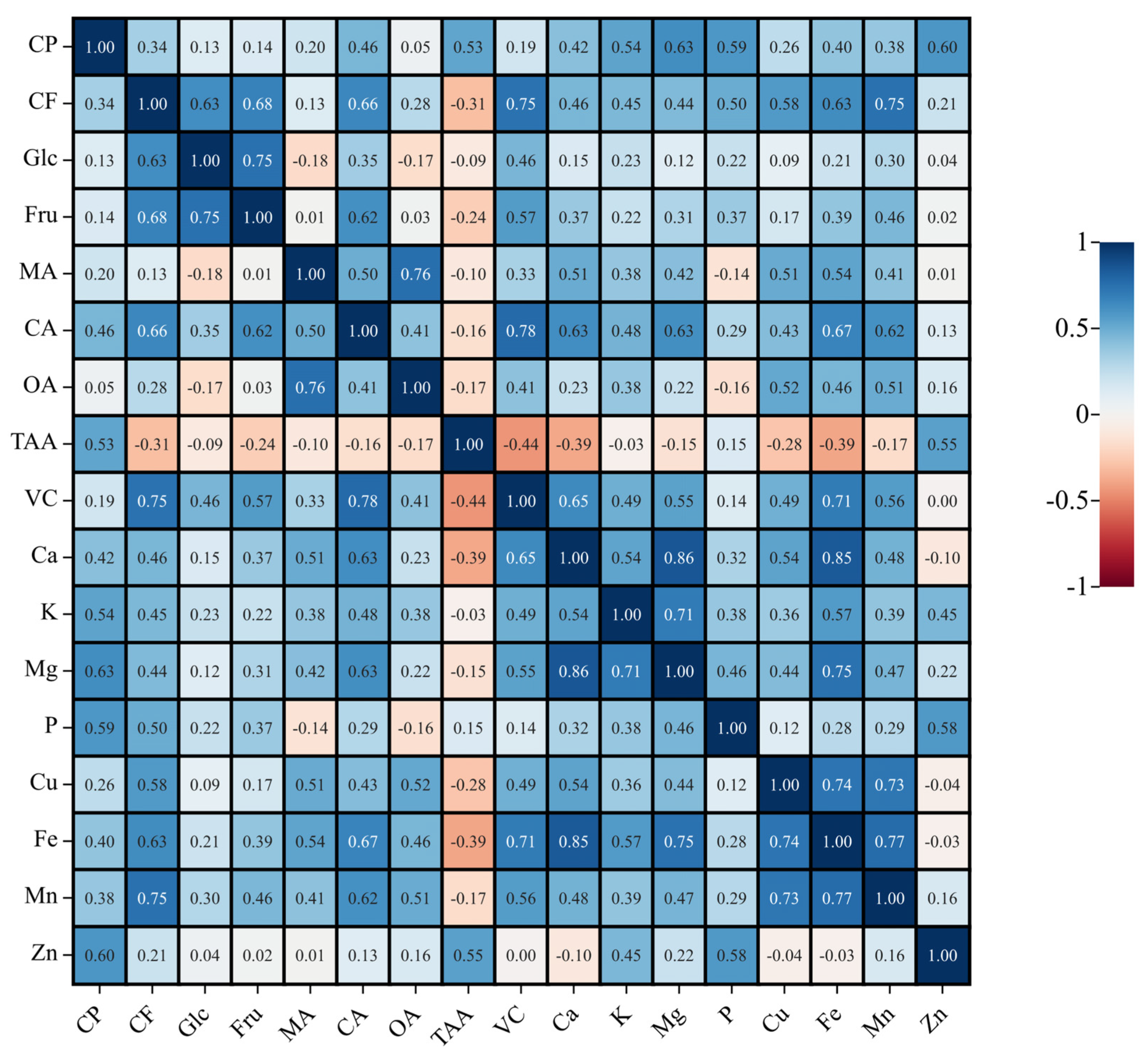
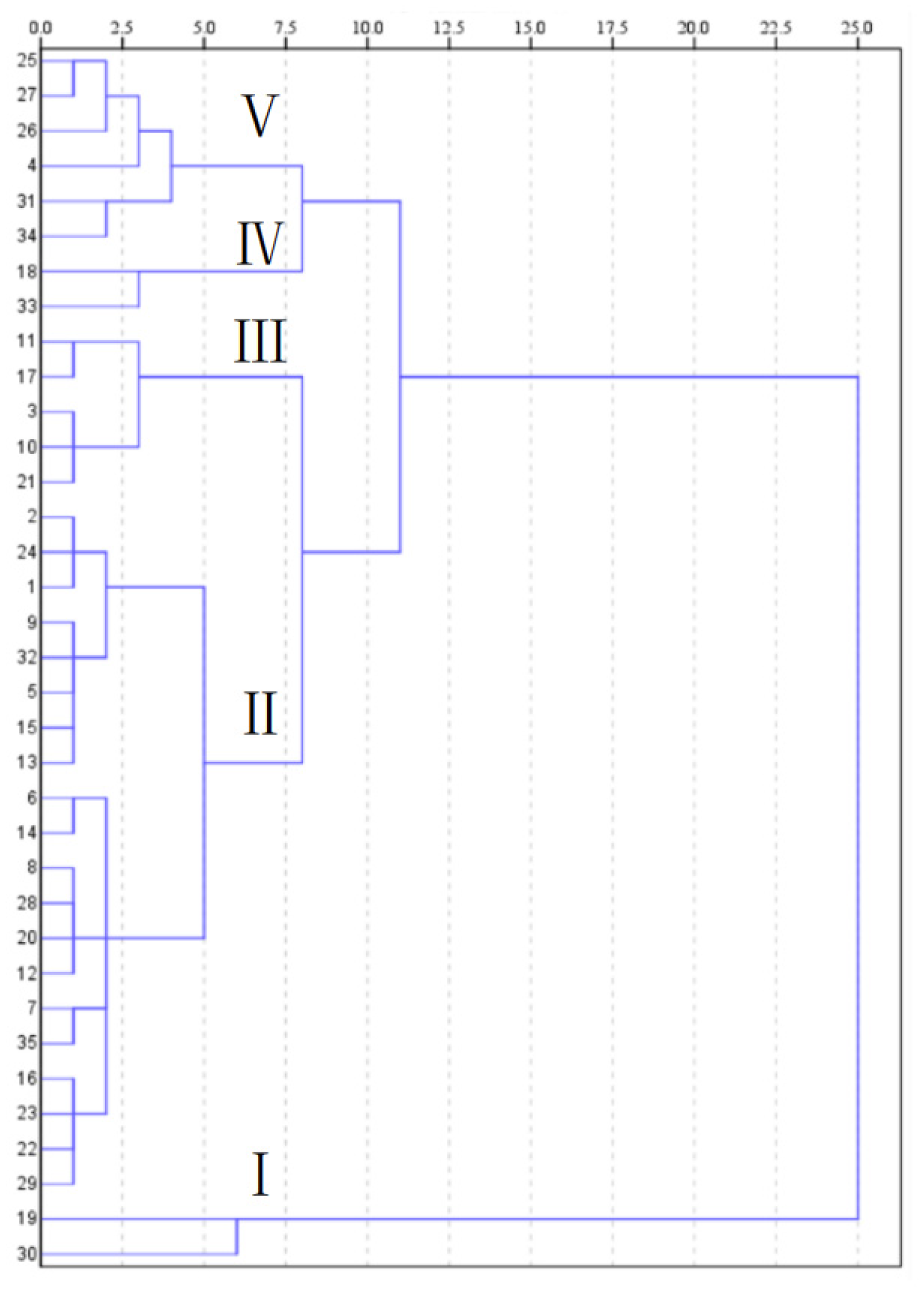
| Index | Range | Min | Max | Mean | SD | SE | CV (%) |
|---|---|---|---|---|---|---|---|
| CP | 6.30 | 10.60 | 16.90 | 13.13 | 1.78 | 0.30 | 13.53 |
| CF | 3.80 | 4.20 | 8.00 | 5.30 | 0.95 | 0.16 | 17.86 |
| Glc | 11.05 | 10.09 | 21.14 | 14.52 | 2.73 | 0.46 | 18.83 |
| Fru | 20.54 | 7.72 | 28.26 | 13.40 | 4.30 | 0.73 | 32.08 |
| MA | 2.21 | 0.22 | 2.43 | 0.84 | 0.46 | 0.08 | 54.34 |
| CA | 1.12 | 0.06 | 1.18 | 0.23 | 0.21 | 0.03 | 91.47 |
| OA | 0.16 | 0.04 | 0.20 | 0.10 | 0.04 | 0.01 | 41.97 |
| VC | 0.38 | 0.16 | 0.54 | 0.25 | 0.08 | 0.01 | 31.26 |
| TAA | 4.60 | 1.29 | 5.89 | 3.08 | 1.21 | 0.20 | 39.25 |
| Ca | 998.28 | 366.33 | 1364.61 | 684.26 | 243.29 | 41.12 | 35.56 |
| K | 922.4 | 1420.33 | 2342.73 | 1952.19 | 45.24 | 39.29 | 11.45 |
| Mg | 92.75 | 87.71 | 180.46 | 125.04 | 0.19 | 3.78 | 39.08 |
| P | 188.20 | 313.94 | 502.14 | 394.98 | 232.44 | 0.11 | 11.91 |
| Cu | 0.91 | 0.25 | 1.16 | 0.50 | 3.68 | 0.03 | 49.83 |
| Fe | 17.52 | 3.82 | 21.34 | 7.38 | 22.36 | 0.62 | 17.89 |
| Mn | 1.81 | 0.85 | 2.66 | 1.35 | 0.38 | 0.06 | 28.38 |
| Zn | 2.22 | 1.20 | 3.42 | 2.14 | 0.45 | 0.08 | 21.02 |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| CP | 0.072 | 0.260 | 0.116 |
| CF | 0.111 | 0.023 | 0.174 |
| Glc | 0.054 | 0.056 | 0.327 |
| Fru | 0.079 | 0.018 | 0.288 |
| MA | 0.072 | 0.120 | 0.270 |
| CA | 0.114 | 0.003 | 0.029 |
| OA | 0.066 | 0.130 | 0.231 |
| TAA | 0.038 | 0.274 | 0.133 |
| VC | 0.111 | 0.087 | 0.103 |
| Ca | 0.110 | 0.054 | 0.037 |
| K | 0.095 | 0.099 | 0.100 |
