Survey of the Influences of Microbial Biostimulants on Horticultural Crops: Case Studies and Successful Paradigms
Abstract
1. Introduction to Microbial Biostimulants
2. Mechanisms of Microbial Biostimulant Action
2.1. Modes of Action of Plant Growth-Promoting Rhizobacteria
2.2. Modes of Action of Arbuscular Mycorrhizal Fungi
2.3. Indirect Effects of Microbial Biostimulants
3. Case Studies and Practical Application of Microbial Biostimulants on Horticultural Crops
3.1. Plant Growth-Promoting Rhizobacterias (PGPRs)
3.2. Arbuscular Mycorrhizal Fungi (AMFs)
4. Future Remarks and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Using bacteria and fungi as plant biostimulants for sustainable agricultural production systems. Recent Pat Biotechnol. 2022, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Sustainable approaches to boost yield and chemical constituents of aromatic and medicinal plants by application of biostimulants. Recent Adv. Food Nutr. Agric. 2022, 13, 72–92. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Olarewaju, O.O.; Arthur, G.D.; Fajinmi, O.O.; Coopoosamy, R.M.; Naidoo, K.K. Biostimulants: Potential benefits of enhancing nutrition efficiency in agronomic and horticultural crops. In Biostimulants for Crops from Seed Germination to Plant Development; Gupta, S., van Staden, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 427–443. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Joly, P.; Calteau, A.; Wauquier, A.; Dumas, R.; Beuvin, M.; Vallenet, D.; Crovadore, J.; Cochard, B.; Lefort, F.; Berthon, J.-Y. From strain characterization to field authorization: Highlights on Bacillus velezensis strain B2 beneficial properties for plants and its activities on phytopathogenic fungi. Microorganisms 2021, 9, 1924. [Google Scholar] [CrossRef]
- Tomas, M.S.J.; Carrasco, M.G.; Lobo, C.B.; Alessandrello, M.J.; Sanchez, L.; Ferrero, M.A. PAH removal by simultaneous and sequential inoculation of Pseudomonas monteilii P26 and Gordonia sp. H19 in the presence of biostimulants. Int. Biodeterior. Biodegrad. 2019, 144, 104752. [Google Scholar] [CrossRef]
- Barros-Rodriguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-aree, W.; Manzanera, M. Regulatory risks associated with bacteria as biostimulants and biofertilizers in the frame of the European Regulation (EU) 2019/1009. Sci. Total Environ. 2020, 740, 140239. [Google Scholar] [CrossRef]
- Mickan, B.S.; Alsharmani, A.R.; Solaiman, Z.M.; Leopold, M.; Abbott, L.K. Plant-dependent soil bacterial responses following amendment with a multispecies microbial biostimulant compared to rock mineral and chemical fertilizers. Front. Plant Sci. 2021, 11, 550169. [Google Scholar] [CrossRef] [PubMed]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2020, 777, 146204. [Google Scholar] [CrossRef]
- Shukla, D.; Shukla, P.; Tandon, A.; Singh, P.C.; Johri, J.K. Chapter 1- Role of microorganism as new generation plant biostimulants: An assessment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; De Pascale, S.; Colla, G. Implications of microbial and non-microbial biostimulatory action on the quality of leafy and fruit vegetables. Acta Hortic. 2020, 1268, 13–18. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; Pascale, S.D.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Techol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Paterson, J.; Jahanshah, G.; Li, Y.; Wang, Q.; Mehnaz, S.; Gross, H. The contribution of genome mining strategies to the under-standing of active principles of PGPR strains. FEMS Microbiol. Ecol. 2017, 93, fiw249. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial biostimulants as response to modern agriculture needs: Composition, role and application of these innovative products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Macik, M.; Gryta, A.; Frac, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Sakthieaswari, P.; Kannan, A.; Baby, S. Chapter 14—Role of mycorrhizosphere as a biostimulant and its impact on plant growth, nutrient uptake and stress management. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 319–336. [Google Scholar] [CrossRef]
- Rillig, M.C.; Sosa-Hernández, M.A.; Roy, J.; Aguilar-Trigueros, C.A.; Vályi, K.; Lehmann, A. Towards an integrated mycorrhizal technology: Harnessing mycorrhiza for sustainable intensification in agriculture. Front. Plant Sci. 2016, 7, 1625. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.; Rai, K.K.; Zehra, A.; Dubey, M.K.; Kumar, S.; Shukla, V.; Upadhyay, R.S. Microbial Bioformulation-Based Plant Biostimulants: A Plausible Approach Toward Next Generation of Sustainable Agriculture. In Microbial Endophytes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–225. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Palenzuela, J.; Ineichen, K.; da Silva, G.A. Advances in Glomeromycota taxonomy and classification. IMA Fungus 2011, 2, 191–199. [Google Scholar] [CrossRef]
- Tedersoo, L.; Ko, U.; Bahram, M.; Sa, S.; Do, M.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. The Symbionts Forming Arbuscular Mycorrhizas. In Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 13–41. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Song, J.; Han, Y.; Bai, B.; Jin, S.; He, Q.; Ren, J. Diversity of arbuscular mycorrhizal fungi in rhizosphere soils of the Chinese medicinal herb Sophora flavescens Ait. Soil Tillage Res. 2019, 195, 104423. [Google Scholar] [CrossRef]
- Thamsurakul, S.; Nopamonbodi, O.; Charoensook, S.; Roenrungroeng, S. Increasing pineapple yield using VA mycorrhizal fungi. Acta Hortic. 2000, 529, 199–202. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Srivastava, A.K.; Zou, Y.-N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Mattarozzi, M.; Di Zinno, J.; Montanini, B.; Manfredi, M.; Marengo, E.; Fornasier, F.; Ferrarini, A.; Careri, M.; Visioli, G. Biostimulants applied to maize seeds modulate the enzymatic activity and metaproteome of the rhizosphere. Appl. Soil Ecol. 2020, 148, 103480. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Ciriello, M.; Kyriacou, M.C.; De Pascale, S.; Rouphael, Y. An appraisal of critical factors configuring the composition of basil in minerals, bioactive secondary metabolites, micronutrients and volatile aromatic compounds. J. Food Compos. Anal. 2022, 111, 104582. [Google Scholar] [CrossRef]
- Cellini, A.; Spinelli, F.; Donati, I.; Ryu, C.-M.; Kloepper, J.W. Bacterial volatile compound-based tools for crop management and quality. Trends Plant Sci. 2021, 26, 968–983. [Google Scholar] [CrossRef]
