Black Rot of Grapes (Guignardia bidwellii)—A Comprehensive Overview
Abstract
:1. Introduction
2. Classification, Nomenclature, Emergence and Distribution of Black Rot
3. Genetic Diversity and Population Biology of Black Rot Agent
4. Disease Cycle and Environmental Conditions for Development
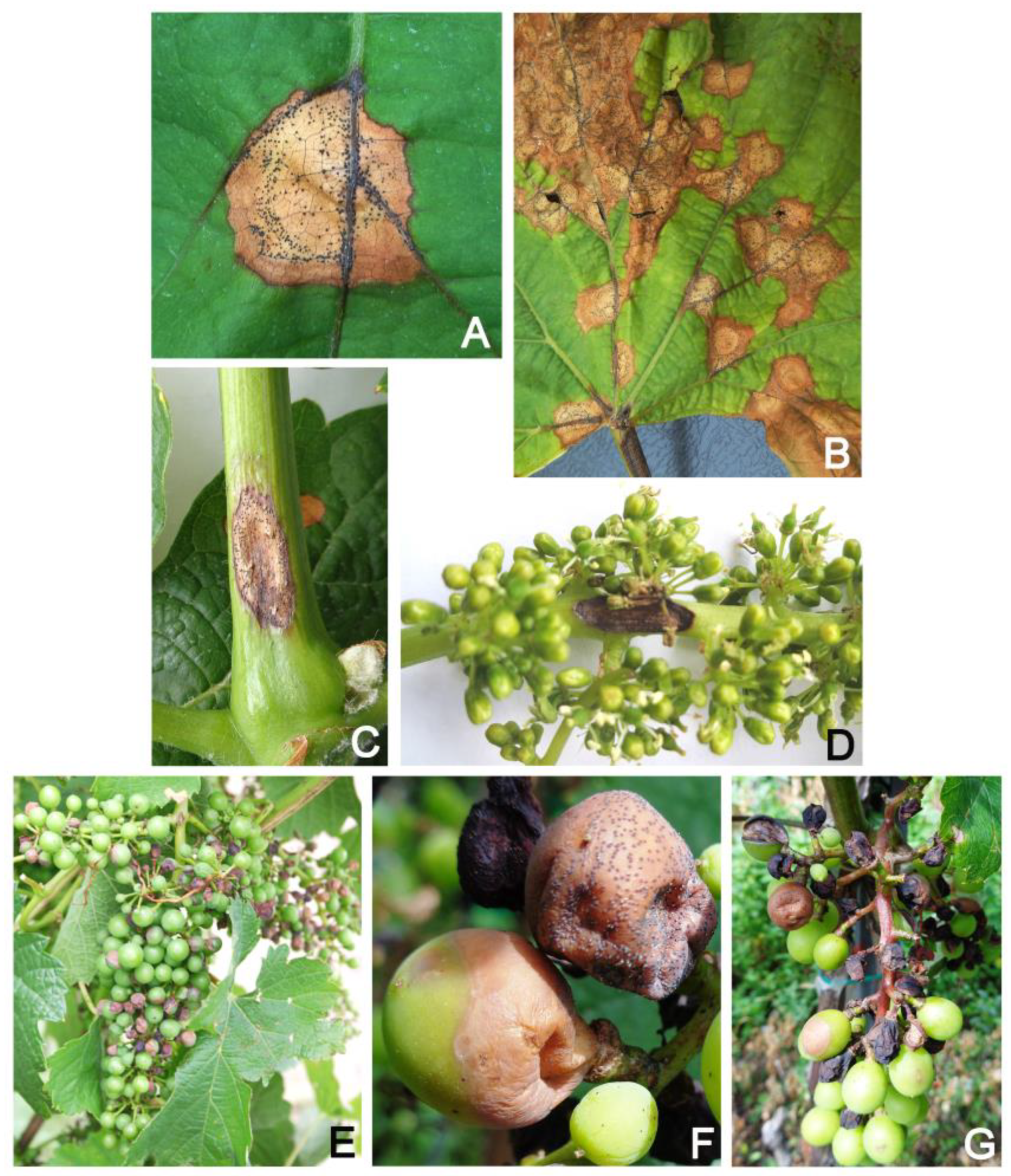
5. Symptomatology
6. Host Plant Community and Black Rot Resistance
6.1. Host Plants
6.2. Genetic Resources of Black Rot Resistance in Grapevine
| Cultivar | Hausmann et al. 1 [3] | Loskill et al. 2 [73] | Rex 1 [74] | Roznik et al. 3 [58] | Tomoiaga and Chedea 4 [75] |
|---|---|---|---|---|---|
| Amurg | T | ||||
| Astra | S | ||||
| Baron | MS | ||||
| Beta | 8 | ||||
| Bianca | 5 | HS | |||
| Blasius | T | ||||
| Börner * | 9 | ||||
| Bronner | MS | ||||
| Brumariu | T | ||||
| Cabernet carbon | MS | ||||
| Cabernet carol | LS | ||||
| Cabernet cortis | MS | ||||
| Cabernet sauvignon | HS | ||||
| Campbell early | 5 | ||||
| Carman | 9 | ||||
| Catawba | 7 | ||||
| Cayuga white | 6 | ||||
| Champanel | 8 | ||||
| Chancellor | 9 | ||||
| Chardonnay | HS | ||||
| Clinton | 9 | ||||
| Cloeta | 9 | ||||
| Concord | 6 | ||||
| Cynthiana | 6 | ||||
| Csillám | SR | ||||
| De Chaunac | 9 | ||||
| Delaware | 5 | ||||
| Emerald | 5 | ||||
| Esther | HS | ||||
| Etta | 8 | ||||
| Felicia | 7 | MR | |||
| Feteasca alba | S | ||||
| Feteasca regale | S | ||||
| Fredonia | 9 | ||||
| Furmint | HS | ||||
| Hanover | 9 | ||||
| Helios | MS | ||||
| Iordana | MT | ||||
| Ironclad | 9 | ||||
| Isabella | 4 | ||||
| Isaura | HS | ||||
| Jefferson | 3 | ||||
| Johanniter | HS | ||||
| Lemberger | 1 | ||||
| Malverina | MR | ||||
| Manito | 5 | ||||
| Mars | 6 | ||||
| Merlot | HS | ||||
| Merzling | LS | 9 | MR | ||
| Mills | 5 | ||||
| Missouri riesling | 9 | ||||
| Moldova | MS | ||||
| Monarch | MS | ||||
| Muscat Ottonel | S | ||||
| Müller-Thurgau | 1 | HS | 1 | ||
| Nero | MS | ||||
| Neuburger | MT | ||||
| Pearls | 5 | ||||
| Pinot gris | S | ||||
| Pinot noir | 1 | ||||
| Pinotin | HS | ||||
| Primitivo | 3 | ||||
| Prior | MS | ||||
| Radames | T | ||||
| Reberger | 3 | ||||
| Regent | HS | 3 | |||
| Riesling | 1 | HS | MT | ||
| Rommel | 8 | ||||
| Rubin | T | ||||
| Selena | MT | ||||
| Sauvignon blanc | S | ||||
| Seyval blanc | HR | ||||
| Solaris | LS | ||||
| Spätburgunder | HS | ||||
| Suelter | 9 | ||||
| Suzy | MS | ||||
| Teréz | HR | ||||
| Traminer Rot | MT | ||||
| Triumph | 6 | ||||
| Trollinger | 1 | ||||
| Viktória gyöngye | MS | ||||
| Villard blanc | 7 | ||||
| Villaris | 5 | MR | |||
| Wapanuka | 7 | ||||
| Welschriesling | S | ||||
| Xlnta | 9 | ||||
| Zalán | HS |
6.3. Genetic Factors Determining Resistance to Black Rot in Grapes
7. The Control of Black Rot
7.1. Changes in Cultivation Practices in Central Europe
- With the reduction of manual labour and increasing mechanization (e.g., mechanical pruning and mechanical harvesting), infected grapes and grape parts remain frequently on the vine.
- The decline of cover crops and inter-row cultivation has led to the spread of mechanical shredding of prunings. Thus, infected plant parts remain between vine rows, on the soil or on the surface of the inter-row vegetation, facilitating the persistence and accumulation of the infective material within the plantation.
