Acidovorax citrulli Type IV Pili PilR Interacts with PilS and Regulates the Expression of the pilA Gene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. Construction of the pilR and pilS Mutants and their Complementary Strains
2.3. Pathogenicity Assay
2.4. Assays of Twitching and Swimming Motility Assays
2.5. Biofilm Formation Assay
2.6. In Vitro Growth Assay
2.7. Quantitative Reverse Transcription PCR (qRT-PCR) Analysis of Gene Expression
2.8. Assay for pilA Promoter Activity
2.9. Yeast Two-Hybrid Assay
2.10. Glutathione S-Transferase (GST) Pull-Down Assay
2.11. Luciferase Complementation Imaging (LCI) Assay
2.12. Statistical Analysis
3. Results
3.1. Successful Construction of Mutants and Complementary Strains
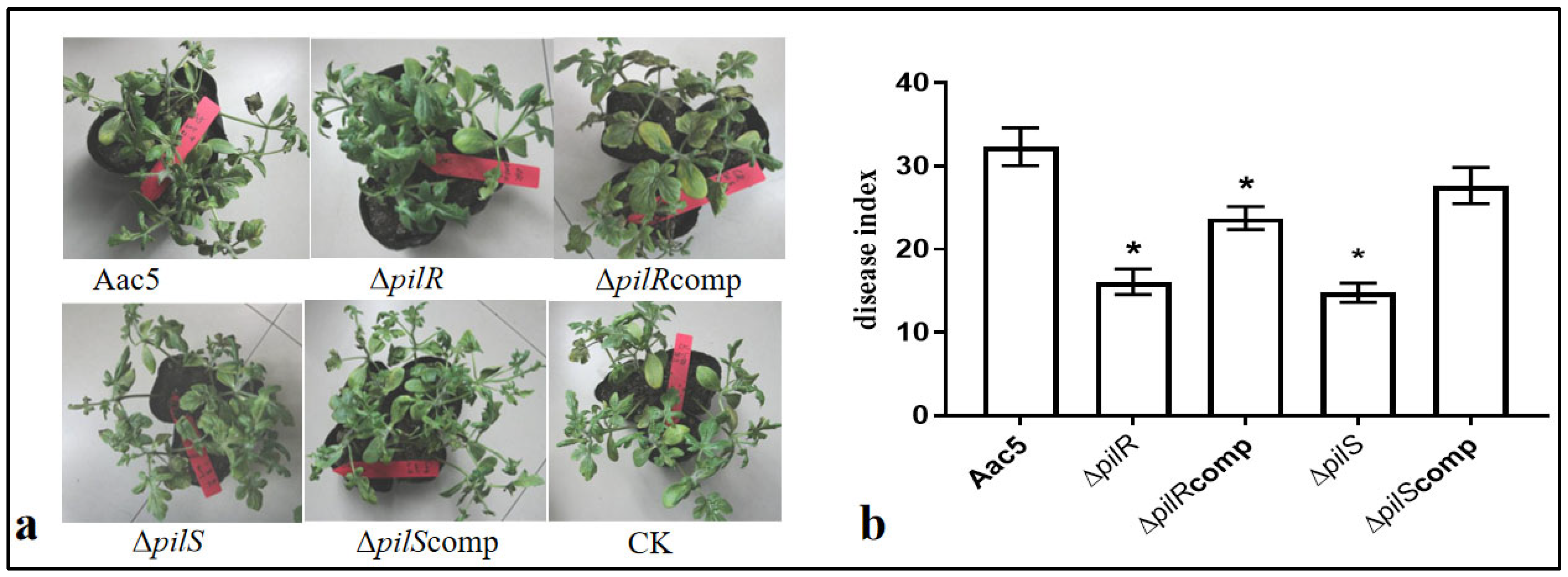
3.2. pilR and pilS Genes Are Required for Virulence in Watermelon
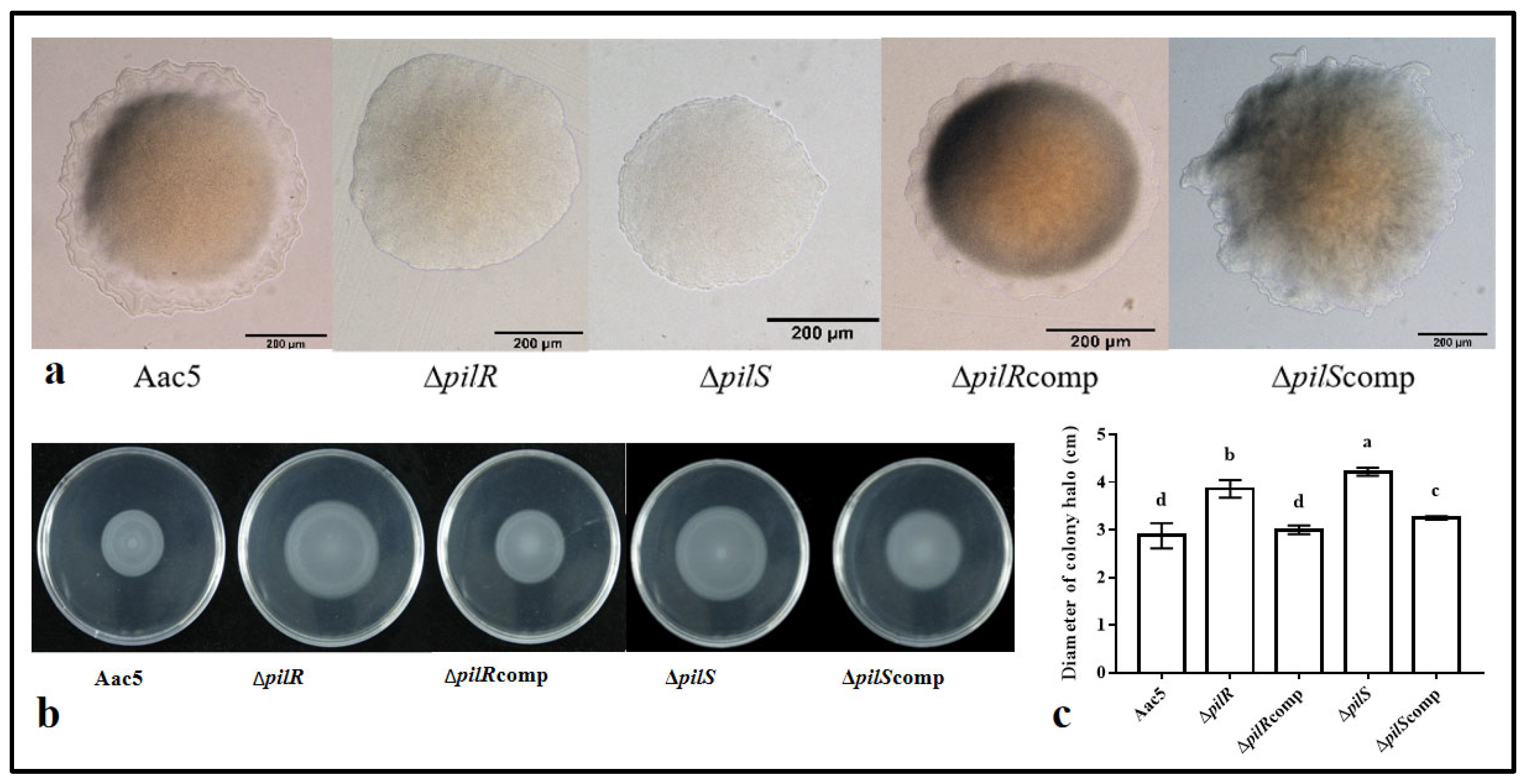
3.3. The Effects of the Deletion of pilR and pilS on the Motility of A. citrulli
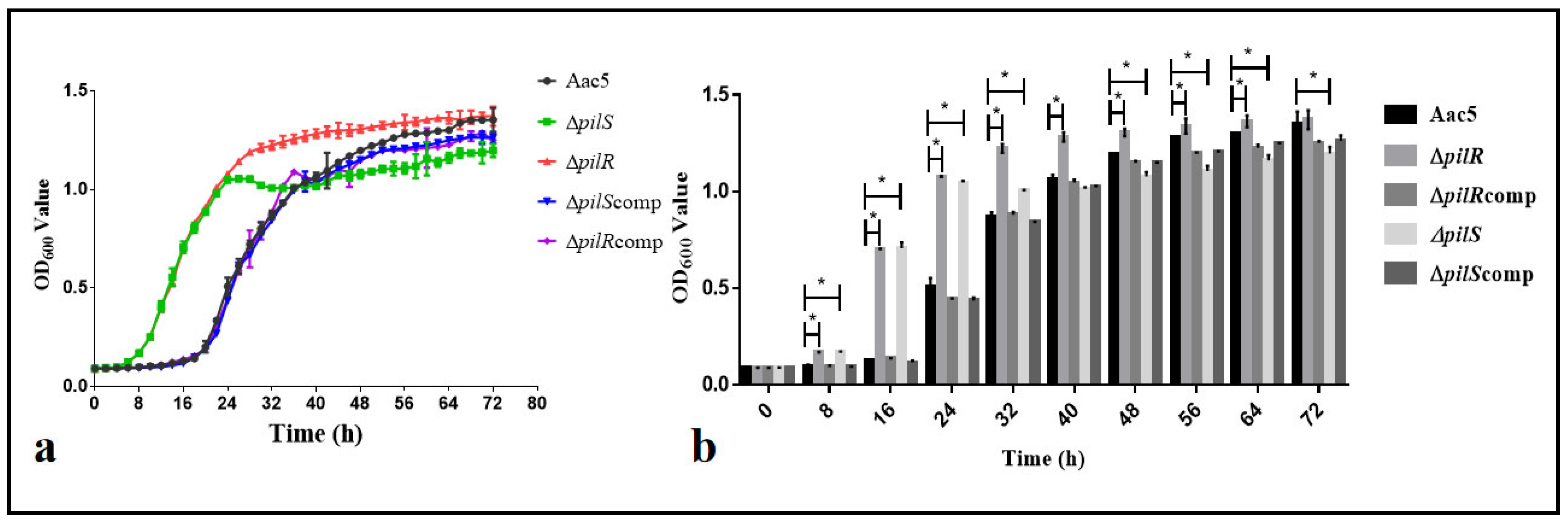
3.4. Deletion of pilR and pilS Enhanced the Biofilm Formation of A. citrulli
3.5. The Deletion of the pilR or pilS Genes Enhanced the Early Growth Ability of A. citrulli In Vitro
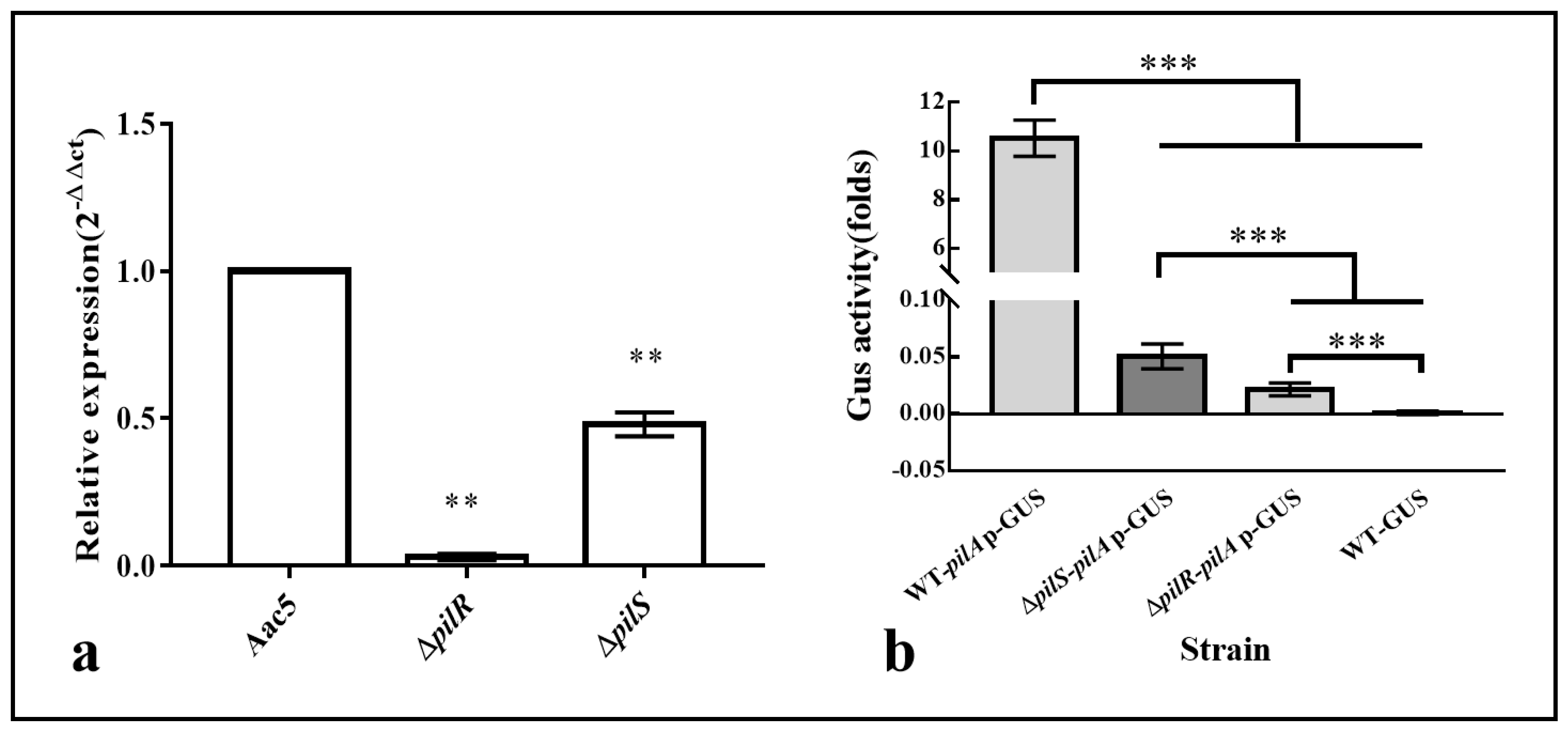
3.6. The Deletion of pilR and pilS Affects the Transcription of pilA
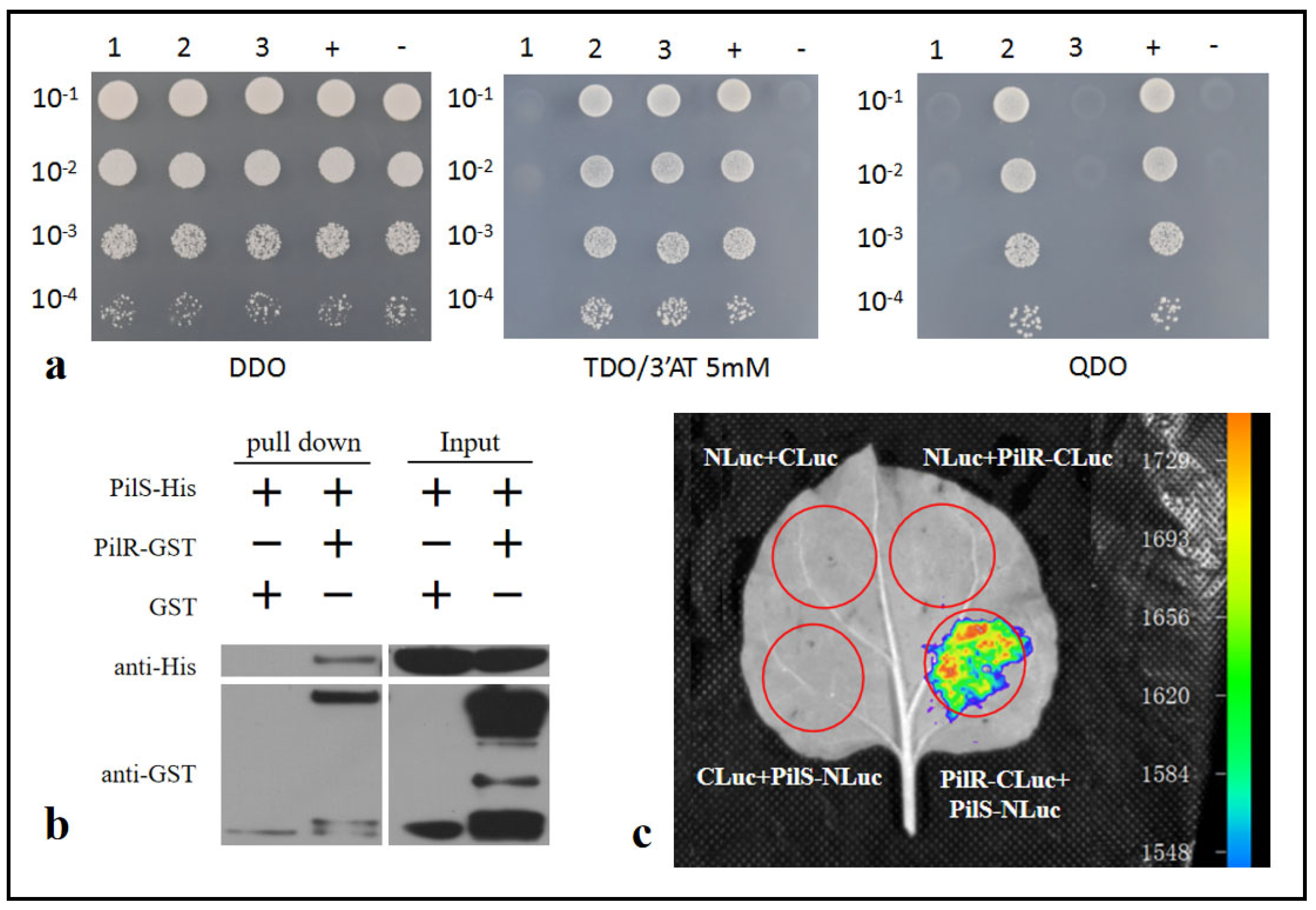
