Bio-Fertilizers Reduced the Need for Mineral Fertilizers in Soilless-Grown Capia Pepper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Bio-Fertilizer Materials and Experimental Conditions
- Common nutrient solution, 100% mineral fertilization (as control) (Table 1),
- 60% mineral fertilization (MF) + PGPR,
- 60% MF + AMF
- 60% MF + PGPR + AMF
- 80% MF + PGPR
- 80% MF + AMF
- 80% MF + PGPR + AMF
2.2. Nutrient Solution and Irrigation
2.3. Parameters Examined in the Experiment
2.3.1. Determination of Leaf Potassium (K), Calcium (Ca), Magnesium (Mg), Iron (Fe), Zinc (Zn), Manganese (Mn), and Copper (Cu) by Atomic Absorption Spectrophotometry
2.3.2. Determination of Leaf Total Nitrogen (N) by the Kjeldahl Method
2.3.3. Determination of Leaf Phosphorus (P) by the Barton Method
2.4. Statistical Analysis
3. Results
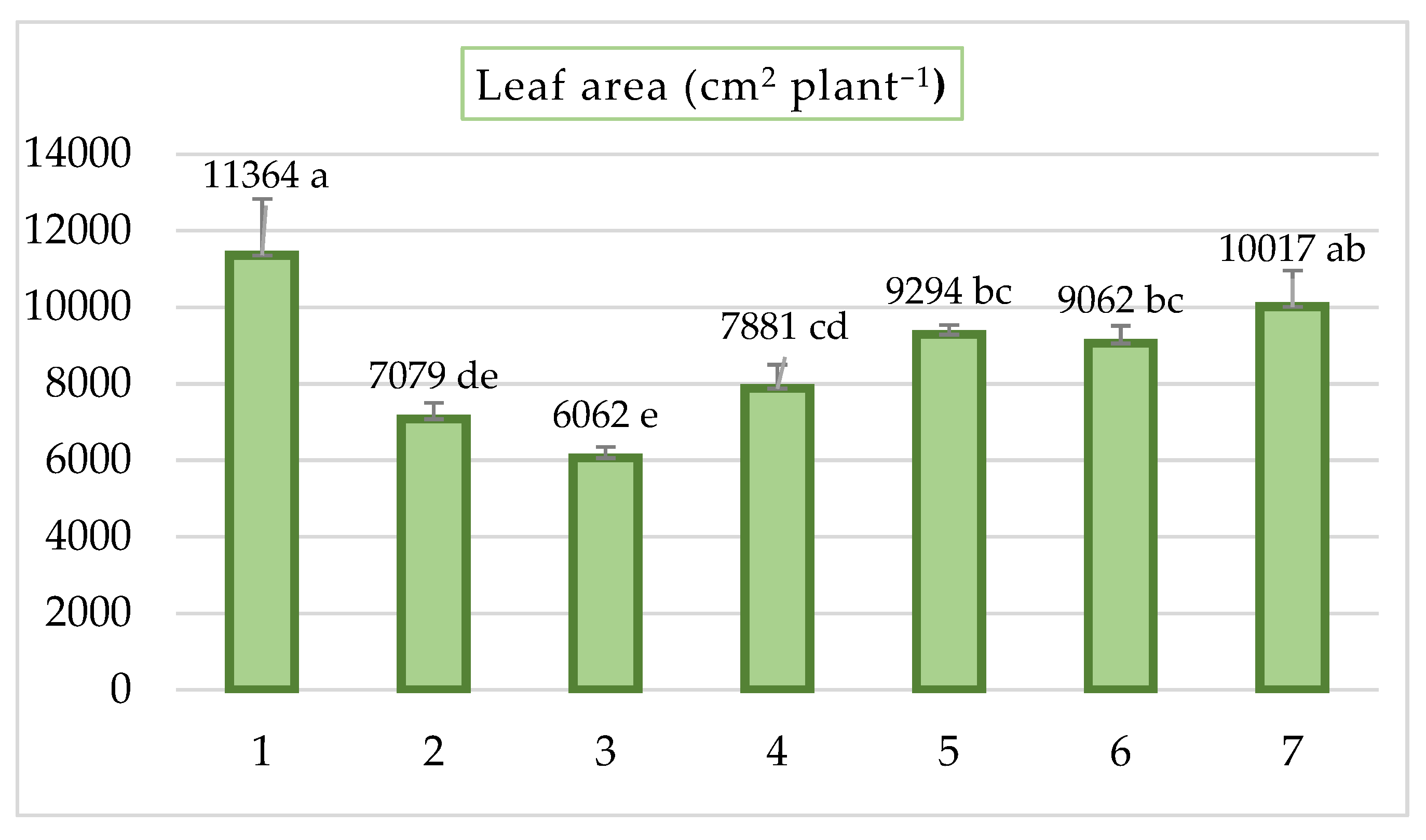
3.1. Effects of Bio-Fertilizers on Plant Growth
3.2. Effects of Bio-Fertilizers on Total Fruit Yield and Fruit Number
3.3. Effects of Bio-Fertilizers on Capia Pepper Fruit Quality Properties
3.4. Effects of Bio-Fertilizers on Mineral Nutrient Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Guo, X.; Guo, Q.; Tan, X.; Wang, Z. Long-term chili monoculture alters environmental variables affecting the dominant microbial community in rhizosphere Soil. Front. Microbiol. 2021, 12, 681953. [Google Scholar]
- Dere, S.; Coban, G.A.; Akhoundnejad, Y.; Ozsoy, Y.S.; Dasgan, H.Y. Use of mycorrhiza to reduce mineral fertilizers in soilless melon (Cucumis melo L) Cultivation. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1331–1336. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Gulia, U.; Shukla, J.; Nishanth, S.; Kokila, V.; Bharti, A.; Singh, A.K.; Shivay, Y.S.; Prasanna, R. Fortifying nursery soil-less media with cyanobacteria for enhancing the growth of tomato. S. Afr. J. Bot. 2022, 146, 564–572. [Google Scholar] [CrossRef]
- Aini, N.; Yamika WS, D.; Ulum, B. Effect of nutrient concentration, PGPR and AMF on plant growth, yield and nutrient uptake of hydroponic lettuce. Int. J. Agric. Biol. 2019, 21, 175–183. [Google Scholar]
- Gul, A.; Kidioglu, F.; Tuzel, Y. Effects of nutrition and Bacillus amyloliquefaciens on tomato (Solanum lycopersicum L.). Span. J. Agric. Res. 2008, 6, 422–429. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Kusvuran, S.; Ortas, I. Responses of soilless grown tomato plants to arbuscular mycorrhizal fungal (Glomus fasciculatum) colonization in re-cycling and open systems. Afr. J. Biotechnol. 2008, 7, 3606–3613. [Google Scholar]