- Dense vine spacing and low vine heights create more favourable microclimatic conditions for pathogens, resulting in an increased risk of plant diseases.
- Organic farming is spreading, but with no effective control options against black rot, as the permitted copper and sulphur-based products are ineffective. Systemic pesticides are effective against black rot but banned in organic farming. Therefore, the control of black rot in organic crops is slowly becoming unmanageable.
- Strong increase in the cultivation area of inter- and intraspecific varieties resistant to powdery mildew and downy mildew, but highly susceptible to black rot, in lowland viticultural regions and in organic farming, creating an ecological niche for emerging pathogens such as G. bidwellii.
- The official withdrawal of certain active substances e.g., sterol biosynthesis inhibitors such as demethylation inhibitors, strobilurin, and contact dithiocarbamates, has made it increasingly difficult to control the pathogen effectively in Integrated Production and in European national agricultural programs such as Agri-Environment Scheme (AES) that supports biodiversity, quality of water, air and soil.
- The growth of abandoned and uncultivated vineyards is a major and growing problem in Central Europe, as black rot and other diseases spread unhindered in these areas. Infectious material can easily be carried by wind from these areas to areas still free of infection.
7.2. Prevention of Black Rot Infection by Agrotechnology
- After harvesting, the amount of infective material can be reduced below critical level by cutting and burning mummified clusters [80] and by turning infected plant debris that falls to the ground during pruning into the soil [57,81]. Experiments have shown that this method is very effective when used in heavily infected areas for several, but at least for two consecutive years [42].
- The removal and destruction (e.g., burying) of the first infected, symptomatic leaves found during shoot thinning is a beneficial method for cluster protection.
- An airy, thin canopy and keeping rows free of weeds will ensure that foliage dries quickly after rainfall, reducing the risk of infection. Low cover cropping can also reduce relative humidity in the plantation.
- Nearby abandoned vineyards should be treated or eradicated to reduce the chance of infection.
- Any measure that limits or prevents physical damage to grapes and clusters is important.
- A balanced supply of nutrients reduces the susceptibility of the vine to diseases. It is strongly advisable to avoid excessive nitrogen supply to prevent extreme growth of vegetative parts.
7.3. Biological Control of Black Rot
7.4. Models for Predicting Black Rot Epidemics
7.5. Chemical Crop Protection
- Contact agents should be applied before infection, although they may not be fully effective. Unfortunately, organic growers are still in a difficult position, because the sulphur and copper they commonly use are virtually ineffective against black rot. Dithiocarbamates are generally considered to have excellent efficacy against black rot, although there are conflicting experiences from farmers. Unfortunately, fungicides containing mancozeb and myclobutanil have already been withdrawn from commercial distribution in the EU. Metiram, phthalimide fungicides such as captan and folpet, dithianon and fluxapyroxad are still approved, but other new plant protection products containing active substances such as the combination of fluopyram + tebuconazole and the recently registered mefentrifluconazole are also permitted in the EU [106].
- Fungal sterol biosynthesis inhibitors: difenoconazole, flusilazole, tetraconazole and tebuconazole have excellent activity against powdery mildew, and many of them are also very effective against black rot [49]. Their preventive effect is weak, but they can block the disease process through their rapid absorption, stopping the development of symptoms in both leaves and berries. The long-lasting curative effect of the withdrawn myclobutanil and perhaps the equally effective triazole fungicides, tebuconazoles, provides flexibility in the timing of black rot control. They should therefore be used mainly after infection, in the first half of the incubation period of G. bidwellii. Their weak preventive effect can be improved by combining them with the still available dithiocarbamate agents.
- Of the strobilurins, pyraclostrobin is the best preventive and curative agent for both leaves and berries [107]. This active ingredient, sprayed after flower caps have fallen off, binds well to the waxy layer and follows surface growth of black rot on leaves and berries; therefore, has a long duration of action of up to 3 weeks. Although the efficacy of azoxystrobin and kresoxim-methyl has been confirmed [81,108], the excellent efficacy of pyraclostrobin unfortunately does not automatically apply to other strobilurin-type agents such as azoxystrobin [107]. In any case, it is reassuring that the development of strobilurin resistance in black rot is not a concern [109].
7.6. On the Road to Sustainability
8. Metabolite Analyses Associated with Black Rot—Grapevine Interactions
8.1. Secondary Metabolite Identifications
| Secondary Metabolite | Compound Summary Active PubChem Weblinks | Compound Phytotoxicity | Producing Phyllosticta Strains, Host Plants and Fungal Lifestyle | Mode of Compound Detection | |||
|---|---|---|---|---|---|---|---|
| Submerged Culture Fermentation | In planta V. vinifera—Fungus Interaction | ||||||
| Guignardic acid | https://pubchem.ncbi.nlm.nih.gov/compound/Guignardic-acid (accessed on 30 September 2022) | Highly phytotoxic, not host-specific | P. telopeae | Telopea speciosissima | Endophyte | [132] | NDA |
| P. parthenocissi CBS 111645 | Parthenocissus quinquefolia | Plant pathogen | [134] | [131,135] | |||
| P. musarum IMI 147360 | Musa sp. | Plant pathogen | [131] | NDA | |||
| P. elongata CBS 126.22 | Vaccinium sp. | Casual plant pathogen | [131] | NDA | |||
| P. capitalensis CBS 123405 | Recorded on 70 plant families * | Endophyte and plant pathogen | [131] | NDA | |||
| Phenguignardic acid | https://pubchem.ncbi.nlm.nih.gov/compound/72204238 (accessed on 30 September 2022) | Highly phytotoxic, not host-specific | P. parthenocissi CBS 111645 | Parthenocissus quinquefolia | Plant pathogen | [134] | NDA |
| P. sphaeropsoidea CBS 756.70 | Aesculus sp. | Plant pathogen | [131] | NDA | |||
| P. musarum IMI 147360 | Musa sp. | Plant pathogen | [131] | NDA | |||
| P. elongata CBS 126.22 | Vaccinium sp. | Casual plant pathogen | [131] | NDA | |||
| Alaguignardic acid | https://pubchem.ncbi.nlm.nih.gov/compound/102435402 (accessed on 30 September 2022) | Highly phytotoxic, not host-specific | P. parthenocissi CBS 111645 | Parthenocissus quinquefolia | Plant pathogen | [136] | NDA |
| Guignardianone A | https://pubchem.ncbi.nlm.nih.gov/compound/102435403 (accessed on 30 September 2022) | Phytotoxic, not host-specific | NDA | ||||
| Guignardianone B | https://pubchem.ncbi.nlm.nih.gov/compound/102435404 (accessed on 30 September 2022) | Non-phytotoxic | NDA | ||||
| Guignardianone C | https://pubchem.ncbi.nlm.nih.gov/compound/102435405 (accessed on 30 September 2022) | Non-phytotoxic | NDA | ||||
| Guignardianone D | https://pubchem.ncbi.nlm.nih.gov/compound/102435406 (accessed on 30 September 2022) | Non-phytotoxic | NDA | ||||
| Guignardianone E | https://pubchem.ncbi.nlm.nih.gov/compound/71682251 (accessed on 30 September 2022) | Phytotoxic, not host-specific | NDA | ||||
| Guignardianone F | https://pubchem.ncbi.nlm.nih.gov/compound/71682252 (accessed on 30 September 2022) | Phytotoxic, not host-specific | NDA | ||||
| (6S,9R)-vomifoliol | https://pubchem.ncbi.nlm.nih.gov/compound/5280462 (accessed on 30 September 2022) | Non-phytotoxic | P. ampelicida PSU-G11 | Garcinia hombroniana | Endophyte | [137] | NDA |
| Guignarenone A | NDA | NDA | |||||
| Guignarenone B | NDA | NDA | |||||
| Guignarenone C | https://pubchem.ncbi.nlm.nih.gov/compound/139585121 (accessed on 30 September 2022) | NDA | |||||
| Guignarenone D | NDA | NDA | |||||

8.2. Metabolomics, a Promising Tool to Describe the Grapevine—Black Rot Pathosystem
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statistical Report on World Vitiviniculture. Available online: https://www.oiv.int/en/statistiques/recherche (accessed on 30 September 2022).