- Gupta, S.; Stirk, W.A.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Interactive effects of plant growth-promoting rhizobacteria and a seaweed extract on the growth and physiology of Allium cepa L. (onion). J. Plant Physiol. 2021, 262, 153437. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Poonam; Ahmad, S.; Singh, R. Plant Growth Promoting Microbes: Diverse Roles for Sustainable and Ecofriendly Agriculture. Energy Nexus 2022, 7, 100133. [Google Scholar] [CrossRef]
- Kumari, M.; Swarupa, P.; Kesari, K.K.; Kumar, A. Microbial inoculants as plant biostimulants: A review on risk status. Life 2023, 13, 12. [Google Scholar] [CrossRef]
- Joshi, M.; Parewa, H.P.; Joshi, S.; Sharma, J.K.; Shukla, U.N.; Paliwal, A.; Gupta, V. Chapter 5- Use of Microbial Biostimulants in Organic Farming. In Advances in Organic Farming; Meena, V.S., Meena, S.K., Srinivasarao, C., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 59–73. [Google Scholar] [CrossRef]
- Ganugi, P.; Martinelli, E.; Lucini, L. Microbial biostimulants as a sustainable approach to improve the functional quality in plant-based foods: A review. Curr. Opin. Food Sci. 2021, 41, 217–223. [Google Scholar] [CrossRef]
- Mansoor, S.; Sharma, V.; Mir, M.A.; Mir, J.I.; Nabi, S.U.; Ahmed, N.; Alkahtani, J.; Alwahibi, M.S.; Masoodi, K.Z. Quantification of polyphenolic compounds and relative gene expression studies of phenylpropanoid pathway in apple (Malus domestica Borkh) in response to Venturia inaequalis infection. Saudi J. Biol. Sci. 2020, 27, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Bostock, R. Induced systemic resistance (ISR) against pathogens in the contect of induced plant defences. Ann. Bot. 2002, 89, 503–512. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Tanveer, Y.; Jahangir, S.; Shah, Z.A.; Yasmin, H.; Nosheen, A.; Hassan, M.N.; Illyas, N.; Bajguz, A.; El-Sheikh, M.A.; Ahmad, P. Zinc oxide nanoparticles mediated biostimulant impact on cadmium detoxification and in silico analysis of zinc oxide-cadmium networks in Zea mays L. regulome. Environ. Pollut. 2023, 316, 120641. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Skz, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; Garcia-Estevez, I.; Jimenez-Gomez, A.; Flores-Felix, J.D.; Escribano-Bailon, M.T.; Rivas, R. Rhizobium laguerreae improves productivity and phenolic content of lettuce (Lactuca sativa L.) under saline stress conditions. Foods 2020, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic responses of maize shoots and roots elicited by combinatorial seed treatments with microbial and non-microbial biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.-B.F.; Aldehaish, H.A.; Egamberdieva, D.; Allah, E.F.A. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Pellegrini, M.; Spera, D.; Ercole, C.; del Gallo, M. Allium cepa L. seed inoculation with a consortium of plant growth-promoting bacteria: Effects on plant growth and development and soil fertility status and microbial community. Proceedings 2020, 6, 20. [Google Scholar]
- Mangman, J.S.; Deaker, R.; Rogers, G. Optimal plant growth-promoting concentration of Azospirillum brasilense inoculated to cucumber, lettuce, and tomato seeds varies between bacterial strains. Isr. J. Plant Sci. 2015, 62, 145–152. [Google Scholar] [CrossRef]
- He, Y.; Pantigoso, H.A.; Wu, Z.; Vivanco, J.M. Co-inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimultant to promote growth, yield, and nutrient uptake of tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef]
- Kumar, P.; Erturk, V.S.; Almusawa, H. Mathematical structure of mosaic disease using microbial biostimulants via Caputo and Atangana-Baleanu derivatives. Results Phys. 2021, 24, 104186. [Google Scholar] [CrossRef]
- Ruiu, L. Insect Pathogenic bacterial in integrated pest management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.D.; Zheng, Y.; Zheng, L.; Jiang, C.H.; Zhou, D.M.; Guo, J.H. Application of PSX biocontrol preparation confers root-knot nematode management and increased fruit quality in tomato under field conditions. Biocont. Sci. Technol. 2016, 26, 174–180. [Google Scholar] [CrossRef]
- Munhoz, L.D.; Fonteque, J.P.; Santos, I.M.O.; Navarro, M.O.P.; Simionato, A.S.; Goya, E.T.; Rezende, M.I.; Balbi-Pena, M.L.; de Oliveira, A.G.; Andrade, G. Control of bacterial stem rot on tomato by extracellular bioactive compounds produced by Pseudomonas aeruginosa LV strain. Cogent Food Agric. 2017, 31, 1282592. [Google Scholar] [CrossRef]
- Goutam, J.; Singh, R.; Vijayaraman, R.S.; Meena, M. Endophytic Fungi: Carrier of Potential Antioxidants. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Sinagpore, 2018; pp. 539–551. [Google Scholar]
- Liu, K.; Garrett, C.; Fadamiro, H.; Kloepper, J.W. Induction of systemic resistance in Chinese cabbage against black rot by plant growth-promoting rhizobacteria. Biol. Control. 2016, 99, 8–13. [Google Scholar] [CrossRef]
- Shekoofeh, E.; Sepideh, H.; Roya, R. Role of mycorrhizal fungi and salicylic acid in salinity tolerance of Ocimum basilicum resistance to salinity. J. Biotech. 2012, 11, 2223–2235. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Zhang, C.L.; Lin, F.C. Role of diverse non-systemic fungal endophytes in plant performance and response to stress: Progress and approaches. J. Plant Growth Regul. 2010, 29, 116–126. [Google Scholar] [CrossRef]
- Yang, H.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The combined effects of Arbuscular Mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef]
- Qun, H.Z.; Zing, H.C.; Bin, Z.Z.; Rong, Z.Z.; Song, W.H. Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCL stress. Coll. Surf. B Bioint. 2007, 59, 128–133. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [PubMed]
- Parre, E.; Ghars, M.A.; Leprince, A.S.; Thiery, L.; Lefebvre, D.; Bordenave, M.; Richard, L.; Mazars, C.; Abdelly, C.; Savoure, A. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol. 2007, 144, 503–512. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Ahmad, A.; Hemant Ganie, A.H.; Aref, I.M.; Iqbal, M. Potassium and calcium application ameliorates growth and oxidative homeostasis in salt-stressed Indian mustard (Brassica juncea) plants. Pak. J. Bot. 2015, 47, 1629–1639. [Google Scholar]
- Estrada, B.; Aroca, R.; Maathuis, F.J.M.; Barea, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 2013, 36, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Moodley, V.; Piater, L.; Steenkamp, P.; Buthelezi, N.; Dubery, I.; Burgess, K.; Huyser, J.; Tugizimana, F. A metabolomic landscape of mize plants treated with a microbial biostimulant under well-watered and drought conditions. Front. Plant Sci. 2021, 12, 676632. [Google Scholar] [CrossRef]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular mechanisms associated with microbial biostimulant-mediated growth enhancement, priming and drought stress tolerance in maize plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef]