- Ikiz, O.; Abak, K.; Dasgan, H.Y.; Ortas, I. Effects of mycorrhizal inoculation in soilless culture on pepper plant growth. Acta Hortic. 2009, 807, 533–540. [Google Scholar] [CrossRef]
- El-Tohamy, W.A.; El-Abagy, H.M.; El-Greadly NH, M.; Gruda, N. Hormonal changes, growth and yield of tomato plants in response to chemical and bio-fertilization application in sandy soils. J. Appl. Bot. Food Qual. 2009, 82, 179–182. [Google Scholar]
- Abak, K.; Dasgan, H.Y.; Rehber, Y.; Ortas, I. Effect of vesicular arbuscular mycorrhizas on plant growth of soilless grown muskmelon. Acta Hortic. 2010, 871, 301–306. [Google Scholar] [CrossRef]
- Aini, N.; Yamika, W.S.D.; Pahlevi, R.W. The effect of nutrient concentration and inoculation of PGPR and AMF on the yield and fruit quality of hydroponic cherry tomatoes (Lycopersicum esculentum Mill. var. cerasiforme). J. Appl. Hortic. 2019, 21, 116–122. [Google Scholar] [CrossRef]
- Nasiri, A.; Yarnia, M.; Hassanpanah, D.; Farahvash, F.; Khalivand, E. The response of different potato cultivars to plant growth-promoting rhizobacteria (PGPRs) and chemical fertilizers in aeroponic culture conditions. J. Plant Nutr. 2022, 45, 2975–2985. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aldiyab, A.; Elgudayem, F.; Ikiz, B.; Gruda, N.S. Effect of biofertilizers on leaf yield, nitrate amount, mineral content and antioxidants of basil (Ocimum basilicum L.) in a floating culture. Sci. Rep. 2022, 12, 20917. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables—A review. Eur. J. Hortic. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aydoner, G.; Akyol, M. Use of some microorganisms as bio-fertilizers in soilless grown squash for saving chemical nutrients. Acta Hortic. 2012, 927, 155–162. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Raffo, A.; Baiamonte, I.; Nardo, N.; Paoletti, F. Internal quality and antioxidants content of coldstored red sweet peppers as affected by polyethylene bag packaging and hot water treatment. Eur. Food Res. Technol. 2007, 225, 395–405. [Google Scholar] [CrossRef]
- Karaagac, O.; Balkaya, A. Bafra red pepper populations (Capsicum annuum L. var. conoides (Mill.) Irish] identification and evaluation of variation available. Anadolu J. Agric. Sci. 2010, 25, 10–20. [Google Scholar]
- Tüik. Turkish Statistical Institute. 2022. Available online: https://data.tuik.gov.tr/Bulten/Index?p=Bitkisel-Uretim-Istatistikleri-2022-45504 (accessed on 21 January 2023).