- Liu, X.-Q.; Ickert-Bond, S.M.; Nie, Z.-L.; Zhou, Z.; Chen, L.-Q.; Wen, J. Phylogeny of the Ampelocissus-Vitis Clade in Vitaceae Supports the New World Origin of the Grape Genus. Mol. Phylogenet. Evol. 2016, 95, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausmann, L.; Rex, F.; Töpfer, R. Evaluation and Genetic Analysis of Grapevine Black Rot Resistances. Acta Hortic. 2017, 1188, 285–290. [Google Scholar] [CrossRef]
- Rinaldi, P.; Broggini, G.A.L.; Gessler, C.; Molitor, D.; Sofia, J.; Mugnai, L. Guignardia bidwellii, the Agent of Grape Black Rot of Grapevine, Is Spreading in European Vineyards. In Proceedings of the 10th International Congress of Plant Pathology (ICPP 2013), Beijing, China, 25–30 August 2013; Volume 211. [Google Scholar]
- Viala, P.; Ravaz, L. Mémoire Sur Une Nouvelle Maladie de la Vigne: Le Black Rot (Pourriture Noire); Progrès Agricole et Viticole" et C. Coulet: Montpellier, France, 1886; pp. 17–58. [Google Scholar]
- Sivanesan, A.; Holliday, P. CMI. Descriptions of Pathogenic Fungi and Bacteria. Commonw. Mycol. Inst. Kew Surrey Engl. 1981, 71, 701–710. [Google Scholar]
- Schoch, C.L.; Shoemaker, R.A.; Seifert, K.A.; Hambleton, S.; Spatafora, J.W.; Crous, P.W. A Multigene Phylogeny of the Dothideomycetes Using Four Nuclear Loci. Mycologia 2006, 98, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, N.F. A Modern Account of the Genus Phyllosticta. Plant Pathol. Quar. 2013, 3, 145–159. [Google Scholar] [CrossRef]
- Wilcox, W.F.; Hoffmann, L.E. Black Rot. In Compendium of Grape Diseases, Disorders, and Pests, Second Edition; Wilcox, W.F., Gubler, W.D., Uyemoto, J.K., Eds.; Amer Phytopathological Society: St. Paul, MN, USA, 2015; pp. 28–33. ISBN 978-0-89054-479-2. [Google Scholar]
- Lamson-Scribner, F.; Smith, E.F.; Pearson, A.W. Report on the Fungus Diseases of the Grape Vine; Department of Agriculture, Botanical Division: Washington, DC, USA, 1886. [Google Scholar]
- Reddick, D. Black Rot Disease of Grapes; Cornell University: New York, NY, USA, 1911. [Google Scholar]
- Lüstner, G. Auftreten Der Schwarzfaule (Black Rot) Der Rebe in Deutschland. Nachrichtenbl Dtsch Pflanzenschutzd. 1935, 15, 27. [Google Scholar]
- Rui, D. Il Black Rot o Marciume Nero Dell’uva Nell’isola Di Veglia (Jugoslavia). Annu. Della Regia Stazione Vitic. Enol. Conegliano 1935, 5, 285–289. [Google Scholar]
- Jermini, M.; Gessler, C. Epidemiology and Control of Grape Black Rot in Southern Switzerland. Plant Dis. USA 1996, 80, 322–325. [Google Scholar] [CrossRef]
- Harms, M.; Holz, B.; Hoffmann, C.; Lipps, H.P.; Silvanus, W. Occurrence of Guignardia bidwellii, the Causal Fungus of Black Rot on Grapevine, in the Vine-Growing Areas of Rhineland-Palatinate, Germany. In Proceedings of the Plant Protection and Plant Health in Europe: Introduction and Spread of Invasive Species, Berlin, Germany, 9–11 June 2005; pp. 127–132. [Google Scholar]
- Mikulás, J.; Tomcsányi, E. Megjelent hazánkban is a feketerothadás. Kert. És Szőlészet 1999, 49, 11–12. [Google Scholar]
- Tomoioaga, L.; Comsa, M. The Strategy of Optimization for Combat the Black Rot of Vine (Guignardia bidwelli), in the Ecoclimatic Conditions from Vineyard Târnave. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 2010, 67, 500. [Google Scholar] [CrossRef]
- Váczy, Z.; Váczy, K.Z.; Schmidt, Á.; Kiss, L. A Guignardia bidwellii által okozott feketerothadás magyarországi szőlőültetvényekben. In 58. Növényvédelmi Tudományos Napok Összefoglalók; Kőmíves Tamás Magyar Növényvédelmi Társaság: Budapest, Hungary, 2012; p. 38. [Google Scholar]
- Dula, B.; Lázár, J.; Kölber, M. A szőlő növényvédelme II: Betegségek. Növényvédelem 2016, 5, 230–233. [Google Scholar]
- Plant Health Australia. Black Rot. Available online: https://www.planthealthaustralia.com.au/pests/black-rot/ (accessed on 1 October 2022).
- Ramsdell, D.C.; Milholland, R. Black-Rot. In Compendium of Grape Diseases; Pearson, R.C., Goheen, A.C., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1988; pp. 15–17. [Google Scholar]
- Zhang, K.; Zhang, N.; Cai, L. Typification and Phylogenetic Study of Phyllosticta ampelicida and P. vaccinii. Mycologia 2013, 105, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Chen, Q.; Carroll, G.; Zhang, N.; Shivas, R.G.; Cai, L. Polyphasic characterization of four new plant pathogenic Phyllosticta species from China, Japan, and the United States. Fungal Biol. 2015, 119, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Wicht, B.; Petrini, O.; Jermini, M.; Gessler, C.; Broggini, G.A.L. Molecular, Proteomic and Morphological Characterization of the Ascomycete Guignardia bidwellii, Agent of Grape Black Rot: A Polyphasic Approach to Fungal Identification. Mycologia 2012, 104, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.A.; Paffetti, D.; Comparini, C.; Broggini, G.A.L.; Gessler, C.; Mugnai, L. Genetic Variability of Phyllosticta ampelicida, the Agent of Black Rot Disease of Grapevine. Phytopathology 2017, 107, 1406–1416. [Google Scholar] [CrossRef]
- Moreno-Velázquez, M.; Hernández-Ramos, L.; Preuss-Angeles, A.K.; Ronces-Frutos, L.E.; Morales-González, I.; Carrillo-Ortiz, N.; Cárcamo-Rodríguez, A. PCR Detection of Guignardia bidwellii, Causal Agent of Grape Black Rot. Mex. J. Phytopathol. 2019, 37, 383–398. [Google Scholar] [CrossRef]
- Canaveira, F.; Reis, P.; Nascimento, T.; Rego, C. Characterization of Phyllosticta ampelicida Isolates of Grapevine. In 1º Simpósio SCAP (Novos Desafios na Protecção de Plantas) e 7ª Congresso da SPFAt; Oeiras, Portugal; 2014; Available online: https://www.researchgate.net/publication/294580126 (accessed on 1 October 2022).