- Romano, I.; Ventorino, V.; Pepe, O. Effectiveness of plant beneficial microbes: Overview of the methodological approaches for the assessment of root colonization and persistence. Front. Plant Sci. 2020, 11, 6. [Google Scholar] [CrossRef]
- Bhalerao, R.P.; Eklof, J.; Ljung, K.; Marchant, A.; Bennett, M.; Sandberg, G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002, 29, 32–332. [Google Scholar] [CrossRef]
- Theocharis, A.; Bordiec, S.; Fernandez, O.; Paquis, S.; Dhondt-Cordelier, S.; Baillieul, F.; Clément, C.; Barka, E.A. Burkholderia phytofirmans PsJN Primes Vitis vinifera L. and Confers a Better Tolerance to Low Nonfreezing Temperatures. Mol. Plant Microbe Interact. 2012, 25, 241–249. [Google Scholar] [CrossRef]
- Tiryaki, D.; Aydin, I.; Atici, O. Psychrotolerant bacteria isolated from the lead apoplast of cold-adapted wild plants improve the cold resistance of bean (Phaseolus vulgaris L.) under low temperature. Cyrobiology 2019, 86, 111–119. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, S.; Selvakumar, G.; Bisht, S.C.; Bisht, J.K.; Kundu, S.; Gupta, H.S. Characterisation of a psychrotolerant plant growth promotingPseudomonas sp. strain PGERs17 (MTCC 9000) isolated from North Western Indian Himalayas. Ann. Microbiol. 2008, 58, 561–568. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth wheat (Triticum spp.) under heat stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Improved heat stress tolerance of wheat seedlings by bacteria seed treatment. Plant Soil. 2014, 379, 337–350. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Env. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathology J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Saia, S.; Colla, G.; Raimondi, G.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. An endophytic fungi-based biostimulant modulated lettuce yield, physiological and functional quality responses to both moderate and severe water limitation. Sci. Hortic. 2019, 256, 108595. [Google Scholar] [CrossRef]
- Aroca, R.; Rosa, P.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? N. Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.J.; Park, J.M.; Kim, B.R.; Shin, D.H.; Lee, I.J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of “Orange rubis®” apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Tejada, M.; Rodriguez-Morgado, B.; Gomez, I.; Parrado, J. Degradation of chlorpyrifos using different biostimulants/biofertilizers: Effects on soil biochemical properties and microbial community. Appl. Soil. Ecol. 2014, 84, 158–165. [Google Scholar] [CrossRef]
- Seymen, M.; Erdinc, C.; Kurtar, E.S.; Kal, U.; Sensoy, S.; Turkmen, O. Chapter 12—Potential effect of microbial biostimulants in sustainable vegetable production. In Microbiome Stimulants for Crops; While, J., Kumar, A., Droby, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 193–237. [Google Scholar] [CrossRef]
- Tekaya, M.; Dahmen, S.; Mansour, M.B.; Ferhout, H.; Chehab, H.; Hammami, M.; Attia, F.; Mechri, B. Foliar application of fertilizers and biostimulant has a strong impact on the olive (Olea europaea) rhizosphere microbial community profile and the abundance of arbuscular mycorrhizal fungi. Rhizosphere 2021, 19, 100402. [Google Scholar] [CrossRef]
- Prado, D.Z.D.; Oliveira, S.L.; Okino-Delgado, C.H.; Auer, S.; Ludwig-Muller, J.; Da Silva, M.R.; Fernandes, C.J.D.C.; Carbonari, C.A.; Zambuzzi, W.F.; Fleuri, L.F. Aspergillus flavipes as a novel biostimulant for rooting-enhancement of Eucalyptus. J. Clean Prod. 2019, 234, 681–689. [Google Scholar] [CrossRef]
- Kopta, T.; Pavlikova, M.; Sekara, A.; Pokluda, R.; Marsalek, B. Effect of bacterial-algal biostimulant on the yield and internal quality of lettuce (Lactuca sativa L.) produced for spring and summer crops. Not. Bot. Horti. Agrobot. 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Gaveliene, V.; Socik, B.; Jankovska-Bortkevic, E.; Jurkoniene, S. Plant microbial biostimulants as a promising tool to enhance the productivity and quality of carrot root crops. Microorganisms 2021, 9, 1850. [Google Scholar] [CrossRef]
- Vasseur-Coronado, M.; Boulois, H.D.D.; Pertot, I.; Puopolo, G. Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 2021, 245, 126672. [Google Scholar] [CrossRef]
- Lin, Y.; Jones, M.L. Evaluating the growth-promoting effects of microbial biostimulants on greenhouse floriculture crops. HortScience 2022, 57, 97–109. [Google Scholar] [CrossRef]
- Wazny, R.; Rozpadek, P.; Jedrzejczyk, R.J.; Domka, A.; Nosek, M.; Kidd, P.; Turnau, K. Phytohormone based biostimulant combined with plant growth promoting endophytic fungus enhances Ni phytoextraction of Noccaea goesingensis. Sci. Total Environ. 2021, 789, 147950. [Google Scholar] [CrossRef] [PubMed]
- Aalipour, H.; Nikbakht, A.; Etemadi, N.; Rejali, F.; Soleimani, M. Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci. Hortic. 2020, 261, 108923. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of Casuaria obese (Miq.). Front. Sustain. Food Syst. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Kuyper, T.W.; Boonlue, S. Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L. Sci. Rep. 2020, 10, 4916. [Google Scholar] [CrossRef]
- Khan, M.A.; Asad, S.; Khan, A.L.; Ullah, L.; Ali, S.; Kang, S.-M.; Lee, I.-J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–800. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Ameliorating of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Ait Barka, E.; Nowak, J.; Clement, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef]
- Arkhipova, T.; Prinsen, E.; Veselov, S.; Martinenko, E.; Melentiev, A.; Kudoyarova, G. Cytokinin producing bacteria enhance plant growth and drying soil. Plant Soil Biol. 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Ali, S.; Xie, L. Plant growth promoting and stress mitigating abilities of soil born microorganisms. Recent Pat. Food Nutr. Agric. 2020, 11, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.; Rolli, E.; Ettoumi, B.; Vigani, G.; Mapelli, F.; Borin, S.; Abou-Hadid, A.F.; El-Behairy, U.A.; Sorlini, C.; Cherif, A.; et al. A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE 2012, 7, e48479. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Radhakrishnan, R.; You, Y.-H.; Khan, A.L.; Park, J.-M.; Lee, S.-M.; Lee, I.-J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B Soil Plant. 2015, 65, 36–44. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Sharma, P.K.; Anand, R.C.; Lakshminarayana, L. Construction of Tn5 taged mutants of Rhizobium spp. (Cicer) for ecological studies. Soil Biol. Biochem. 1991, 23, 881–885. [Google Scholar] [CrossRef]