| Mg | 0.108 | 0.063 | 0.085 |
| P | 0.060 | 0.250 | 0.074 |
| Cu | 0.094 | 0.102 | 0.092 |
| Fe | 0.123 | 0.077 | 0.047 |
| Mn | 0.110 | 0.025 | 0.006 |
| Zn | 0.028 | 0.294 | 0.110 |
| Eigenvalue | 7.355 | 2.713 | 2.291 |
| Variance contribution rate | 43.266 | 15.960 | 13.478 |
| Cumulative contribution rate | 43.266 | 60.600 | 3.740 |
| Variety | CI(1) | CI(2) | CI(3) | U(1) | U(2) | U(3) | D Value |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 0.78 | 1.46 | 0.31 | 0.28 | 0.32 | 0.30 |
| 2 | 0.17 | −0.18 | 1.04 | 0.33 | 0.27 | 0.31 | 0.31 |
| 3 | −2.13 | −2.35 | 1.21 | 0.13 | 0.09 | 0.27 | 0.15 |
| 4 | 1.33 | −1.05 | 0.26 | 0.34 | 0.21 | 0.45 | 0.33 |
| 5 | −0.77 | 0.72 | 2.27 | 0.28 | 0.32 | 0.28 | 0.29 |
| 6 | −1.42 | −1.39 | −0.45 | 0.18 | 0.17 | 0.28 | 0.19 |
| 7 | −1.69 | 0.82 | 1.45 | 0.24 | 0.34 | 0.22 | 0.26 |
| 8 | −2.20 | 1.55 | 0.27 | 0.17 | 0.31 | 0.25 | 0.21 |
| 9 | −1.55 | 2.69 | 0.98 | 0.23 | 0.44 | 0.26 | 0.28 |
| 10 | −2.84 | −0.99 | −2.07 | 0.11 | 0.20 | 0.23 | 0.15 |
| 11 | −3.65 | −1.98 | −1.00 | 0.06 | 0.14 | 0.17 | 0.10 |
| 12 | −1.28 | 0.23 | 0.01 | 0.17 | 0.20 | 0.41 | 0.22 |
| 13 | −1.03 | 1.30 | 1.01 | 0.31 | 0.30 | 0.24 | 0.29 |
| 14 | −2.08 | −0.03 | −0.59 | 0.18 | 0.22 | 0.24 | 0.20 |
| 15 | −0.98 | 1.38 | 1.11 | 0.27 | 0.31 | 0.31 | 0.29 |
| 16 | −1.11 | 0.20 | −0.37 | 0.18 | 0.21 | 0.40 | 0.23 |
| 17 | −3.08 | −2.25 | 0.27 | 0.09 | 0.14 | 0.13 | 0.11 |
| 18 | 3.56 | −0.63 | −0.26 | 0.55 | 0.32 | 0.52 | 0.49 |
| 19 | 8.45 | −1.31 | 2.31 | 0.85 | 0.48 | 0.77 | 0.76 |
| 20 | −1.46 | −0.08 | −1.51 | 0.17 | 0.17 | 0.39 | 0.21 |