- Narduzzi-Wicht, B.; Jermini, M.; Gessler, C.; Broggini, G.A.L. Microsatellite Markers for Population Studies of the Ascomycete Phyllosticta ampelicida, the Pathogen Causing Grape Black Rot. Phytopathol. Mediterr. 2014, 53, 470–479. [Google Scholar] [CrossRef]
- Eichmeier, A.; Diaz-Losada, E.; Hakalova, E.; Pecenka, J.; Stuskova, K.; Ojeda, S.; Gramaje, D. Draft Genome Sequence of Phyllosticta ampelicida, the Cause of Grapevine Black Rot. Phytopathol. Mediterr. 2022, 61, 279–282. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle Transitions in Plant Pathogenic Colletotrichum Fungi Deciphered by Genome and Transcriptome Analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- De Silva, N. Mycosphere Essays 9: Defining Biotrophs and Hemibiotrophs. Mycosphere 2016, 7, 545–559. [Google Scholar] [CrossRef]
- Suzuki, S.U.; Sasaki, A. Ecological and Evolutionary Stabilities of Biotrophism, Necrotrophism, and Saprotrophism. Am. Nat. 2019, 194, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Jabco, J.P.; Nesbitt, W.B.; Werner, D.J. Resistance of Various Classes of Grapes to the Bunch and Muscadine Grape Forms of Black Rot. J. Am. Soc. Hortic. Sci. USA 1985, 110, 762–765. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Diseases Caused by Fungi. In Plant Pathology, 5th ed.; Agrios, G.N., Ed.; Academic Press: San Diego, CA, USA, 2005; pp. 385–614. ISBN 978-0-12-044565-3. [Google Scholar] [CrossRef]
- Ferrin, D.M.; Ramsdell, D.C. Ascospore Dispersal and Infection of Grapes by Guignardia bidwellii, the Causal Agent of Grape Black Rot Disease. Phytopathology 1977, 67, 1501–1505. [Google Scholar] [CrossRef]
- Onesti, G.; González-Domínguez, E.; Rossi, V. Production of Guignardia bidwellii Conidia on Grape Leaf Lesions Is Influenced by Repeated Washing Events and by Alternation of Dry and Wet Periods. Eur. J. Plant Pathol. 2017, 147, 949–953. [Google Scholar] [CrossRef]
- Ferrin, D.M.; Ramsdell, D.C. Influence of Conidia Dispersal and Environment on Infection of Grape by Guignardia bidwellii. Phytopathology 1978, 68, 892. [Google Scholar] [CrossRef]
- Onesti, G.; González-Domínguez, E.; Manstretta, V.; Rossi, V. Release of Guignardia bidwellii Ascospores and Conidia from Overwintered Grape Berry Mummies in the Vineyard. Aust. J. Grape Wine Res. 2018, 24, 136–144. [Google Scholar] [CrossRef]
- Hoffman, L.E.; Wilcox, W.F.; Gadoury, D.M.; Seem, R.C. Influence of Grape Berry Age on Susceptibility to Guignardia bidwellii and Its Incubation Period Length. Phytopathology 2002, 92, 1068–1076. [Google Scholar] [CrossRef]
- Dula, B. A szőlő feketerothadása. Gyak. Agrofórum 2012, 46, 24–27. [Google Scholar]
- Spotts, R.A. Infection of Grape by Guignardia bidwellii—Factors Affecting Lesion Development, Conidial Dispersal, and Conidial Populations on Leaves. Phytopathology 1980, 70, 252. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Emmett, R.W.; Wilcox, W.F.; Wicks, T.J. Eradication of Black Rot (Guignardia bidwellii) from Grapevines by Drastic Pruning. Plant Pathol. 2012, 61, 1093–1102. [Google Scholar] [CrossRef]
- Spotts, R.A. Effect of Leaf Wetness Duration and Temperature on the Infectivity of Guignardia bidwellii on Grape Leaves. Phytopathology 1977, 77, 1378. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, C.; Kleespies, R.; Enders, M.; Koch, E. Biology of the Black Rot Pathogen, Guignardia bidwellii, Its Development in Susceptible Leaves of Grapevine Vitis vinifera. J Cultiv. Plants 2009, 61, 82–90. [Google Scholar] [CrossRef]
- Kuo, K.; Hoch, H.C. The Parasitic Relationship between Phyllosticta ampelicida and Vitis vinifera. Mycologia 1996, 88, 626–634. [Google Scholar] [CrossRef]
- Tisch, C. Biologie der Schwarzfäule an Reben und potentielle Abwehrmechanismen bei der Europäischen Wildrebe und Verschieden Resistenten Sorten. Doctoral Dissertation, Karlsruher Instituts für Technologie, Karlsruhe, Germany, 2017. [Google Scholar] [CrossRef]
- Molitor, D.; Berkelmann-Loehnertz, B. Simulating the Susceptibility of Clusters to Grape Black Rot Infections Depending on Their Phenological Development. Crop Prot. 2011, 30, 1649–1654. [Google Scholar] [CrossRef]
- Molitor, D.; Augenstein, B.; Mugnai, L.; Rinaldi, P.A.; Sofia, J.; Hed, B.; Dubuis, P.-H.; Jermini, M.; Kührer, E.; Bleyer, G.; et al. Composition and Evaluation of a Novel Web-Based Decision Support System for Grape Black Rot Control. Eur. J. Plant Pathol. 2016, 144, 785–798. [Google Scholar] [CrossRef]
- Hoffman, L.E.; Wilcox, W.F. Utilizing Epidemiological Investigations to Optimize Management of Grape Black Rot. Phytopathology 2002, 92, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Molitor, D.; Beyer, M. Epidemiology, Identification and Disease Management of Grape Black Rot and Potentially Useful Metabolites of Black Rot Pathogens for Industrial Applications—A Review. Ann. Appl. Biol. 2014, 165, 305–317. [Google Scholar] [CrossRef]
- Pirrello, C.; Mizzotti, C.; Tomazetti, T.C.; Colombo, M.; Bettinelli, P.; Prodorutti, D.; Peressotti, E.; Zulini, L.; Stefanini, M.; Angeli, G.; et al. Emergent Ascomycetes in Viticulture: An Interdisciplinary Overview. Front. Plant Sci. 2019, 10, 1–30. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Tan, Y.-P. National Diagnostic Protocol for Phyllosticta Ampelicida—NDP13 V2; de Alwis, S.K., Baskarathevan, J., Eds.; Subcommittee on Plant Health Diagnostics; 2017; ISBN 978-0-9945113-7-9. Available online: https://www.plantbiosecur itydiagnostics.net.au/app/uploads/2018/11/NDP-13-Black-rot-on-grapevine-Phyllosticta-ampelicida-V2.pdf (accessed on 1 October 2022).
- Crandall, S.G.; Spychalla, J.; Crouch, U.T.; Acevedo, F.E.; Naegele, R.P.; Miles, T.D. Rotting Grapes Don’t Improve with Age: Cluster Rot Disease Complexes, Management, and Future Prospects. Plant Dis. 2022, 106, 2013–2025. [Google Scholar] [CrossRef]
- Kövics, G. Növénybetegséget Okozó Gombák Névtára; Mezőgazda Kiadó: Budapest, Hungary, 2000; ISBN 978-963-9239-81-4. [Google Scholar]
- Wikee, S.; Udayanga, D.; Crous, P.W.; Chukeatirote, E.; McKenzie, E.H.C.; Bahkali, A.H.; Dai, D.; Hyde, K.D. Phyllosticta—An Overview of Current Status of Species Recognition. Fungal Divers. 2011, 51, 43–61. [Google Scholar] [CrossRef]
- Liberato, J.R.; Schilder, A.M.C.; Shivas, R.G. Guignardia bidwellii. PaDIL Datasheet. Available online: https://www.padil.gov.au/pests-and-diseases/pest/136603 (accessed on 12 January 2023).
- Wilcox, W.F. Black Rot. Disease Identification. Sheet No. 102GFSG-D4. Available online: https://ecommons.cornell.edu/bitstream/handle/1813/43076/black-rot-grapes-FS-NYSIPM.pdf?sequence=1 (accessed on 1 October 2022).