- Akyol, M.; Dasgan, H.Y. The Effects of partial root drying in greenhouse soilless bell pepper cultivation. 9th Natl. Veg. Symp. Proc. 2012, 1, 305–309. [Google Scholar]
- Schröder, F.G.; Lieth, J.H. Irrigation Control in Hydroponics. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H., Eds.; Embryo Publishers: Athens, Greece, 2022; pp. 263–298. [Google Scholar]
- Jones, J.B. Laboratory Guid for Conducting Soil Tests Plant Analysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Lucas García, J.A.; Probanza, A.; Ramos, B.; Palomino, M.; Mañero, F.J.G. Effect of inoculation of Bacillus licheniformis on tomato and pepper. Agronomie 2004, 24, 169–176. [Google Scholar] [CrossRef]
- Sinha, R.K.; Valani, D.; Chauhan, K.; Agarwal, S. Embarking on a second green revolution for sustainable agriculture by vermiculture biotechnology using earthworms: Reviving the dreams of Sir Charles Darwin. J. Agric. Biotechnol. Sustain. Dev. 2010, 2, 113–128. [Google Scholar]
- Zayed, M.S. Improvement of growth and nutritional quality of Moringa oleifera using different biofertilizers. Ann. Agric. Sci. 2012, 57, 53–62. [Google Scholar] [CrossRef]
- Prasad, M.R.; Srinivasan, M.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing (Elsevier): Sawston, UK, 2019; pp. 129–157. [Google Scholar]
- Kandiannan, K.; Sivaraman, K.; Anandaraj, M.; Krishnamurthy, K.S. Growth and nutrient content of black pepper (Piper nigrum L.) cuttings as influenced by inoculation with biofertilizers. J. Spices Aromat. Crops 2000, 9, 145–147. [Google Scholar]
- Maboko, M.M.; Bertling, I.; Du Plooy, C.P. Arbuscular mycorrhiza has limited effects on yield and quality of tomatoes grown under soilless cultivation. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2013, 63, 261–270. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Michałojć, Z.; Jarosz, Z.; Pitura, K.; Dzida, K. Effect of mycorrhizal colonization and nutrient solutions concentration on the yielding and chemical composition of tomato grown in rockwool and straw medium. Acta Sci. Pol. Hortorum Cultus 2015, 14, 5–27. [Google Scholar]
- Gruda, N.S. Advances in Soilless Culture and Growing Media in Today’s Horticulture An Editorial. Agronomy 2022, 12, 2773. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, M.; Rajesh, K.; Khushboo, S.; Manoj, K. Evaluation of native PGPR isolates in bell pepper for enhanced growth, yield and fruit quality. Int. J. Farm Sci. 2018, 2, 28–35. [Google Scholar]
- Hochmuth, G.; Maynard, D.; Vavrina, C.; Hanlon, E.; Simonne, E. Plant Tissue Analysis and Interpretation for Vegetable Crops in Florida; IFAS Extension; University of Florida: Gainesville, FL, USA, 2012. [Google Scholar]
- Ortas, I. Do maize and pepper plants depend on mycorrhizae in terms of phosphorus and zinc uptake? J. Plant Nutr. 2012, 35, 1639–1656. [Google Scholar] [CrossRef]