3.7. PilR Interacts with PilS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaad, N.W.; Postnikova, E.; Sechler, A.; Claflin, L.E.; Vidaver, A.K.; Jones, J.B.; Agarkova, I.; Ignatov, A.; Dickstein, E.; Ramundo, B.A. Reclassification of subspecies of Acidovorax avenae as A. avenae (Manns 1905) emend., A. cattleyae (Pavarino, 1911) comb. nov., A. citrulli (Schaad et al., 1978) comb. nov., and proposal of A. oryzae sp. nov. Syst. Appl. Microbiol. 2008, 31, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.E.; Goth, R.W. A seedborne bacterium isolated from watermelon. Plant Dis. Rep. 1965, 49, 818–821. [Google Scholar]
- Wall, G.C.; Santos, V.M. A new bacterial disease on watermelon in the Mariana Islands. Phytopathology 1988, 78, 1605. [Google Scholar]
- Evans, T.A.; Mulrooney, R.P. First report of watermelon fruit blotch in Delaware. Plant Dis. 1991, 75, 1074. [Google Scholar] [CrossRef]
- Black, M.C.; Isakeit, T.; Barnes, L.W. First report of bacterial fruit blotch of watermelon in Texas. Plant Dis. 1994, 78, 831. [Google Scholar] [CrossRef]
- O’Brien, R.G.; Martin, H.L. Bacterial blotch of melons caused by strains of Acidovorax avenae subsp. citrulli. Aust. J. Exp. Agric. 1999, 39, 479–485. [Google Scholar] [CrossRef]
- Burdman, S.; Kots, N.; Kritzman, G.; Kopelowitz, J. Molecular, physiological, and host-range characterization of Acidovorax avenae subsp. citrulli isolates from watermelon and melon in Israel. Plant Dis. 2005, 89, 1339–1347. [Google Scholar] [CrossRef]
- Ren, Y.Z.; Li, H.; Li, G.Y.; Wang, Q.Y.; Li, J.Q. First report of Acidovorax avenae subsp. citrulli infecting edible seed watermelon (Citrullus lanatus var. lanatus) in China. Plant Dis. 2006, 90, 1112. [Google Scholar] [CrossRef]