- Roznik, D.; Hoffmann, S.; Kozma, P. Screening a Large Set of Garpe Accessions for Resistance against Black Rot (Guignardia bidwellii/(Ell.)). Mitt. Klosterneubg. Rebe Und Wein Obs. Und Früchteverwertung 2017, 67, 149–157. [Google Scholar]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New Horizons for Grapevine Breeding. In Methods in Temperate Fruit Breeding; Flachowsky, H., Hanke, M.V., Eds.; Global Science Books: Isleworth, UK, 2011; pp. 79–100. [Google Scholar]
- Gessler, C.; Philippe, B.; Mauro, J. Black Rot on the Hybrid Vitis Cultivar Isabella; Integrated Protection in Viticulture; IOBC-WPRS Bulletin: Montfavet Cedex, France, 2006; Volume 29, pp. 95–102. [Google Scholar]
- Barrett, H.C. Survey of Black Rot Resistance of the Foliage of Wild Grape Species. Proc. Am. Soc. Hortic. Sci. 1953, 62, 319–622. [Google Scholar]
- Kummuang, N.; Diehl, S.V.; Smith, B.J.; Graves, C.H., Jr. Muscadine Grape Berry Rot Diseases in Mississippi: Disease Epidemiology and Crop Reduction. Plant Dis. 1996, 80, 244–247. [Google Scholar] [CrossRef]
- Dalbó, M.A.; Weeden, N.F.; Reisch, B.I. QTL Analysis of Disease Resistance in Interspecific Hybrid Grapes. Acta Hortic. 2000, 528, 217–222. [Google Scholar] [CrossRef]
- Rex, F.; Fechter, I.; Hausmann, L.; Töpfer, R. QTL Mapping of Black Rot (Guignardia bidwellii) Resistance in the Grapevine Rootstock ‘Börner’ (V. Riparia Gm183 × V. Cinerea Arnold). Theor. Appl. Genet. 2014, 127, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Rex, F.; Töpfer, R.; Hausmann, L. Characterization of the Black Rot Resistance Loci (Rgb1 and Rgb2) of ‘Börner’ and Development of Associated Markers Suitable for Marker-Assisted Selection in Grapevine Breeding. In Proceedings of the XIII. International Symposium on Grapevine Breeding and Genetics, Pioneering Wines (PIWIs)—Innovation and Tradition, Institute for Grapevine Breeding Geilweilerhof I Siebeldingen,, Siebeldingen, Germany, 10–15 July 2022. [Google Scholar] [CrossRef]
- Kiss, E.; Tóth-Lencsés, K.; Szőke, A.; Kerekes, A.; Veres, A.; Roznik, D.; Kozma, P. Origin of “Csillám”, a Promising Source for Black Rot Resistance. VITIS-J. Grapevine Res. 2017, 56, 53–54. [Google Scholar] [CrossRef]
- Roznik, D. Identification and Application of Resistance Sources against Black Rot (Guignardia bidwellii (Ellis) Viala et Ravaz) in Grape Resistance Breeding. Doctoral Dissertation, Szent István University, Budapest, Hungary, 2019. [Google Scholar]
- Vitis vinifera subsp. Sylvestris. Available online: https://www.ipni.org/n/urn:lsid:ipni.org:names:60460144-2 (accessed on 21 October 2022).
- Coleman, C.; Copetti, D.; Cipriani, G.; Hoffmann, S.; Kozma, P.; Kovács, L.; Morgante, M.; Testolin, R.; Di Gaspero, G. The Powdery Mildew Resistance Gene REN1 Co-segregates with an NBS-LRR Gene Cluster in Two Central Asian Grapevines. BMC Genet. 2009, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, S.; Di Gaspero, G.; Kovács, L.; Howard, S.; Kiss, E.; Galbács, Z.; Testolin, R.; Kozma, P. Resistance to Erysiphe necator in the Grapevine ‘Kishmish Vatkana’ Is Controlled by a Single Locus through Restriction of Hyphal Growth. Theor. Appl. Genet. 2008, 116, 427–438. [Google Scholar] [CrossRef]
- Pap, D.; Riaz, S.; Dry, I.B.; Jermakow, A.; Tenscher, A.C.; Cantu, D.; Oláh, R.; Walker, M.A. Identification of Two Novel Powdery Mildew Resistance Loci, Ren6 and Ren7, from the Wild Chinese Grape Species Vitis Piasezkii. BMC Plant Biol. 2016, 16, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Schröder, S.; Kortekamp, A.; Heene, E.; Daumann, J.; Valea, I.; Nick, P. Crop Wild Relatives as Genetic Resources—The Case of the European Wild Grape. Can. J. Plant Sci. 2015, 95, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Loskill, B.; Molitor, D.; Koch, E.; Harms, M.; Berkelmann-Löhnertz, B.; Hoffmann, C.; Kortekamp, A.; Porten, M.; Louis, F.; Maixner, M. Strategien zur Regulation der Schwarzfäule (Guignardia bidwellii) im ökologischen Weinbau; Julius Kühn-Instititut, Bundesforschungsinstitut für Kulturpflanzen, D-Bernkastel-Kues. 2009. Available online: https://orgprints.org/id/eprint/17072/1/17072-04OE032-jki-maixner-2009-schwarzfaeule.pdf (accessed on 1 October 2022).
- Rex, F. Resistenz Gegen Die Schwarzfäule (Guignardia bidwellii) in Der Weinrebe (Vitis Spec.)—Etablierung Phänotypischer Erfassungsmethoden Und Genetische Kartierung von Resistenzloci. Doctoral Dissertation, Julius Kühn-lnstitut, Bundesforschungsinstitut für Kulturpflanzen, Quedlinburg, Germany, 2012. [Google Scholar] [CrossRef]
- Tomoiaga, L.; Chedea, V.S. The Behaviour of Some Grapevine Varieties to the Guignardia bidwellii Fungus Attack. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 2020, 77, 122. [Google Scholar] [CrossRef]
- Băileştianu, N.A.; Mitrea, R. The Black Rot—A New Challenge for Vine Crops. Ann. Univ. Craiova-Agric. Mont. Cadastre Ser. 2019, 49, 20–25. [Google Scholar]
- Jones, D.S.; McManus, P.S. Susceptibility of Cold-Climate Wine Grape Cultivars to Downy Mildew, Powdery Mildew, and Black Rot. Plant Dis. 2017, 101, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettinelli, P.; Nicolini, D.; Giovannini, O.; Costantini, L.; Stefanini, M.; Hausmann, L.; Vezzulli, S. Towards Marker-Assisted Breeding for Black Rot Resistance: High-Density Linkage Mapping and Identification of a Major QTL in the Cultivar ‘Merzling’ (V. rupestris × V. aestivalis Var. lincecumii). In Proceedings of the XIII. International Symposium on Grapevine Breeding and Genetics, Pioneering Wines (PIWIs) – Innovation and Tradition, Institute for Grapevine Breeding Geilweilerhof I Siebeldingen, Germany, 10–15 July 2022. [Google Scholar] [CrossRef]
- Dula, B. Elméleti és gyakorlati alapismeretek a szőlő járványos betegségeiről és leküzdésükről (4.)—A Szőlő Feketerothadása. Agrofórum 2021, 32, 122–129. [Google Scholar]
- Holz, B. Schwarzfäule Der Rebe in Mosel-Saar-Ruwer: Plötzlich Und Unverhofft. Dtsch. Weinmagazin 2003, 6, 26–29. [Google Scholar]
- Molitor, D. Untersuchungen zur Biologie und Bekämpfung der Schwarzfäule (Guignardia bidwellii) an Weinreben; Geisenheimer Berichte; Gesellschaft zur Förderung der Hochschule Geisenheim: Geisenheim, Germany, 2009; ISBN 978-3-934742-54-3. [Google Scholar]
- Linhart, G.; Mezey, G. Szőlőbetegségek; Magyar Királyi Földművelésügyi Miniszter—Czéh Sándor Nyomda: Magyar-Óvár, Hungary, 1895. [Google Scholar]
- Strategy for the Minimisation of Copper in Organic Farming in Europe. Available online: https://www.organicseurope.bio/content/uploads/2020/10/ifoam_eu_copper_minimisation_in_organic_farming_may2018_0.pdf?dd (accessed on 30 September 2022).
- Panagos, P.; Ballabio, C.; Lugato, E.; Jones, A.; Borrelli, P.; Scarpa, S.; Orgiazzi, A.; Montanarella, L. Potential Sources of Anthropogenic Copper Inputs to European Agricultural Soils. Sustainability 2018, 10, 2380. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2021/1165 Authorising Certain Products and Substances for Use in Organic Production and Establishing Their Lists. Available online: https://leap.unep.org/countries/eu/national-legislation/commission-implementing-regulation-eu-20211165-authorising (accessed on 1 October 2022).
- Schilder, A.M.C.; Gillett, J.M.; Sysak, R.W.; Wise, J.C. Evaluation of environmentally friendly products for control of fungal diseases of grapes. In 10th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing and Viticulture, Proceedings of the Conference, Weinsberg/Germany, 4–7 Febuary 2002; Boos, M., Ed.; 2002; pp. 163–167. Available online: https://orgprints.org/id/eprint/14620/ (accessed on 1 October 2022).
- Rutto, L.K.; Mersha, Z.; Nita, M. Evaluation of Cultivars and Spray Programs for Organic Grape Production in Virginia. HortTechnology 2021, 31, 166–173. [Google Scholar] [CrossRef]
- Schönbeck, F.; Schlösser, E. Preformed Substances as Potential Protectants. In Physiological Plant Pathology; Heitefuss, R., Williams, P.H., Eds.; Encyclopedia of Plant Physiology; Springer: Berlin/Heidelberg, Germany, 1976; pp. 653–678. ISBN 978-3-642-66279-9. [Google Scholar]
- Travis, J.; Hed, B.; Muza, A. Control of Black Rot in Greenhouse and Field Trials Using Organic Approved Materials, 2005; Research Report to the New York Wine/Grape Foundation, The Grape Production Research Fund and The Viticulture Consortium-East; Penn State College of Agricultural Science, 2005; Available online: https://agsci.psu.edu/research/centers-facilities/extension/erie/research/plant-pathology/organic-grape-disease-management-trials/organicgrapecontrol05.pdf/@@download/file/OrganicGrapeControl05.pdf (accessed on 1 October 2022).