- Sreenivasa, M.N.; Krishnaraj, P.U. Synergistic interaction between VA mycorrhizal fungi and a phosphate solubilizing bacterium in chilli (Capsicum annuum). Zent. Für Mikrobiol. 1992, 147, 126–130. [Google Scholar] [CrossRef]
- Kim, K.; Yim, W.; Trivedi, P.; Madhaiyan, M.; Deka Boruah, H.P.; Rashedul Islam, M.; Lee, G.; Sa, T.M. Synergistic effects of inoculating arbuscular mycorrhizal fungi and Methylobacterium oryzae strains on growth and nutrient uptake of red pepper (Capsicum annuum L.). Plant Soil 2010, 327, 429–440. [Google Scholar] [CrossRef]
- Tajini, F.; Trabelsi, M.; Drevon, J.J. Co-inoculation with Glomus intraradices and Rhizobium tropici CIAT899 increases P use efficiency for N2 fixation in the common bean (Phaseolus vulgaris L.) under P deficiency in hydro-aeroponic culture. Symbiosis 2011, 53, 123–129. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, D.P.; Alström, S.; Lundquist, P.O. Interactions among Glomus irregulare, arbuscular mycorrhizal spore-associated bacteria, and plant pathogens under in vitro conditions. Mycorrhiza 2012, 22, 437–447. [Google Scholar] [CrossRef]



| N | P | K | Ca | Mg | Fe | Mn | Zn | B | Cu | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| 100–239 | 40–81 | 96–370 | 150–250 | 50–92 | 5–10 | 1.97 | 0.25 | 0.7 | 0.07 | 0.05 |
| Treatments | Plant Height (cm) | Stem Diameter (mm) | Number of Branches |
|---|---|---|---|
| 100%MF | 81.08 b | 10.66 | 6.42 |
| 60%MF + PGPR | 78.75 b | 9.61 | 5.75 |
| 60%MF + AMF | 66.50 c | 9.00 | 5.67 |
| 60%MF + PGPR + AMF | 83.25 b | 9.75 | 5.33 |
| 80%MF + PGPR | 90.83 a | 10.04 | 5.20 |
| 80%MF + AMF | 94.92 a | 10.03 | 5.33 |
| 80%MF+ PGPR + AMF | 98.13 a | 10.09 | 4.92 |
| LSD0.05 | 7.398 | NS | NS |
| P | <0.0001 | 0.1620 | 0.5873 |
| Treatments | Shoot Fresh Weight (g Plant−1) | Leaf Fresh Weight (g Plant−1) |
|---|---|---|
| 100%MF | 635.2 a | 297.5 a |
| 60%MF + PGPR | 377.4 cd | 185.3 de |
| 60%MF + AMF | 308.2 d | 157.7 e |
| 60%MF + PGPR + AMF | 412.8 bcd | 206.3 cd |
| 80%MF + PGPR | 530.6 abc | 258.0 b |
| 80%MF + AMF | 516.8 abc | 243.3 bc |
| 80%MF+ PGPR + AMF | 545.0 ab | 262.3 ab |
| LSD0.05 | 156.484 | 34.008 |
| P | 0.0053 | <0.0001 |
| Treatments | Weight (g) | Height (cm) | Diameter (mm) | Volume (cm3) | Firmness (kg cm−3) | FLESH Thickness (mm) |
|---|---|---|---|---|---|---|
| 100%MF | 105.11 c | 20.33 a | 49.69 cd | 201 bc | 4.21 | 4.33 |
| 60%MF + PGPR | 109.70 bc | 19.95 a | 51.59 bc | 208 ab | 4.00 | 4.23 |
| 60%MF + AMF | 91.96 d | 19.25 a | 47.68 d | 163 c | 4.13 | 3.93 |
| 60%MF + PGPR + AMF | 81.81 d | 16.78 b | 48.24 d | 168 c | 4.16 | 3.65 |
| 80%MF + PGPR | 112.54 bc | 19.70 a | 55.40 a | 231 ab | 3.81 | 4.03 |
| 80%MF + AMF | 118.86 ab | 19.40 a | 54.88 ab | 230 ab | 4.04 | 4.05 |
| 80%MF+ PGPR + AMF | 125.16 a | 20.73 a | 54.94 a | 246 a | 3.98 | 4.12 |