- Drouin, P.; Prevost, D.; Antoun, H. Physiological adaptation to low temperatures of strains of Rhizobium leguminosarum bv. Viciae associated with Lathyrus spp. FEMS Microbiol. Ecol. 2000, 32, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, D.N.; Buendia, A.M.; Camacho, M.; Lucas, M.M.; Santamaria, C. Characterization of Rhizobium spp. bean isolates from South-West Spain. Soil Biol Biochem. 2000, 32, 1601–1613. [Google Scholar] [CrossRef]
- Young, J.M.; Park, D.-C.; Weir, B.S. Diversity of 16S rDNA sequences of Rhizobium spp. Implications for species determinations. FEMS Microbiol. Lett. 2004, 238, 125–131. [Google Scholar] [CrossRef]
- Yates, R.J.; Howieson, J.G.; Reeve, W.G.; Brau, L.; Speijers, J.; Nandasena, K.; Real, D.; Sezmis, E.; O’Hara, G.W. Hot-strain mediated selection for an effective nitrogen-fixing symbiosis between Trifolium spp. And Rhizobium leguminosarum biovar trifolii. Soil Biol. Biochem. 2008, 40, 822–833. [Google Scholar] [CrossRef]
- Shah, A.S.; Wakelin, S.A.; Moot, D.J.; Blond, C.; Noble, A.; Ridgway, H.J. High throughput pH bioassay demonstrates pH adaptation of Rhizobium strains isolated from the nodules of Trifolium subterraneum and T. repens. J. Microbiol. Methods. 2022, 195, 106455. [Google Scholar] [CrossRef] [PubMed]
- Ham, R.V.; O’Callaghan, M.; Geurts, R.; Ridgway, H.J.; Ballard, R.; Noble, A.; Macara, G.; Wakelin, S.A. Soil moisture deficit selects for desiccation tolerant Rhizobium leguminosarum bv. trifolii. Appl. Soil Ecol. 2016, 108, 371–380. [Google Scholar] [CrossRef]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Bini, Y.K.; Subila, K.P.; Aravind, R. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res. 2015, 173, 34–43. [Google Scholar] [CrossRef]
- Aslantas, R.; Cakmakci, R.; Sahin, F. Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci. Hortic. 2007, 111, 371–377. [Google Scholar] [CrossRef]
- Leidi, E.O.; Navarro, D.N.R.; Fernandez, M.; Sarmiento, R.; Semedo, J.; Marques, N.; Matos, A.; Machado, A.P.; Orting, B.; Sorensen, M.; et al. Factors affecting root and seed yield in ahipa (Pachyrhizus ahipa (Wedd.) Parodi), a multipurpose legume crop. Eur. J. Agron. 2004, 20, 395–403. [Google Scholar] [CrossRef]
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130. [Google Scholar] [CrossRef]
- El-Esawi, M.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Env. Exp. Bot. 2018, 159, 55–65. [Google Scholar] [CrossRef]
- Fasciglione, G.; Casanovas, E.M.; Quillehauquy, V.; Yommi, A.K.; Goñi, M.G.; Roura, S.I.; Barassi, C.A. Azospirillum inoculation effects on growth, product quality and storage life of lettuce plants grown under salt stress. Sci. Hortic. 2015, 195, 154–162. [Google Scholar] [CrossRef]
- Barassi, C.A.; Ayrault, G.; Creus, C.M.; Sueldo, R.J.; Sobrero, M.T. Seed inoculation with Azospirillum mitigates NaCl effects on lettuce. Sci. Hortic. 2006, 109, 8–14. [Google Scholar] [CrossRef]
- Rodrigues, G.L.; Matteoli, F.P.; Gazara, R.K.; Rodrigues, P.S.L.; Santos, S.T.D.; Alves, A.F.; Pedrosa-Silva, F.; Oliveira-Pinheiro, I.; Candeo-Alvarenga, D.; Olivares, F.L.; et al. Characterization of cellular, biochemical and genomic features of the diazotrophic plant growth-promoting bacterium Azospirillum sp. UENF-412522, a novel member of the Azospirillum genus. Microbiol. Res. 2022, 254, 126896. [Google Scholar] [CrossRef] [PubMed]
- Gadagi, R.S.; Krishnaraj, P.U.; Kulkarni, J.H.; Sa, T. The effect of combined Azospirillum inoculation and nitrogen fertilizer on plant growth promotion and yield response of the blanket flower Gaillardia pulchella. Sci. Hortic. 2004, 100, 323–332. [Google Scholar] [CrossRef]
- Rabiei, Z.; Hosseini, S.J.; Pirdashti, H.; Hazrati, S. Physiological and biochemical traits in coriander affected by plant growth-promoting rhizobacteria under salt stress. Heliyon 2020, 6, e05321. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Sumbul, A.; Ansari, R.A.; Rizvi, R.; Mahmood, I. Azotobacter: A potential bio-fertilizer for soil and plant health management. Saudi J. Biol. Sci. 2020, 27, 3634–3640. [Google Scholar] [CrossRef]
- Abdel-Aziez, S.; Eweda, W.E.; Girgis, M.G.Z.; Ghany, B.F.A. Improving the productivity and quality of black cumin (Nigella sativa) by using Azotobacter as N2 biofertilizer. Ann. Agric. Sci. 2014, 59, 95–108. [Google Scholar] [CrossRef]
- Ahmed, B.; Syed, A.; Rizvi, A.; Shahid, M.; Bahkali, A.H.; Khan, M.S.; Musarrat, J. Impact of metal-oxide nanoparticles on growth, physiology and yield of tomato (Solanum lycopersicum L.) modulated by Azotobacter salinestris strain ASM. Environ. Poll. 2021, 269, 116218. [Google Scholar] [CrossRef]
- Kumar, A.; Naqvi, S.D.Y.; Kaushik, P.; Khojah, E.; Amir, M.; Alam, P.; Samra, B.N. Rhizophagus irregularis and nitrogen fixing azotobacter enhances greater yam (Dioscorea alata) biochemical profile and upholds yield under reduced fertilization. Saudi J. Biol. Sci. 2022, 29, 3694–3703. [Google Scholar] [CrossRef]
- Kiran, S.; Furtana, G.B.; Ellialtioglu, S.S. Physiological and biochemical assay of drought stress responses in eggplant (Solanum melongena L.) inoculated with commercial inoculant of Azotobacter chroococum and Azotobacter vinelandii. Sci. Hortic. 2022, 305, 111394. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kumar, P.; Raj, H.; Bhardwaj, S.K. Isolation of arbuscular mycorrhizal fungi and Azotobacter chroococum from local litchi orchards and evaluation of their activity in the air-layers system. Sci. Hortic. 2009, 123, 117–123. [Google Scholar] [CrossRef]
- Kashyap, S.; Sharma, S.; Vasudevan, P. Role of bioinoculants in development of salt-resistant saplings of Morus alba (var. Sujanpuri) in vivo. Sci. Hortic. 2004, 100, 291–307. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kumar, P.; Bhardwaj, S.K.; Yadav, S.K. Screening and selecting novel AM fungi and Azotobacter strain for inoculating apple under soil solarization and chemical disinfestation with mulch practices for sustainable nursery management. Sci. Hortic. 2011, 130, 164–174. [Google Scholar] [CrossRef]
- Kumar, A.; Vandana Singh, M.; Singh, P.P.; Singh, S.K.; Kumar, P.K.; Pandey, K.D. Isolation of plant growth promoting rhizobacteria and their impact on growth and curcuin content in Curcuma longa L. Biocatal. Agric. Biotechnol. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Essalimi, B.; Esserti, S.; Rifai, L.A.; Koussa, T.; Makroum, K.; Belfaiza, M.; Rifai, S.; Venisse, J.S.; Faize, L.; Alburquerque, N.; et al. Enhancement of plant growth, acclimatization, salt stress tolerance and verticillium wilt disease resistance using plant growth-promoting rhizobacteria (PGPR) associated with plum trees (Prunus domestica). Sci. Hortic. 2022, 291, 110621. [Google Scholar] [CrossRef]