- Molitor, D.; Heibertshausen, D.; Baus, O.; Loskill, B.; Maixner, M.; Berkelmann-Löhnertz, B. Einsatz Eines Sapindus Mukorossi-Extraktes Zur Regulierung von Pilzlichen Pathogenen an Weinreben—Eine Alternative Für Den Ökologischen Rebschutz? J. Für Kult.–J. Cultiv. Plants 2010, 62, 444–450. [Google Scholar]
- Koch, E.; Enders, M.; Ullrich, C.; Molitor, D.; Berkelmann-Löhnertz, B. Effect of Primula Root and Other Plant Extracts on Infection Structure Formation of Phyllosticta ampelicida (Asexual Stage of Guignardia bidwellii) and on Black Rot Disease of Grapevine in the Greenhouse. J. Plant Dis. Prot. 2013, 120, 26–33. [Google Scholar] [CrossRef]
- Stafne, E.T.; Carroll, B.; Smith, D. Black Rot Control and Bud Cold Hardiness of “Noiret” Winegrape. J. Appl. Hortic. 2015, 17, 106–108. [Google Scholar] [CrossRef]
- Pálfi, X.; Karácsony, Z.; Csikós, A.; Bencsik, O.; Szekeres, A.; Zsófi, Z.; Váczy, K. The Potential Use of the Culture Filtrate of an Aspergillus niger Strain in the Management of Fungal Diseases of Grapevine. J. Cent. Eur. Agric. 2020, 21, 839–850. [Google Scholar] [CrossRef]
- Fenu, G.; Malloci, F.M. Forecasting Plant and Crop Disease: An Explorative Study on Current Algorithms. Big Data Cogn. Comput. 2021, 5, 2. [Google Scholar] [CrossRef]
- Maurin, G.; Cartolaro, P.; Clerjeau, M.; Benac, G. Black-rot: Vers une methode de previsions des risques. Resultats de six annees d’etudes. Phytoma Déf. Végétaux 1991, 433, 39–42. [Google Scholar]
- Ellis, M.A.; Madden, L.V.; Wilson, L.L. Electronic Grape Black Rot Predictor for Scheduling Fungicides with Curative Activity. Plant Dis. 1986, 70, 938. [Google Scholar] [CrossRef]
- Northover, P.R.; Travis, J.W.; Hickey, K.D. Use of a Degree-Day Model to Predict Sporulation of Guignardia bidwellii on Grape. Phytopathology 1997, 87, S70. [Google Scholar]
- Smith, D.L. Grape Pathology Research Update: Black-Rot Advisory. Le Vigneron 2009, 4, 7–8. [Google Scholar]
- Smith, D.L.; Sutherland, A. Mesonet Grape Black-Rot Advisor; Oklahoma State University, 2012. Available online: https://www.mesonet.org/images/site/Grape%20Black%20Rot%20User%20Guide%20-%20Final(1).pdf (accessed on 1 October 2022).
- Black Rot Advisor. Available online: http://mesonet.org/index.php/agriculture/category/horticulture/grape/black_rot_advisor (accessed on 1 October 2022).
- Rossi, V.; Caffi, T.; Onesti, G.; Legler, S.E. Use of Systems Analysis to Develop Plant Disease Models Based on Literature Data: Grape Black-Rot as a Case-Study. Eur. J. Plant Pathol. 2015, 141, 427–444. [Google Scholar] [CrossRef]
- Onesti, G.; González-Domínguez, E.; Rossi, V. Accurate Prediction of Black Rot Epidemics in Vineyards Using a Weather-Driven Disease Model. Pest Manag. Sci. 2016, 72, 2321–2329. [Google Scholar] [CrossRef]
- Prognosemodelle, Wetterdaten Und Monitoring Für Den Weinbau. Available online: https://www.vitimeteo.de/vitimeteo/default/index (accessed on 1 October 2022).
- Defenso. Grape Protection Forecasts. Available online: https://defenso.hu/szolo (accessed on 1 October 2022).
- Hoffman, L.E.; Wilcox, W.F.; Gadoury, D.M.; Seem, R.C.; Riegel, D.G. Integrated Control of Grape Black Rot: Influence of Host Phenology, Inoculum Availability, Sanitation, and Spray Timing. Phytopathology 2004, 94, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Active Substances, Safeners and Synergists. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 10 October 2022).
- Molitor, D.; Baus, O.; Berkelmann-Löhnertz, B. Protective and Curative Grape Black Rot Control Potential of Pyraclostrobin and Myclobutanil. J. Plant Dis. Prot. 2011, 118, 161–167. [Google Scholar] [CrossRef]
- Hoffman, L.E.; Wilcox, W.F. Factors Influencing the Efficacy of Myclobutanil and Azoxystrobin for Control of Grape Black Rot. Plant Dis. 2003, 87, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miessner, S.; Mann, W.; Stammler, G. Guignardia bidwellii, the Causal Agent of Black Rot on Grapevine Has a Low Risk for QoI Resistance. J. Plant Dis. Prot. 2011, 118, 51–53. [Google Scholar] [CrossRef]
- List of Frist Confirmed Cases of Plant Pathogenic Organisms Resistant to Disease Control Agents. Available online: https://www.frac.info/docs/default-source/publications/list-of-resistant-plant-pathogens/list-of-first-confirmed-cases-of-plant-pathogenic-organisms-resistant-to-disease-control-agents_05_2020.pdf (accessed on 4 October 2022).
- Grapevine—Protection Methods. Available online: http://ephytia.inra.fr/en/C/6971/Grapevine-Protection-methods (accessed on 1 October 2022).
- European Parliament; Directorate-General for Parliamentary Research; Kempenaar, C.; Wenneker, M.; Bai, Y.; van Apeldoorn, D.; Riemens, M.; Bremmer, J.; Reinders, M.; Allema, B. The Future of Crop Protection in Europe. Appendix 1, Overview of Current and Emerging Crop Protection Practices; European Parliament, 2021. Available online: https://op.europa.eu/en/publication-detail/-/publication/a4bf1868-410d-11ec-89db-01aa75ed71a1/language-en (accessed on 1 October 2022).