| LSD0.05 | 11.382 | 1.845 | 3.321 | 38.531 | NS | NS |
| P | <0.0001 | 0.0075 | 0.0001 | 0.0013 | 0.4228 | 0.3261 |
| Treatments | EC (µS cm−1) | pH | TSS (%) | TA (%) |
|---|---|---|---|---|
| 100%MF | 2070 b | 5.27 | 5.90 d | 1.35 |
| 60%MF + PGPR | 2642 a | 5.01 | 7.00 a | 1.79 |
| 60%MF + AMF | 2671 a | 5.04 | 7.10 a | 1.67 |
| 60%MF + PGPR + AMF | 2487 a | 5.10 | 6.93 ab | 1.78 |
| 80%MF + PGPR | 2542 a | 5.16 | 6.10 cd | 1.61 |
| 80%MF + AMF | 2577 a | 5.00 | 6.23 bcd | 1.71 |
| 80%MF+ PGPR + AMF | 2445 a | 5.11 | 6.85 ab | 1.61 |
| LSD0.05 | 288.059 | NS | 0.326 | NS |
| p | 0.007 | 0.193 | 0.0222 | 0.242 |
| Treatments | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| 100%MF | 3.46 c | 0.37 c | 4.94 d | 1.28 d | 1.10 c |
| 60%MF + PGPR | 3.73 c | 0.40 c | 5.04 cd | 1.94 ab | 1.16 abc |
| 60%MF + AMF | 3.98 bc | 0.61 a | 5.32 bc | 1.88 abc | 1.21 ab |
| 60%MF + PGPR + AMF | 4.34 b | 0.52 b | 5.51 b | 1.64 bc | 1.11 bc |
| 80%MF + PGPR | 5.65 a | 0.49 b | 5.61 ab | 1.59 c | 1.22 a |
| 80%MF + AMF | 5.62 a | 0.48 b | 5.88 a | 2.06 a | 1.26 a |
| 80%MF+ PGPR + AMF | 5.57 a | 0.42 c | 5.89 a | 1.88 abc | 1.23 a |
| LSD0.05 | 0.536 | 0.0496 | 0.341 | 0.296 | 0.104 |
| p | ˂0.0001 | ˂0.0001 | ˂0.0001 | 0.0005 | 0.0255 |
| Treatments | Fe | Mn | Zn | Cu |
|---|---|---|---|---|
| 100%MF | 71.48 c | 23.30 e | 41.41 cd | 8.36 b |
| 60%MF + PGPR | 82.65 bc | 43.27 de | 46.88 c | 10.75 a |
| 60%MF + AMF | 171.67 a | 86.04 ab | 39.69 d | 10.97 a |
| 60%MF + PGPR + AMF | 174.81 a | 75.40 abc | 43.44 cd | 10.72 a |
| 80%MF + PGPR | 154.25 a | 92.04 a | 55.31 b | 11.94 a |
| 80%MF + AMF | 147.06 a | 64.70 bcd | 58.44 ab | 12.25 a |
| 80%MF+ PGPR + AMF | 107.63 b | 54.22 cd | 61.56 a | 11.25 a |
| LSD0.05 | 31.121 | 25.501 | 5.598 | 1.768 |
| p | ˂0.0001 | 0.0002 | ˂0.0001 | 0.0060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgan, H.Y.; Yilmaz, M.; Dere, S.; Ikiz, B.; Gruda, N.S. Bio-Fertilizers Reduced the Need for Mineral Fertilizers in Soilless-Grown Capia Pepper. Horticulturae 2023, 9, 188. https://doi.org/10.3390/horticulturae9020188
Dasgan HY, Yilmaz M, Dere S, Ikiz B, Gruda NS. Bio-Fertilizers Reduced the Need for Mineral Fertilizers in Soilless-Grown Capia Pepper. Horticulturae. 2023; 9(2):188. https://doi.org/10.3390/horticulturae9020188
Chicago/Turabian StyleDasgan, Hayriye Yildiz, Mehmet Yilmaz, Sultan Dere, Boran Ikiz, and Nazim S. Gruda. 2023. "Bio-Fertilizers Reduced the Need for Mineral Fertilizers in Soilless-Grown Capia Pepper" Horticulturae 9, no. 2: 188. https://doi.org/10.3390/horticulturae9020188
APA StyleDasgan, H. Y., Yilmaz, M., Dere, S., Ikiz, B., & Gruda, N. S. (2023). Bio-Fertilizers Reduced the Need for Mineral Fertilizers in Soilless-Grown Capia Pepper. Horticulturae, 9(2), 188. https://doi.org/10.3390/horticulturae9020188