- He, X.; Xu, M.; Wei, Q.; Tang, M.; Guan, L.; Lou, L.; Xu, X.; Hu, Z.; Chen, Y.; Shen, Z.; et al. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2020, 205, 111333. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Shaharoona, B.; Arshad, M.; Crowley, D.E. Population density and functional diversity of plant growth promoting rhizobacteria associated with avocado trees in saline soils. Appl. Soil Ecol. 2012, 62, 147–154. [Google Scholar] [CrossRef]
- Kaloterakis, N.; Delden, S.H.V.; Hartley, S.; Deyn, G.B.D. Silicon application and plant growth promoting rhizobacteria consisting of six pure Bacillus species alleviate salinity stress in cucumber (Cucumis sativus L.). Sci. Hortic. 2021, 288, 110383. [Google Scholar] [CrossRef]
- Pinter, M.I.F.; Salomon, M.V.; Berli, F.; Gil, R.; Bottini, R.; Piccoli, P. Plant growth promoting rhizobacteria alleviate stress by AsIII in grapevine. Agric. Ecosyst. Environ. 2018, 267, 100–108. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Murali, M.; Singh, S.B.; Lakshmeesha, T.R.; Murthy, K.N.; Amruthesh, K.N.; Niranjana, S.R. Plant growth promoting rhizobacteria-Bacillus amyloliquefaciens improves plant growth and induces resistance in chilli against anthracnose disease. Biol. Control. 2018, 126, 209–217. [Google Scholar] [CrossRef]
- Pinter, I.F.; Salomon, M.V.; Berli, F.; Bottini, R.; Piccoli, P. Characterization of the As(III) tolerance conferred by plant growth promoting rhizobacteria to in vitro-grown grapevine. Appl. Soil Ecol. 2017, 109, 60–68. [Google Scholar] [CrossRef]
- Esitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci. Hortic. 2006, 110, 324–327. [Google Scholar] [CrossRef]
- Visen, A.; Singh, P.N.; Chakraborty, B.; Singh, A.; Bisht, T.S. Scanning electron microscopy indicates Pseudomonad strains facilitate AMF mycorrhization in litchi (Litchi chinensis Sonn.) air-layers and improving survivability, growth and leaf nutrient status. Curr. Res. Microb. Sci. 2021, 2, 100063. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, P.R.; Moradi, R.; Mansoori, H. Influence of planting date, intercropping and plant growth promoting rhizobacteria on cumin (Cuminum cyminum L.) with particular respect to disease infestation in Iran. J. Appl. Res. Med. Aromat. Plants 2014, 1, 134–143. [Google Scholar] [CrossRef]
- Karlidag, H.; Esitken, A.; Turan, M.; Sahin, F. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Sci. Hortic. 2007, 114, 16–20. [Google Scholar] [CrossRef]
- Sang, M.K.; Kim, K.D. Plant growth-promoting rhizobacteria suppressive to Phytophthora blight affect microbial activities and communities in the rhizosphere of pepper (Capsicum annuum L.) in the field. Appl. Soil Ecol. 2012, 62, 88–97. [Google Scholar] [CrossRef]
- Banchio, E.; Bogino, P.C.; Zygadlo, J.; Giordano, W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008, 36, 766–771. [Google Scholar] [CrossRef]
- Chenniappan, C.; Narayanasamy, M.; Daniel, G.M.; Ramaraj, G.B.; Ponnusamy, P.; Sekar, J.; Ramalingam, P.V. Biocontrol efficiency of native plant growth promoting rhizobacteria against rhizome rot disease of turmeric. Biol. Control. 2019, 129, 55–64. [Google Scholar] [CrossRef]
- Kurabachew, H.; Wydra, K. Characterization of plant growth promoting rhizobacteria and their potential as bio-protectant against tomato bacterial wilt caused by Ralstonia solancearum. Biol. Control. 2013, 67, 75–83. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.D.R.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crop. Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Henandez-Soberano, C.; Ruiz-Herrera, L.F.; Valencia-Cantero, E. Endophytic bacteria Arthrobacter agilis UMCV2 and Bacillus methylotrophicus M4-96 stimulate achene germination, in vitro growth, and greenhouse yield of strawberry (Fragaria × ananassa). Sci. Hortic. 2020, 261, 109005. [Google Scholar] [CrossRef]
- James, N.; Umesh, M.; Sarojini, S.; Shanmugam, S.; Nasif, O.; Alharbi, S.A.; Chi, N.T.L.; Brindhadevi, K. Unravelling the potential plant growth activity of halotolerant Bacillus licheniformis NJ04 isolated from soil and its possible use as a green bioinoculant on Solanum lycopersicum L. Environ. Res. 2023, 216, 114620. [Google Scholar] [CrossRef]
- Balderas-Ruiz, K.A.; Gomez-Guerrero, C.I.; Trujillo-Roldan, M.; Valdez-Cruz, N.A.; Aranda-Ocampo, S.; Juarez, A.M.; Leyva, E.; Galindo, E.; Serrano-Carreon, L. Bacillus velezensis 83 increases productivity and quality of tomato (Solanum lycopersicum L.): Pre and postharvest assessment. Curr. Res. Microb. Sci. 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Parra, L.; Mutis, A.; Parada, M.; Quiroz, A. Volatiles emitted by Bacillus sp. BCT9 act as growth modulating agents on Lactuca sativa seedlings. Microbiol. Res. 2017, 203, 47–56. [Google Scholar] [CrossRef]
- Xue, Q.-Y.; Chen, Y.; Li, S.-M.; Chen, L.-F.; Ding, G.-C.; Guo, D.-A.; Guo, J.-H. Evaluation of the strains of Acinetobacter and Enterobacter as potential biocontrol agents against Ralstonia wilt of tomato. Biol. Control. 2009, 48, 252–258. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.; Ming, R.; Huang, D.; Yao, Y.; Li, L.; Huang, R. Burkholderia cenocepacia ETR-B22 volatile organic compounds suppress postharvest grey mould infection and maintain aroma quality of tomato fruit. LWT 2022, 165, 113715. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Q.; Wang, P. Phosphate-solubilizing bacterium Burkholderia sp. strain N3 facilitates the regulation of gene expression and improves tomato seedling growth under cadmium stress. Ecotoxicol. Environ. Saf. 2021, 217, 112268. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for varition in the colonizatio strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Yang, H.; Zang, Y.; Yuan, Y.; Tang, J.; Chen, X. Selectivity by host plants affects the distribution of arbuscular mycorrhizal funi: Evidence from ITS rDNA sequence metadata. BMC Evol. Biol. 2012, 12, 50. [Google Scholar] [CrossRef]
- Cruz, C.; Vishwakarma, K.; Kumar, D.; Varma, A. Soil Nitrogen Ecology; Cruz, C., Vishwakarma, K., Kumar, D., Varma Cham, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Redecker, D.; Schussler, A.; Stockinger, H.; Sturmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Zhu, B.; Gao, T.; Zhang, D.; Fing, K.; Li, C.; Ma, F. Functions of arbuscular mycorrhizal fungi in horticultural crops. Sci. Hortic. 2022, 303, 111219. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, N.; Yu, K.; Zhao, C. The effects of fine roots and arbuscular mycorrhizal fungi on soil macropores. Soil Tillage Res. 2023, 225, 105528. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, B. Arbuscular mycorrhizal fungi reduce soil nitrous oxide emission. Geoderma 2021, 402, 115179. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, L.; Shakeel, M.; Wan, Y.; Song, Z.; Wang, D. Screening of the plant growth-promoting mycorrhizal fungi in Guizhou blueberry. Rhizosphere 2021, 19, 100389. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Xie, M.-M.; Li, Y.; Liu, B.-Y.; Liu, C.-Y.; Wu, Q.-S.; Kuca, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2022, 170, 104308. [Google Scholar] [CrossRef]