- Shapiro-Ilan, D.I.; Fuxa, J.R.; Lacey, L.A.; Onstad, D.W.; Kaya, H.K. Definitions of Pathogenicity and Virulence in Invertebrate Pathology. J. Invertebr. Pathol. 2005, 88, 1–7. [Google Scholar] [CrossRef]
- Heesemann, J. Pathogenität und Virulenz. In Medizinische Mikrobiologie und Infektiologie; Suerbaum, S., Burchard, G.-D., Kaufmann, S.H.E., Schulz, T.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 13–26. ISBN 978-3-662-48678-8. [Google Scholar]
- Stein, J. Molekulare Mikrobiologie und Immunpathogenese von Helicobacter pylori. In Infektiologie des Gastrointestinaltraktes; Caspary, W.F., Kist, M., Stein, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 151–157. ISBN 978-3-540-37211-0. [Google Scholar]
- Bezerra, J.D.P.; Santos, M.G.S.; Svedese, V.M.; Lima, D.M.M.; Fernandes, M.J.S.; Paiva, L.M.; Souza-Motta, C.M. Richness of Endophytic Fungi Isolated from Opuntia ficus-indica Mill. (Cactaceae) and Preliminary Screening for Enzyme Production. World J. Microbiol. Biotechnol. 2012, 28, 1989–1995. [Google Scholar] [CrossRef]
- Kothe, E. Pilze. In Allgemeine Mikrobiologie; Fuchs, G., Schlegel, H.G., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2007; pp. 78–79. ISBN 978-3-13-444608-1. [Google Scholar]
- Wenzel, C. Identifizierung und Charakterisierung von Putativen Virulenzfaktoren des Gerstenpathogens Rhynchosporium Commune. Doctoral Thesis, Universitäts- und Landesbibliothek Sachsen-Anhalt, Halle, Germany, 2014. [Google Scholar] [CrossRef]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Regulation of Secondary Metabolism in Fungi. Pure Appl. Chem. 1986, 58, 219–226. [Google Scholar] [CrossRef]
- Sutton, B.C. A Century of Mycology; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0-521-57056-5. [Google Scholar]
- Möbius, N.; Hertweck, C. Fungal Phytotoxins as Mediators of Virulence. Curr. Opin. Plant Biol. 2009, 12, 390–398. [Google Scholar] [CrossRef]
- van der Does, H.C.; Rep, M. Virulence Genes and the Evolution of Host Specificity in Plant-Pathogenic Fungi. Mol. Plant-Microbe Interact. 2007, 20, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camañes, G.; Scalschi, L.; Vicedo, B.; González-Bosch, C.; García-Agustín, P. An Untargeted Global Metabolomic Analysis Reveals the Biochemical Changes Underlying Basal Resistance and Priming in Solanum lycopersicum, and Identifies 1-Methyltryptophan as a Metabolite Involved in Plant Responses to Botrytis cinerea and Pseudomonas syringae. Plant J. 2015, 84, 125–139. [Google Scholar] [CrossRef]
- Hu, Z.; Chang, X.; Dai, T.; Li, L.; Liu, P.; Wang, G.; Liu, P.; Huang, Z.; Liu, X. Metabolic Profiling to Identify the Latent Infection of Strawberry by Botrytis cinerea. Evol. Bioinforma. 2019, 15, 1176934319838518. [Google Scholar] [CrossRef] [Green Version]
- Pontes, J.G.D.M.; Fernandes, L.S.; dos Santos, R.V.; Tasic, L.; Fill, T.P. Virulence Factors in the Phytopathogen–Host Interactions: Afn Overview. J. Agric. Food Chem. 2020, 68, 7555–7570. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Andolfi, A. Phytopathogenic Fungi and Toxicity. Toxins 2021, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cornelissen, B.; Rep, M. Host-Specificity Factors in Plant Pathogenic Fungi. Fungal Genet. Biol. 2020, 144, 103447. [Google Scholar] [CrossRef] [PubMed]
- Kellner, N.; Antal, E.; Szabó, A.; Matolcsi, R. The Effect of Black Rot on Grape Berry Composition. Acta Aliment. 2022, 51, 126–133. [Google Scholar] [CrossRef]
- Poitou, X.; Redon, P.; Pons, A.; Bruez, E.; Delière, L.; Marchal, A.; Cholet, C.; Geny-Denis, L.; Darriet, P. Methyl Salicylate, a Grape and Wine Chemical Marker and Sensory Contributor in Wines Elaborated from Grapes Affected or Not by Cryptogamic Diseases. Food Chem. 2021, 360, 130120. [Google Scholar] [CrossRef]
- Buckel, I.; Andernach, L.; Schüffler, A.; Piepenbring, M.; Opatz, T.; Thines, E. Phytotoxic Dioxolanones Are Potential Virulence Factors in the Infection Process of Guignardia bidwellii. Sci. Rep. 2017, 7, 8926. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Heerklotz, K.F.; Drandarov, K.; Heerklotz, J.; Hesse, M.; Werner, C. Guignardic Acid, a Novel Type of Secondary Metabolite Produced by the Endophytic Fungus Guignardia sp.: Isolation, Structure Elucidation, and Asymmetric Synthesis. Helv. Chim. Acta 2001, 84, 3766–3772. [Google Scholar] [CrossRef]
- Yip, H.-Y. Five New Species of Phyllosticta on Australian Native Plants. Mycol. Res. 1989, 93, 489–496. [Google Scholar] [CrossRef]
- Molitor, D.; Liermann, J.C.; Berkelmann-Löhnertz, B.; Buckel, I.; Opatz, T.; Thines, E. Phenguignardic Acid and Guignardic Acid, Phytotoxic Secondary Metabolites from Guignardia bidwellii. J. Nat. Prod. 2012, 75, 1265–1269. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-Endophyte Symbiosis, an Ecological Perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef]
- Buckel, I.; Molitor, D.; Liermann, J.C.; Sandjo, L.P.; Berkelmann-Löhnertz, B.; Opatz, T.; Thines, E. Phytotoxic Dioxolanone-Type Secondary Metabolites from Guignardia bidwellii. Phytochemistry 2013, 89, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sommart, U.; Rukachaisirikul, V.; Trisuwan, K.; Tadpetch, K.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Tricycloalternarene Derivatives from the Endophytic Fungus Guignardia bidwellii PSU-G11. Phytochem. Lett. 2012, 1, 139–143. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Bhat, J.D.; Mortimer, P.E.; Xu, J.; Promputtha, I.; Doilom, M.; Yang, J.-B.; Tang, A.M.C.; Karunarathna, S.C. Identification of Endophytic Fungi from Leaves of Pandanaceae Based on Their Morphotypes and DNA Sequence Data from Southern Thailand. MycoKeys 2018, 33, 25–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PubChem. Alaguignardic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/102435402 (accessed on 30 September 2022).
- PubChem. Guignardianone A. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/102435403 (accessed on 30 September 2022).
- PubChem. Guignardianone E. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/71682251 (accessed on 30 September 2022).
- PubChem. Guignardianone F. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/71682252 (accessed on 30 September 2022).
- PubChem. Guignardic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11268495 (accessed on 30 September 2022).
- PubChem. Phenguignardic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/72204238 (accessed on 30 September 2022).
- Hennings, P.C. Fungi S. Paulenses IV. a Cl. Puttmans Collecti. Hedwigia 1908, 48, 13. [Google Scholar]
- McCoy, P. Radical Mycology: A Treatise on Seeing & Working With Fungi, 1st ed.; Chthaeus Press: Portland, OR, USA, 2016; ISBN 978-0-9863996-0-2. [Google Scholar]
- Yamano, Y.; Ito, M. Synthesis of Optically Active Vomifoliol and Roseoside Stereoisomers. Chem. Pharm. Bull. 2005, 53, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Shimada, H.; Saka, M.; Yoshizumi, S.; Yamahara, J.; Matsuda, H. Medicinal Foodstuffs. V. Moroheiya. (1): Absolute Stereostructures of Corchoionosides A, B, and C, Histamine Release Inhibitors from the Leaves of Vietnamese Corchorus olitorius L. (Tiliaceae). Chem. Pharm. Bull. 1997, 45, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, C.L.; Alarcón-Chaidez, F.; Gross, D.C. Pseudomonas Syringae Phytotoxins: Mode of Action, Regulation, and Biosynthesis by Peptide and Polyketide Synthetases. Microbiol. Mol. Biol. Rev. 1999, 63, 266–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckel, I. Beschreibung von phytotoxischen Dioxolanonen als konstitutive Virulenzfaktoren und Etablierung eines Transformationssystems zur zielgerichteten Mutagenese in Guignardia bidwellii. Doctoral Dissertation, Johannes Gutenberg-Universität Mainz, Mainz, Germany, 2015. [Google Scholar] [CrossRef]
- Methyl Salicylate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4133 (accessed on 30 September 2022).