| 21 | −3.06 | −0.36 | −1.03 | 0.09 | 0.26 | 0.13 | 0.13 |
| 22 | −1.37 | 1.62 | 0.44 | 0.16 | 0.35 | 0.33 | 0.24 |
| 23 | −1.48 | 0.01 | 2.22 | 0.14 | 0.30 | 0.42 | 0.23 |
| 24 | −0.46 | 3.03 | 1.82 | 0.25 | 0.45 | 0.36 | 0.31 |
| 25 | 2.09 | −1.11 | −0.65 | 0.37 | 0.26 | 0.47 | 0.36 |
| 26 | 2.13 | 0.73 | −2.83 | 0.38 | 0.26 | 0.54 | 0.38 |
| 27 | 1.82 | −1.43 | −0.48 | 0.33 | 0.40 | 0.44 | 0.36 |
| 28 | −0.60 | −0.98 | −2.15 | 0.14 | 0.19 | 0.50 | 0.22 |
| 29 | −0.42 | 0.05 | −2.14 | 0.17 | 0.28 | 0.42 | 0.24 |
| 30 | 7.07 | 0.44 | −3.09 | 0.74 | 0.32 | 0.79 | 0.66 |
| 31 | 1.81 | 3.11 | −0.07 | 0.43 | 0.47 | 0.45 | 0.44 |
| 32 | −0.42 | 1.48 | −1.96 | 0.26 | 0.32 | 0.31 | 0.28 |
| 33 | 3.83 | 2.51 | −0.41 | 0.63 | 0.37 | 0.45 | 0.54 |
| 34 | 2.99 | −3.51 | 2.80 | 0.45 | 0.15 | 0.59 | 0.41 |
| 35 | −0.46 | −3.01 | 0.14 | 0.34 | 0.06 | 0.23 | 0.26 |
| Index | I | II | III | IV | V |
|---|---|---|---|---|---|
| CP | 15.90 | 15.75 | 12.93 | 13.17 | 11.10 |
| CF | 7.50 | 6.20 | 6.12 | 4.93 | 4.56 |
| Glc | 18.48 | 15.42 | 15.87 | 13.93 | 13.30 |
| Fru | 22.84 | 17.73 | 13.77 | 12.55 | 10.87 |
| MA | 1.73 | 0.82 | 0.96 | 0.76 | 0.68 |
| CA | 0.93 | 0.38 | 0.26 | 0.16 | 0.11 |
| OA | 0.16 | 0.10 | 0.12 | 0.09 | 0.07 |
| VC | 0.44 | 0.34 | 0.32 | 0.22 | 0.20 |
| TAA | 2.70 | 2.71 | 2.14 | 3.58 | 2.53 |
| Ca | 1153.17 | 1091.79 | 847.72 | 585.77 | 531.49 |
| K | 2235.44 | 2095.42 | 2156.55 | 1945.40 | 1563.52 |
| Mg | 164.60 | 164.29 | 137.10 | 120.31 | 97.95 |
| P | 430.14 | 448.97 | 408.25 | 391.71 | 356.44 |
| Cu | 0.86 | 0.57 | 0.69 | 0.42 | 0.40 |