- Merlin, E.; Melato, E.; Lourenco, E.L.B.; Jacomassi, E.; Gasparotto Junior, A.; Sete da Cruz, R.M.; Otenio, J.K.; Da Silva, C.; Alberton, O. Inoculation of arbuscular mycorrhizal fungi and phosphorus addition increase coarse mint (Plectranthus amboinicus Lour.) plant growth and essential oil content. Rhizosphere 2020, 15, 100217. [Google Scholar] [CrossRef]
- Alves de Assis, R.M.; Carneiro, J.J.; Medeiros, A.P.R.; Carvalho, A.A.D.; Honorato, A.D.C.; Carneiro, M.A.C.; Bertolucci, S.K.V.; Pinto, J.E.B.P. Arbuscular mycorrhizal fungi and organic manure enhance growth and accumulation of citral, total phenols, and flavonoids in Melissa officinalis L. Ind. Crops Prod. 2020, 158, 112981. [Google Scholar] [CrossRef]
- Baczek, K.B.; Wisniewska, M.; Przybyl, J.L.; Kosakowska, O.; Weglarz, Z. Arbuscular mycorrhizal fungi in chamomile (Matricaria recutita L.) organic cultivation. Ind. Crops Prod. 2019, 140, 111562. [Google Scholar] [CrossRef]
- Chen, K.; Kleijn, D.; Scheper, J.; Fijen, T.P.M. Additive and synergistic effects of arbuscular mycorrhizal fungi, insect pollination and nutrient availability in a perennial fruit crop. Agric Ecosyst. Environ. 2022, 325, 107742. [Google Scholar] [CrossRef]
- Ndiate, N.I.; Qun, C.L.; Nkoh, J.N. Importance of soil amendments with biochar and/or Arbuscular Mycorrhizal fungi to mitigate aluminum toxicity in tamarind (Tamarindus indica L.) on an acidic soil: A greenhouse study. Heliyon 2022, 8, e09009. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Annapurna, K.; Al-Sadi, A.; Alharbi, S.A.; Datta, R.; Zuan, A.T.K. Biochar and arbuscular mycorrhizal fungi mediated enhanced drought tolerance in Okra (Abelmoschus esculentus) plant growth, root morphological traits and physiological properties. Saudi J. Biol. Sci. 2021, 28, 5490–5499. [Google Scholar] [CrossRef]
- Sharma, M.; Saini, I.; Kaushik, P.; Aldawsari, M.M.; Balawi, T.; Alam, P. Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi J. Biol. Sci. 2021, 28, 3685–3691. [Google Scholar] [CrossRef]
- Ziane, H.; Hamza, N.; Meddad-Hamza, A. Arbuscular mycorrhizal fungi and fertilization rates optimize tomato (Solanum lycopersicum L.) growth and yield in a Mediterranean agroecosystem. J. Saudi Soc. Agric. Sci. 2021, 20, 454–458. [Google Scholar] [CrossRef]
- El-Sherbeny, T.M.S.; Mousa, A.M.; El-Sayed, E.-S. Use of mycorrhizal fungi and phosphorus fertilization to improve the yield of onion (Allium cepa L.) plant. Saudi J. Biol. Sci. 2022, 29, 331–338. [Google Scholar] [CrossRef]
- Poveda, J.; Baptista, P. Filamentous fungi as biocontrol agents in olive (Olea europaea L.) diseases: Mycorrhizal and endophytic fungi. Crop. Prot. 2021, 146, 105672. [Google Scholar] [CrossRef]
- Alipour, A.; Rahimi, M.M.; Hosseini, S.M.A.; Bahrani, A. Mycorrhizal fungi and growth-promoting bacteria improves fennel essential oil yield under water stress. Ind. Crops Prod. 2021, 170, 113792. [Google Scholar] [CrossRef]
- Lahbouki, S.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Ait-El-Mokhtar, M.; Gabardi, S.; Douira, A.; Wahbi, S.; Outzourhit, A.; et al. Arbuscular mycorrhizal fungi and/or organic amendment enhance the tolerance of prickly pear (Opuntia ficus-indica) under drought stress. J. Arid. Env. 2022, 199, 104703. [Google Scholar] [CrossRef]
- Leventis, G.; Tsiknia, M.; Feka, M.; Ladikou, E.V.; Papadakis, I.E.; Chatzipavlidis, L.; Papadopoulou, K.; Ehaliotis, C. Arbuscular mycorrhizal fungi enhance growth of tomato under normal and drought conditions, via different water regulation mechanisms. Rhizosphere 2021, 19, 100394. [Google Scholar] [CrossRef]
- Wu, Q.-C.; Xia, R.-X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Tekaya, M.; Dabbaghi, O.; Guesmi, A.; Attia, F.; Chehab, H.; Khezami, L.; Algathami, F.K.; Hamadi, N.B.; Hammadi, M.; Prinsen, E.; et al. Arbuscular mycorrhizas modulate carbohydrate, phenolic compounds and hormonal metabolism to enhance water deficit tolerance of olive trees (Olea europaea). Agric. Water Manag. 2022, 274, 107947. [Google Scholar] [CrossRef]
- El-Nashar, Y.; Hassan, B.A.; Aboelsaadat, E.M. Response of Nemesia (Nemesia × hybridus) plants to different irrigation water sources and arbuscular mycorrhizal fungi inoculation. Agric. Water Manag. 2021, 243, 106416. [Google Scholar] [CrossRef]
- Sensoy, S.; Demir, S.; Turkmen, P.; Erdinc, C.; Burak Savur, O. Responses of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Sci. Hortic. 2007, 113, 92–95. [Google Scholar] [CrossRef]
- Singh, N.V.; Singh, S.K.; Singh, A.K.; Meshram, D.T.; Suroshe, S.S.; Mishra, D.C. Arbuscular mycorrhizal fungi (AMF) induced hardening of micropropagated pomegranate (Punica granatum L.) plantlets. Sci. Hortic. 2012, 136, 122–127. [Google Scholar] [CrossRef]
- Qiu, Y.-J.; Zhang, N.-L.; Zhang, L.-L.; Zhang, X.-L.; Wu, A.-P.; Huang, J.-Y.; Yu, S.-Q.; Wang, Y.-H. Mediation of arbuscular mycorrhizal fungi on growth and biochemical parameters of Ligustrum vicaryi in response to salinity. Physiol. Mol. Plant Pathol. 2020, 112, 101522. [Google Scholar] [CrossRef]
- Pankaj, U.; Kurmi, A.; Lothe, N.B.; Verma, R.K. Influence of the seedlings emergence and initial growth of palmarosa (Cymbopogon martinii (Roxb.) Wats. var. Motia Burk) by arbuscular mycorrhizal fungi in soil salinity conditions. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100317. [Google Scholar] [CrossRef]
- Krishna, H.; Singh, S.K.; Sharma, R.R.; Khawale, R.N.; Grover, M.; Patel, V.B. Biochemical changes in micropropagated grape (Vitis vinifera L.) pantlets due to arbuscular-mycorhhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci. Hortic. 2005, 106, 554–567. [Google Scholar] [CrossRef]
- Arpanahi, A.A.; Feizian, M.; Mehdipourian, G.; Khojasteh, D.N. Arbuscular mycorrhizal fungi inoculation improve essential oil and physiological parameters and nutritional values of Thymus daenensis Celak and Thymus vulgaris L. under normal and drought stress conditions. Eur. J. Soil Biol. 2020, 100, 103217. [Google Scholar] [CrossRef]
- Caser, M.; Victorino, I.M.M.; Demasi, S.; Berruti, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular mycorrhizal fungi association promotes corm multiplication in potted saffron (Crocus sativus L.) plants. Acta. Hortic. 2020, 1287, 441–446. [Google Scholar] [CrossRef]