- Tyagi, P.; Singh, A.; Gupta, A.; Prasad, M.; Ranjan, R. Chapter 4—Mechanism and Function of Salicylate in Plant toward Biotic Stress Tolerance. In Emerging Plant Growth Regulators in Agriculture; Aftab, T., Naeem, M., Eds.; Academic Press: Cambridge, CA, USA, 2022; pp. 131–164. ISBN 978-0-323-91005-7. [Google Scholar]
- Karban, R.; Yang, L.H.; Edwards, K.F. Volatile Communication between Plants That Affects Herbivory: A Meta-Analysis. Ecol. Lett. 2014, 17, 44–52. [Google Scholar] [CrossRef]
- Kim, J.; Jang, M.; Shin, E.; Kim, J.; Lee, S.H.; Park, C.G. Fumigant and Contact Toxicity of 22 Wooden Essential Oils and Their Major Components against Drosophila suzukii (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2016, 133, 35–43. [Google Scholar] [CrossRef]
- Gadino, A.N.; Walton, V.M.; Lee, J.C. Evaluation of Methyl Salicylate Lures on Populations of Typhlodromus pyri (Acari: Phytoseiidae) and Other Natural Enemies in Western Oregon Vineyards. Biol. Control 2012, 63, 48–55. [Google Scholar] [CrossRef]
- Chalal, M.; Winkler, J.B.; Gourrat, K.; Trouvelot, S.; Adrian, M.; Schnitzler, J.-P.; Jamois, F.; Daire, X. Sesquiterpene Volatile Organic Compounds (VOCs) Are Markers of Elicitation by Sulfated Laminarine in Grapevine. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nick, P.; Kortekamp, A.; Eibach, R.; Trapp, O. Nutzung genetischer Ressourcen der Europäischen Wildrebe für die Züchtung von Mehltau- und Schwarzfäule-resistenten Reben; Karlsruher Institut für Technologie, Botanisches Institut, D-Karlsruhe; Dienstleistungszentrum Ländlicher Raum (DLR) Rheinpfalz, Abt. Phytomedizin D-Neustadt a. d. W. und Julius Kühn-Institut, Institut für Rebenzüchtung, D-Siebeldingen. 2017. Available online: https://orgprints.org/id/eprint/31980/1/31980-10OE067-Verbund-nick-2017-genetische-ressourcen-wildrebe.pdf (accessed on 30 September 2022).
- Goetz, G.; Fkyerat, A.; Métais, N.; Kunz, M.; Tabacchi, R.; Pezet, R.; Pont, V. Resistance Factors to Grey Mould in Grape Berries: Identification of Some Phenolics Inhibitors of Botrytis Cinerea Stilbene Oxidase. Phytochemistry 1999, 52, 759–767. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Buchenauer, H. Inhibition of Phenylalanine Ammonia-Lyase Suppresses the Resistance Induced by Benzothiadiazole in Wheat to Blumeria graminis f. sp. tritici. Physiol. Mol. Plant Pathol. 2000, 57, 25–34. [Google Scholar] [CrossRef]
- Paolocci, M.; Muganu, M.; Alonso-Villaverde Iglesias, V.; Gindro, K. Leaf Morphological Characteristics and Stilbene Production Differently Affect Downy Mildew Resistance of Vitis vinifera Varieties Grown in Italy. Vitis-J. Grapevine Res. 2014, 53, 155–161. [Google Scholar]
- Polesani, M.; Bortesi, L.; Ferrarini, A.; Zamboni, A.; Fasoli, M.; Zadra, C.; Lovato, A.; Pezzotti, M.; Delledonne, M.; Polverari, A. General and Species-Specific Transcriptional Responses to Downy Mildew Infection in a Susceptible (Vitis vinifera) and a Resistant (V. riparia) Grapevine Species. BMC Genomics 2010, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, D.; Halter, D.; Baltenweck, R.; Tisch, C.; Tröster, V.; Kortekamp, A.; Hugueney, P.; Nick, P. Genetic Diversity of Stilbene Metabolism in Vitis sylvestris. J. Exp. Bot. 2015, 66, 3243–3257. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Fischer, S.; Merz, P.; Bogs, J.; Riemann, M.; Nick, P. An Ancestral Allele of Grapevine Transcription Factor MYB14 Promotes Plant Defence. J. Exp. Bot. 2016, 67, 1795–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karahalil, B. Overview of Systems Biology and Omics Technologies. Curr. Med. Chem. 2016, 23, 4221–4230. [Google Scholar] [CrossRef] [PubMed]
- Solairaj, D.; Yang, Q.; Guillaume Legrand, N.N.; Routledge, M.N.; Zhang, H. Molecular Explication of Grape Berry-Fungal Infections and Their Potential Application in Recent Postharvest Infection Control Strategies. Trends Food Sci. Technol. 2021, 116, 903–917. [Google Scholar] [CrossRef]
- Singh, N.; Mansoori, A.; Dey, D.; Kumar, R.; Kumar, A. Potential of Metabolomics in Plant Abiotic Stress Management. In Omics Technologies for Sustainable Agriculture and Global Food Security (Vol II); Kumar, A., Kumar, R., Shukla, P., Patel, H.K., Eds.; Springer: Singapore, 2021; pp. 193–214. ISBN 9789811629563. [Google Scholar]
- Feussner, I.; Polle, A. What the Transcriptome Does Not Tell—Proteomics and Metabolomics Are Closer to the Plants’ Patho-Phenotype. Curr. Opin. Plant Biol. 2015, 26, 26–31. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Vinayavekhin, N.; Saghatelian, A. Untargeted Metabolomics. Curr. Protoc. Mol. Biol. 2010, 90, 30.1.1–30.1.24. [Google Scholar] [CrossRef]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Ohtani, K.; Kodama, M.; Akagi, Y.; Egusa, M.; Yamamoto, M.; Otani, H. Host-Selective Toxins Produced by the Plant Pathogenic Fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an Emerging Tool for the Study of Plant–Pathogen Interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Peyraud, R.; Dubiella, U.; Barbacci, A.; Genin, S.; Raffaele, S.; Roby, D. Advances on Plant-Pathogen Interactions from Molecular toward Systems Biology Perspectives. Plant J. Cell Mol. Biol. 2017, 90, 720–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Ma, R.; Chen, X.-L. Advances of Metabolomics in Fungal Pathogen–Plant Interactions. Metabolites 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Jones, O.A.H.; Maguire, M.L.; Griffin, J.L.; Jung, Y.-H.; Shibato, J.; Rakwal, R.; Agrawal, G.K.; Jwa, N.-S. Using Metabolic Profiling to Assess Plant-Pathogen Interactions: An Example Using Rice (Oryza sativa) and the Blast Pathogen Magnaporthe grisea. Eur. J. Plant Pathol. 2011, 129, 539–554. [Google Scholar] [CrossRef]
- Pang, Q.; Zhang, T.; Wang, Y.; Kong, W.; Guan, Q.; Yan, X.; Chen, S. Metabolomics of Early Stage Plant Cell–Microbe Interaction Using Stable Isotope Labeling. Front. Plant Sci. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Misra, B.B.; Langefeld, C.D.; Olivier, M.; Cox, L.A. Integrated Omics: Tools, Advances, and Future Approaches. J. Mol. Endocrinol. 2019, 62, R21–R45. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants Metabolome Study: Emerging Tools and Techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Razzaq, A.; Sadia, B.; Raza, A.; Khalid Hameed, M.; Saleem, F. Metabolomics: A Way Forward for Crop Improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, M.; Ferreira, A.E.N.; Nascimento, R.; Monteiro, F.; Traquete, F.; Marques, A.P.; Cunha, J.; Eiras-Dias, J.E.; Cordeiro, C.; Figueiredo, A.; et al. Integrating Metabolomics and Targeted Gene Expression to Uncover Potential Biomarkers of Fungal/Oomycetes-Associated Disease Susceptibility in Grapevine. Sci. Rep. 2020, 10, 15688. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N. Recent Applications of Metabolomics in Plant Breeding. Breed. Sci. 2022, 72, 56–65. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, M.; Csikász-Krizsics, A.; Dula, T.; Farkas, E.; Roznik, D.; Kozma, P.; Deák, T. Black Rot of Grapes (Guignardia bidwellii)—A Comprehensive Overview. Horticulturae 2023, 9, 130. https://doi.org/10.3390/horticulturae9020130
Szabó M, Csikász-Krizsics A, Dula T, Farkas E, Roznik D, Kozma P, Deák T. Black Rot of Grapes (Guignardia bidwellii)—A Comprehensive Overview. Horticulturae. 2023; 9(2):130. https://doi.org/10.3390/horticulturae9020130
Chicago/Turabian StyleSzabó, Márton, Anna Csikász-Krizsics, Terézia Dula, Eszter Farkas, Dóra Roznik, Pál Kozma, and Tamás Deák. 2023. "Black Rot of Grapes (Guignardia bidwellii)—A Comprehensive Overview" Horticulturae 9, no. 2: 130. https://doi.org/10.3390/horticulturae9020130
APA StyleSzabó, M., Csikász-Krizsics, A., Dula, T., Farkas, E., Roznik, D., Kozma, P., & Deák, T. (2023). Black Rot of Grapes (Guignardia bidwellii)—A Comprehensive Overview. Horticulturae, 9(2), 130. https://doi.org/10.3390/horticulturae9020130