| Fe | 16.74 | 13.26 | 9.55 | 5.87 | 4.75 |
| Mn | 2.39 | 1.69 | 1.51 | 1.24 | 1.08 |
| Zn | 2.15 | 2.31 | 2.17 | 2.26 | 1.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Ye, X.; Liu, G.; Zhang, S.; Li, G.; Zhang, H.; Li, F.; Sun, R.; Wang, C.; Xu, D.; et al. Comprehensive Evaluation of Nutritional Qualities of Chinese Cabbage (Brassica rapa ssp. pekinensis) Varieties Based on Multivariate Statistical Analysis. Horticulturae 2023, 9, 1264. https://doi.org/10.3390/horticulturae9121264
Song C, Ye X, Liu G, Zhang S, Li G, Zhang H, Li F, Sun R, Wang C, Xu D, et al. Comprehensive Evaluation of Nutritional Qualities of Chinese Cabbage (Brassica rapa ssp. pekinensis) Varieties Based on Multivariate Statistical Analysis. Horticulturae. 2023; 9(12):1264. https://doi.org/10.3390/horticulturae9121264
Chicago/Turabian StyleSong, Chao, Xinyu Ye, Guangyang Liu, Shifan Zhang, Guoliang Li, Hui Zhang, Fei Li, Rifei Sun, Chenggang Wang, Donghui Xu, and et al. 2023. "Comprehensive Evaluation of Nutritional Qualities of Chinese Cabbage (Brassica rapa ssp. pekinensis) Varieties Based on Multivariate Statistical Analysis" Horticulturae 9, no. 12: 1264. https://doi.org/10.3390/horticulturae9121264
APA StyleSong, C., Ye, X., Liu, G., Zhang, S., Li, G., Zhang, H., Li, F., Sun, R., Wang, C., Xu, D., & Zhang, S. (2023). Comprehensive Evaluation of Nutritional Qualities of Chinese Cabbage (Brassica rapa ssp. pekinensis) Varieties Based on Multivariate Statistical Analysis. Horticulturae, 9(12), 1264. https://doi.org/10.3390/horticulturae9121264