- Burdman, S.; Bahar, O.; Parker, J.K.; Fuente, L.D.L. Involvement of Type IV Pili in Pathogenicity of Plant Pathogenic Bacteria. Genes 2011, 2, 706–735. [Google Scholar] [CrossRef]
- Kang, Y.W.; Liu, H.L.; Genin, S.; Schell, M.A.; Denny, T.P. Ralstonia solanacearum requires type 4 pili to adhere to multiple surfaces and for natural transformation and virulence. Mol. Microbiol. 2002, 46, 427–437. [Google Scholar] [CrossRef]
- Taguchi, F.; Ichinose, Y. Role of Type IV Pili in Virulence of Pseudomonas syringae pv. tabaci 6605: Correlation of Motility, Multidrug Resistance, and HR-Inducing Activity on a Nonhost Plant. Mol. Plant Microbe Interact. 2011, 24, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, Y.; Ryan, R.P.; O’Donovan, K.; He, Y.Q.; Jiang, B.L.; Feng, J.X.; Tang, J.L.; Dow, J.M. The role of PilZ domain proteins in the virulence of Xanthomonas campestris pv. campestris. Mol. Plant Pathol. 2008, 9, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Roine, E.; Raineri, D.M.; Romantschuk, M.; Wilson, M.; Nunn, D.N. Characterization of type IV pilus genes in Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 1998, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Bahar, O.; De, L.; Fuente, L.; Burdman, S. Assessing adhesion, biofilm formation and motility of Acidovorax citrulli using microfluidic flow chambers. FEMS Microbiol. Lett. 2010, 312, 33–39. [Google Scholar] [CrossRef]
- Tally, R.; Bolaji, B.S.; Saul, B. Association Between Loss of Type IV Pilus Synthesis Ability and Phenotypic Variation in the Cucurbit Pathogenic Bacterium Acidovorax citrulli. Mol. Plant Microbe Interact. 2018, 31, 548–559. [Google Scholar]