- Thokchom, S.D.; Gupta, S.; Kapoor, R. Arbuscular mycorrhiza augments essential oil composition and antioxidant properties of Ocimum tenuiflorum L.—A popular green tea additive. Ind. Crops Prod. 2020, 153, 112418. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, A.L.; Cullu, M.A. The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martinez-Andujar, C.; Lachaal, M.; Karray-Bouraoui, N.; Perez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Elhindi, K.M.; El-Din, A.S.; Elgorban, A.M. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 2017, 24, 170–179. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Shan, L.; Wu, Q.; Liu, Y.; Zhang, Z.; Ma, F.; Li, C. Dopamine and arbuscular mycorrhizal fungi act synergistically to promote apple growth under salt stress. Environ. Exp. Bot. 2020, 178, 104159. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, T.; Guo, S.; Liu, R.; Li, M. Leaf anatomy, photosynthesis and chlorophyll fluorescence of lettuce as influenced by arbuscular mycorrhizal fungi under high temperature stress. Sci. Hortic. 2021, 280, 109933. [Google Scholar] [CrossRef]
- Hassena, A.B.; Zouari, M.; Trabelsi, L.; Decou, R.; Amar, F.B.; Chaari, A.; Sousa, N.; Labrousse, P.; Khabou, W.; Zouari, N. Potential effects of arbuscular mycorrhizal fungi in mitigating the salinity of treated wastewater in young olive plants (Olea europaea L. cv. Chetoui). Agric. Water Manag. 2021, 245, 106635. [Google Scholar] [CrossRef]
- Li, C.; Feng, G.; Zhang, J.-L.; Yang, S.; Meng, J.-J.; Geng, Y.; Wang, Q.; Li, X.-G.; Wan, S.-B. Arbuscular mycorrhizal fungi combined with exogenous calcium improves the growth of peanut (Arachis hypogaea L.) seedlings under continuous cropping. J. Integr. Agric. 2019, 18, 407–416. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, L.; Xiao, Y. The combined use of arbuscular mycorrhizal fungi, biochar and nitrogen fertilizer is most beneficial to cultivate Cichorium intybus L. in Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 217, 112154. [Google Scholar] [CrossRef]
- Hu, J.-L.; Lin, X.-G.; Wang, J.-H.; Shen, W.-S.; Wu, S.; Peng, S.-P.; Mao, T.-T. Arbuscular mycorrhizal fungal inoculation enhances suppression of cucumber Fusarium wilt in greenhouse soils. Pedosphere 2010, 20, 586–593. [Google Scholar] [CrossRef]
- Khabou, W.; Hajji, B.; Zouari, M.; Rigane, H.; Abdallah, F.B. Arbuscular mycorrhizal fungi improve growth and mineral uptake of olive tree under gypsum substrate. Ecol. Engin. 2014, 73, 290–296. [Google Scholar] [CrossRef]
- Bencherif, K.; Djaballah, Z.; Brahimi, F.; Boutekrabt, A.; Dalpe, Y.; Sahraoui, A.L.-H. Arbuscular mycorrhizal fungi affect total phenolic content and antimicrobial activity of Tamarix gallica in natural semi-arid Algerian areas. S. Afr. J. Bot. 2019, 125, 39–45. [Google Scholar] [CrossRef]
- Amanifar, S.; Toghranegar, Z. The efficiency of arbuscular mycorrhizal for improving tolerance of Valeriana officinalis L. and enhancing valerenic acid accumulation under salinity stress. Ind. Crops Prod. 2020, 147, 112234. [Google Scholar] [CrossRef]
- Mohandas, S. Arbuscular mycorrhizal fungi benefit mango (Mangifera indica L.) plant growth in the field. Sci. Hortic. 2012, 143, 43–48. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Alotaibi, M.O.; Elobeid, M. Interactive influence of elevated CO2 and arbuscular mycorrhizal fungi on sucrose and coumarin metabolism in Ammi majus. Plant Physiol. Biochem. 2022, 185, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Darakeh, S.A.S.S.; Weisany, W.; Tahir, N.A.-R.; Schenk, P.M. Physiological and biochemical responses of black cumin to vermicompost and plant biostimulants: Arbuscular mycorrhizal and plant growth-promoting rhizobacteria. Ind. Crops Prod. 2022, 188 (Part A), 115557. [Google Scholar] [CrossRef]
- Aggangan, N.S.; Cortes, A.D.; Reaño, C.E. Growth response of cacao (Theobroma cacao L.) plant as affected by bamboo biochar and arbuscular mycorrhizal fungi in sterilized and unsterilized soil. Biocatal. Agric. Biotechnol. 2019, 22, 101347. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Liu, A.; Zhang, S.; Liu, D.; Wang, F.; Lin, X.; He, C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperatures stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Hilali, R.E.; Symanczik, S.; Kinany, S.E.; Oehl, F.; Ouahmane, L.; Bouamri, R. Cultivation, identification, and application of arbuscular mycorrhizal fungi associated with date palm plants in Draa-Tafilalet oasis. Rhizosphere 2022, 22, 100521. [Google Scholar] [CrossRef]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Caruso, T.; Mafrica, R.; Bruno, M.; Vescio, R.; Sorgona, A. Root architectural traits of rooted cuttings of two fig cultivars: Treatments with arbuscular mycorrhizal fungi formulation. Sci. Hortic. 2021, 283, 110083. [Google Scholar] [CrossRef]
- Aganchich, B.; Wahbi, S.; Yaakoubi, A.; El-Aououad, H.; Bota, J. Effect of arbuscular mycorrhizal fungi inoculation on growth and physiology performance of olive trees under regulated deficit irrigation and partial rootzone drying. S. Afr. J. Bot. 2022, 148, 1–10. [Google Scholar] [CrossRef]
- Metwally, R.A.; Soliman, S.A.; Latef, A.A.H.A.; Abdelhameed, R.E. The individual and interactive role of arbuscular mycorrhizal fungi and Trichoderma viride on growth, protein content, amino acids fractionation, and phosphatases enzyme activities of onion plants amended with fish waste. Ecotoxicol. Environ. Saf. 2021, 214, 112072. [Google Scholar] [CrossRef]
- Paymaneh, Z.; Sarcheshmehpour, M.; Mohammadi, H.; Hesni, M.A. Vermicompost and/or compost and arbuscular mycorrhizal fungi are conducive to improving the growth of pistachio seedlings to drought stress. Appl. Soil Ecol. 2013, 182, 104717. [Google Scholar] [CrossRef]
- Chiomento, J.L.T.; Nardi, F.S.D.; Filippi, D.; Trentin, T.D.S.; Dornelles, A.G.; Fornari, M.; Nienow, A.A.; Calvete, E.O. Morpho-horticultural performance of strawberry cultivated on substrate with arbuscular mycorrhizal fungi and biochar. Sci. Hortic. 2021, 282, 110053. [Google Scholar] [CrossRef]
- Xiao, L.; Lai, S.; Chen, M.; Long, X.; Fun, X.; Yang, H. Effects of grass cultivation on soil arbuscular mycorrhizal fungi community in a tangerine orchard. Rhizosphere 2022, 24, 100583. [Google Scholar] [CrossRef]
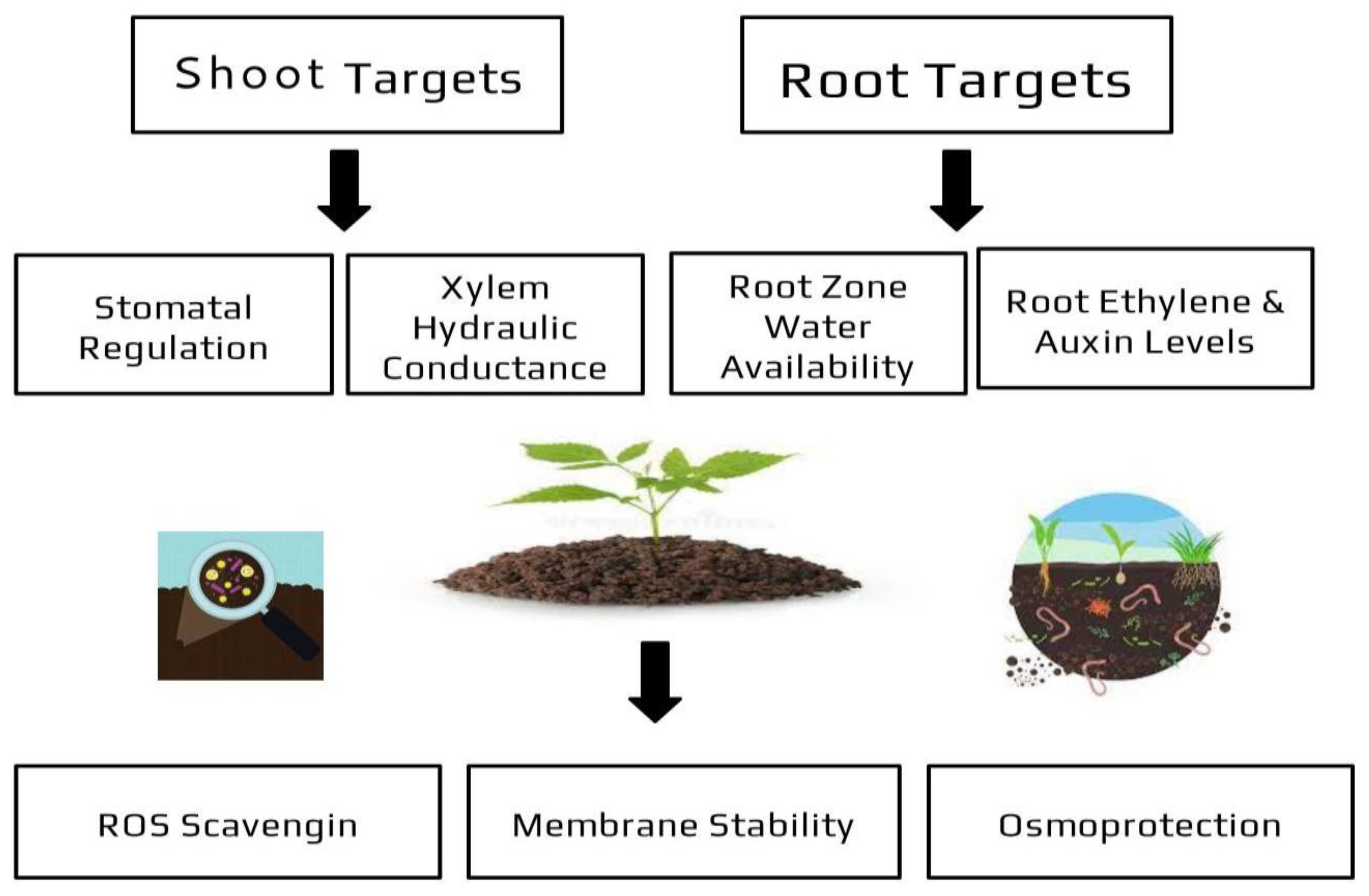
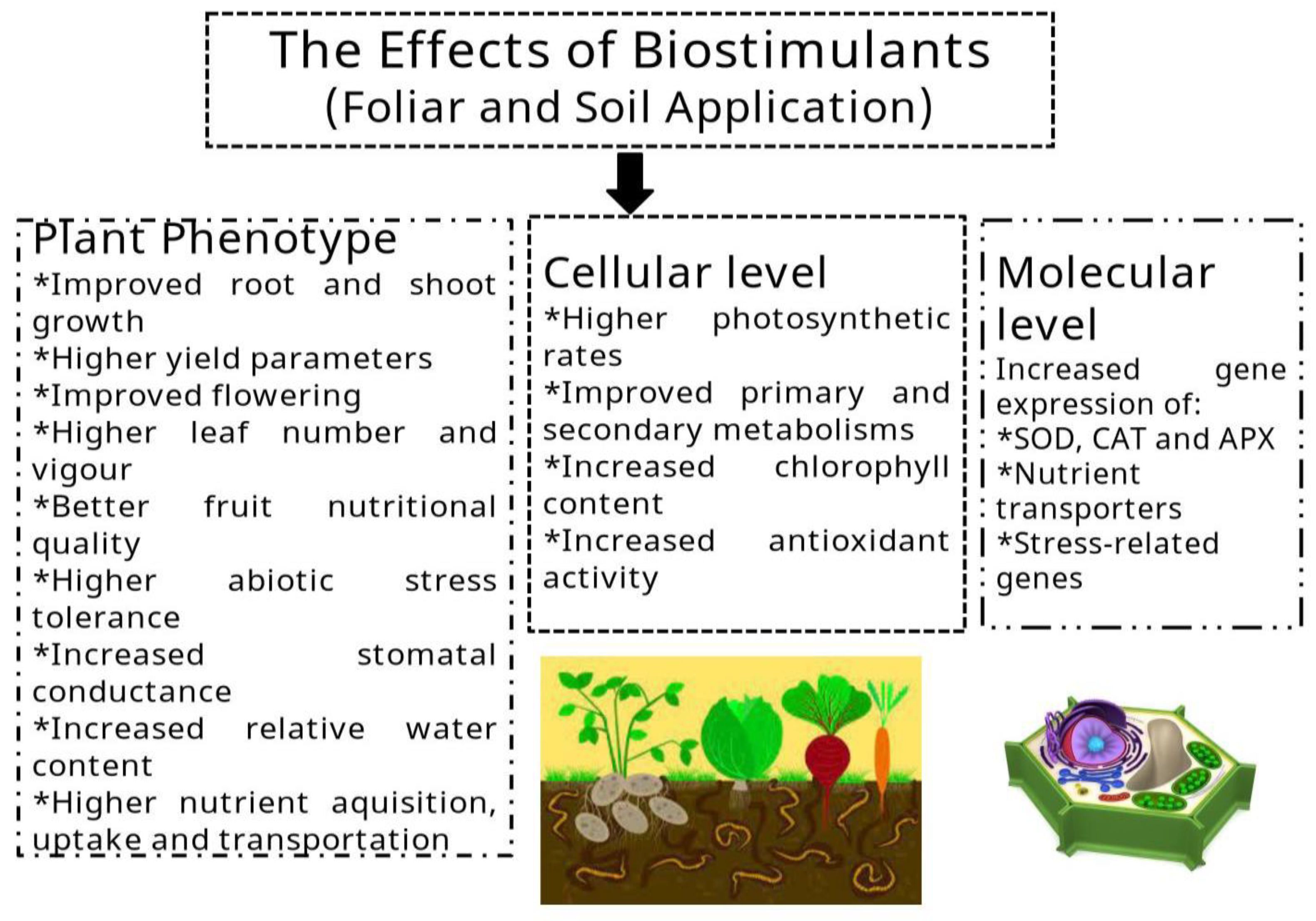
| Stresses | Type of Stresses | Protective Mechanisms | References |
|---|---|---|---|
| Abiotic stress | |||
| Water stress | *Drought *Flooding | *Osmolite production *Increase in antioxidant activity *Phytohormone level modulation *Secretion of Extracellular Polymeric Substances (EPS) | [85,86,87] |
| Thermal stress | *Extreme heat *Freezing | *Emission of volatile organic compounds *Photohormone level modulation *Ice-nucleatin activity antagonism *Osmo and thermal protection *Delay of senescence | [76,77,78,79] |
| Nutrient stress | *Increased soil exploration *Mineral nutrients solubilization | [74,75] | |