- Williams, R.H.; Whitworth, D.E. The genetic organisation of prokaryotic two-component system signalling pathways. BMC Genom. 2010, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Petrova, Q.E.; Sauer, K. A novel signaling network essential for regulting Pseudomonas aeruginosa biofilm development. PLoS Pathog. 2009, 91, e1000668. [Google Scholar]
- Cha, J.Y.; Lee, D.G.; Lee, J.S.; Oh, J.I.; Baik, H.S. GacA directly regulates experssion of several of virulence genes in Pseudomonas syringae pv. tabaci 11528. Biochem. Biophys. Res. Commun. 2012, 417, 665–672. [Google Scholar] [CrossRef]
- Hobbs, M.; Collie, E.S.; Free, P.D.; Livingston, S.P.; Mattick, J.S. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 1993, 7, 669–682. [Google Scholar] [CrossRef]
- Boyd, J.M.; Lory, S. Dual function of pilS during transcriptional activation of the Pseudomonas aeruginosa pilin subunit gene. J. Bacteriol. 1996, 178, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Kaiser, D. Regulation of expression of the pilA gene in Myxococcus xanthus. J. Bacteriol. 1997, 179, 7748–7758. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.J.; West, T.; Engel, J.N. Genetic analysis of the regulation of type IV pilus function by the Chp chemosensory system of Pseudomonas aeruginosa. J. Bacteriol. 2010, 192, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Carmen, L.; Giltner, Y.N.; Lori, L.B. Type IV Pilin Proteins: Versatile Molecular Modules. Microbiol. Mol. Biol. Rev. 2012, 76, 740. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Ji, W.; Zhao, M.; Fei, N.; Yang, L.; Qiao, P.; Walcott, R.; Yang, Y.; Zhao, T. Essential Acidovorax citrulli Virulence Gene hrpE Activates Host Immune Response against Pathogen. Int. J. Mol. Sci. 2022, 23, 9144. [Google Scholar] [CrossRef] [PubMed]
- Bahar, O.; Goffer, T.; Burdman, S. Type IV Pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. citrulli. Mol. Plant Microbe Interact. 2009, 22, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, M.; Qiao, P.; Li, Z.; Chen, G.; Guan, W.; Bai, Q.; Walcott, R.; Yang, Y.; Zhao, T. ntrC Contributes to Nitrogen Utilization, Stress Tolerance, and Virulence in Acidovorax citrulli. Microorganisms 2023, 11, 767. [Google Scholar] [CrossRef]
- Fei, N.; Ji, W.; Yang, L.; Yu, C.; Qiao, P.; Yan, J.; Guan, W.; Yang, Y.; Zhao, T. Hcp of the Type VI Secretion System (T6SS) in Acidovorax citrulli Group II Strain Aac5 Has a Dual Role as a Core Structural Protein and an Effector Protein in Colonization, Growth Ability, Competition, Biofilm Formation, and Ferric Iron Absorption. Int. J. Mol. Sci. 2022, 23, 9632. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the (2−ΔΔCT) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, P.; Wang, T.; Ji, W.; Fei, N.; Zhang, L.; Guan, W.; Zhao, T. Further Characterization of Host Preference of Acidovorax citrulli Based on Growth Competition between Group I and Group II Strains. Horticulturae 2022, 8, 1173. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wei, C.; Jiang, W.D.; Wang, L.; Li, C.R.; Wang, Y.Y. The HD-GYP domain protein RpfG of Xanthomonas oryzae pv. oryzicola regulates synthesis of extracellular polysaccharides that contribute to biofilm formation and virulence on rice. PLoS ONE 2013, 8, e59428. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Zou, Y.; Shang, Y.L.; Lin, H.Q.; Wang, Y.J.; Cai, R.; Tang, X.Y.; Zhou, J.M. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008, 146, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Bi, G.Z.; Zhou, J.M. Luciferase complementation assay for protein-protein interactions in plants. Curr. Protoc. Plant Biol. 2018, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhao, M.; Zhang, X.; Yang, L.; Fei, N.; Ji, W.; Guan, W.; Walcott, R.; Yang, Y.; Zhao, T. Acidovorax citrulli Effector AopV Suppresses Plant Immunity and Interacts with Aromatic Dehydratase ADT6 in Watermelon. Int. J. Mol. Sci. 2022, 23, 11719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Hao, G.X.; Galvani, C.D.; Meng, Y.Z.; Dela, F.L.; Hoch, H.C.; Burr, T.J. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiology 2007, 153, 719–726. [Google Scholar] [CrossRef]
- Yu, C.; Nguyen, D.; Ren, Z.; Liu, J.; Yang, F.; Tian, F.; Fan, S.; Chen, H. The RpoN2-PilRX regulatory system governs type IV pilus gene transcription and is required for bacterial motility and virulence in Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 2020, 21, 652–666. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, N.; Ji, W.; Qiao, P.; Yang, L.; Liu, D.; Guan, W.; Zhao, T. pilA Gene Contributes to Virulence, Motility, Biofilm Formation, and Interspecific Competition of Bacteria in Acidovorax citrulli. Microorganisms 2023, 11, 1806. [Google Scholar] [CrossRef]
- Yang, Y.; Darbari, V.C.; Zhang, N.; Lu, D.; Glyde, R.; Wang, Y.P.; Winkelman, J.T.; Gourse, R.L.; Murakami, K.S.; Buck, M.; et al. Structures of the RNA polymerase-σ54 reveal new and conserved regulatory strategies. Science 2015, 349, 882–885. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Ji, W.; Qiao, P.; Fei, N.; Yang, L.; Guan, W.; Zhao, T. Acidovorax citrulli Type IV Pili PilR Interacts with PilS and Regulates the Expression of the pilA Gene. Horticulturae 2023, 9, 1296. https://doi.org/10.3390/horticulturae9121296
Yang Y, Ji W, Qiao P, Fei N, Yang L, Guan W, Zhao T. Acidovorax citrulli Type IV Pili PilR Interacts with PilS and Regulates the Expression of the pilA Gene. Horticulturae. 2023; 9(12):1296. https://doi.org/10.3390/horticulturae9121296
Chicago/Turabian StyleYang, Yuwen, Weiqin Ji, Pei Qiao, Nuoya Fei, Linlin Yang, Wei Guan, and Tingchang Zhao. 2023. "Acidovorax citrulli Type IV Pili PilR Interacts with PilS and Regulates the Expression of the pilA Gene" Horticulturae 9, no. 12: 1296. https://doi.org/10.3390/horticulturae9121296
APA StyleYang, Y., Ji, W., Qiao, P., Fei, N., Yang, L., Guan, W., & Zhao, T. (2023). Acidovorax citrulli Type IV Pili PilR Interacts with PilS and Regulates the Expression of the pilA Gene. Horticulturae, 9(12), 1296. https://doi.org/10.3390/horticulturae9121296