| Biotic stress | *Induced system resistance *Phytohormone level modulation *Direct antagonism with pathogens | [47,53,54,55,56] |
| Types | Plant | Effects | Reference |
|---|---|---|---|
| Azotobacter | Eggplant (Solanum melongena L.) | *Azotobacter chroococcum and Azotobacter vinelandii rhizobacteria species have the potential to decrease the adverse impacts of droughts stress by mitigating the drought-related oxidative damage. | [138] |
| Tomato (Solanum lycopersicum L.) | *Azotobacter salinestris strain could be an alternative tool to boost the production of tomato. | [136] | |
| Litchi (Litchi chinensis Sonn.) | *Azotobacter chroococcum strains can be applied for air-layering for better adaptation in different conditions. | [139] | |
| Arthrobacter | Strawberry (Fragaria × ananassa) | *Arthrobacter agilis UMCV2 can be inoculated in micropropagated strawberry plants and increase the yield and fruit quality under greenhouse conditions. | [160] |
| Azospirillum | Lettuce (Lactuca sativa) | *Seed inoculation with Azospirillum could increase product quality and improve storage life in lettuce grown under salt stress. | [163] |
| Bacillus | Tomato (Solanum lycopersicum L.) | *Bacillus licheniformis NJ04 may increase root length and shoot length of treated plants. | [161] |
| Tomato (Solanum lycopersicum L.) | *Bacillus velezensis 83 can be used for biological control of five different genera of phytopathogenic fungi, namely, Botrytis, Sphaerotheca, Leveillula, Erysiphe, and Colletotrichum. | [162] | |
| Lettuce (Lactuca sativa) | *Low concentrations of Bacillus sp. BCT9 improved length and lateral root. | [163] | |
| Enterobactersp. | Tomato (Solanum lycopersicum L.) | *The Xy3 strain of Enterobacter sp. had notable controlling effects against bacterial wilt (Ralstonia solanacearum). | [164] |
| Burkholderia | Tomato (Solanum lycopersicum L.) | *Burkholderia cenocepacia ETR-B22 volatiles suppressed Botrytis cinerea infection. *Microbial volatile organic compounds of Burkholderia cenocepacia ETR-B22 could be used as an important biofumigant for extending postharvest tomato fruit shell life and controlling grey mold disease. | [165] |
| Tomato (Solanum lycopersicum L.) | *Burkholderia sp. strain N3 improved tomato seedling height, dry weight, and nutrient uptake. *It can promote Fe3+ uptake, while Zn2+ absorption accompanied Cd accumulation. *Burkholderia sp. strain N3 facilitated gene expression and alleviated Cd toxicity in tomato plants. | [166] |
| Types | Plant | Effects | Reference |
|---|---|---|---|
| Arbuscular mycorrhizal fungi (AMF) | Bishop’s flower (Ammi majus) | *Its application can induce accumulation of phyto-molecules, coumarin, which might improve its medicinal and pharmacological applications. | [215] |
| Black cumin (Nigella sativa Linn.) | *The colonization can increase relative water content (RWC), Chl b content, and micronutrient uptake. | [216] | |
| Cacao (Theobroma cacao L.) | *It can improve the overall growth and can positively increase the yield of cacao plants in acidic soils. | [217] | |
| Glomus tortuosum | Chicory (Cichorium intybus L.) | *AMF, biochar and N fertilizer applications enhanced chicory biomass.*AMF and biochar applications increased nutrient absorption, and reduced Cd absorption. | [209] |
| Funneliformis mosseae | Cucumber (Cucumis sativus L.) | *The enhanced secondary metabolism and integrated transcriptional regulation might play a crucial role in AMF-mediated alleviation of chilling stress in plants. | [218] |
| Pervetustus simplex, Claroideoglomus etunicatum, Albahypha drummondii, Septoglomus xanthium, Funneliformis mosseae, and Rhizoglomus irregulare | Date palm (Phoenix dactylifera L.) | *Shoot length, and stem diameter were significantly higher in treatments augmented with compost and AMF. | [219] |
| Claroideoglomus etunicatum, Rhizoglomus irregulare, Diversispora versiformis | Eggplant (Solanum melongena L.) | *The inoculation is an effective strategy for alleviating cold stress. | [220] |
| Glomus intraradices | Fig (Ficus carica L.) | *Plants positively responded to the mycorrhizal inoculation, and AMF induced different root architecture models. | [221] |
| Glomus deserticola, Gigaspora margarita | Olive (Olea europaea L.) | *Mycorrhizal symbiosis decreased the Na+ and Cl- contents, and improved the RWC, the total fresh and dry weights and the photosynthetic activity. | [222] |
| Rhizophagus irregularis | *The inoculation exhibited better performance under drought, especially under partial-root zone drying (PRD) treatment.*The combination of 50% deficit irrigation and AMF could cause the resistance of olive to drought. | [222] | |
| Funneliformis mosseae, Funneliformis constrictum, Gigaspora margarita, and Rhizophagus irregularis | Onion (Allium cepa L.) | *Application of AMF and Trichoderma viride, for onion plants assists their growth in nutrient-deficient soils amended with fish waste. | [223] |
| Pistachio (Pistacia vera) | *The use of composted materials improved its seedling’s response to native AMF under drought conditions. | [224] | |
| Glomus mosseae, Acaulospora laevis, Glomus manihotis, and a mixed AMF strain | Pomegranate (Punica granatum L.) | *Growth, physiological, and bio-chemical activities were effectively improved by bio-hardening. | [194] |
| Cetraspora pellucida, Claroideoglomus etunicatum | Strawberry (Fragaria × ananassa Duch.) | *Plants grown with 9% of biochar and inoculated with C. etunicatum showed more profuse root system. | [225] |
| Rhizophagus fasciculatus, Rhizophagus aggregatus, Rhizophagus irregularis | Tangerine orchard (Citrus reticulata L.) | *Inoculation had positive effect on final yield. | [226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the Influences of Microbial Biostimulants on Horticultural Crops: Case Studies and Successful Paradigms. Horticulturae 2023, 9, 193. https://doi.org/10.3390/horticulturae9020193
Shahrajabian MH, Petropoulos SA, Sun W. Survey of the Influences of Microbial Biostimulants on Horticultural Crops: Case Studies and Successful Paradigms. Horticulturae. 2023; 9(2):193. https://doi.org/10.3390/horticulturae9020193
Chicago/Turabian StyleShahrajabian, Mohamad Hesam, Spyridon A. Petropoulos, and Wenli Sun. 2023. "Survey of the Influences of Microbial Biostimulants on Horticultural Crops: Case Studies and Successful Paradigms" Horticulturae 9, no. 2: 193. https://doi.org/10.3390/horticulturae9020193
APA StyleShahrajabian, M. H., Petropoulos, S. A., & Sun, W. (2023). Survey of the Influences of Microbial Biostimulants on Horticultural Crops: Case Studies and Successful Paradigms. Horticulturae, 9(2), 193. https://doi.org/10.3390/horticulturae9020193









