Abstract
Gamma amino butyric acid (GABA), an important free amino acid in plant tissues, plays an essential role in all stages of plant growth and development. In this study, we aimed to explore the effects of GABA on the nutrient absorption of loquat [Eriobotrya japonica (Thunb.) Lindl.] seedlings. The effects of applying exogenous GABA in different concentrations (0.0, 0.5, 1.0, 1.5, and 2 g L−1) on the nutrient uptake of loquat seedlings were studied. GABA increased the biomass (dry weight) and contents of photosynthetic pigments in loquat seedlings to a certain extent. GABA concentration exhibited a quadratic polynomial regression relationship with the biomass. Exogenous GABA in different concentrations increased the total nitrogen (N) and phosphorus (P) contents in loquat seedlings, whereas only 0.5 and 1.0 g L−1 of GABA increased the potassium (K) content. Similarly, GABA concentration also had a polynomial regression relationship with the total N, P, and K contents. Compared to the control, 0.5, 1.0, 1.5, and 2.0 g L−1 of GABA increased the shoot total N content by 27.30, 32.99, 15.41, and 12.93%, respectively, and also increased the shoot total P content by 26.12, 37.52, 21.99, and 9.61%, respectively. Furthermore, correlation and grey relational analyses showed that the carotenoid content, root biomass, and soil alkali-hydrolyzable N concentration were the indicators most closely associated with the uptakes of N, P, and K in shoots. This study shows that exogenous GABA can promote the growth and nutrient uptake of loquat seedlings at an optimum concentration of 1.0 g L−1.
1. Introduction
Mineral nutrients are essential for plant growth and development. Studies have demonstrated that plant nutrient uptake is a complex process influenced by various factors, including the external environment and the plant itself [1,2]. In fruit tree production, most fruit trees are grafted on rootstocks [3], which act as the roots of the plant, taking up nutrients from the soil to enhance plant growth [4]. However, fruit tree cultivation is hampered by several challenges, such as poor rootstock resistance, weak growth of grafted plants, and insufficient nutrient uptake by plants [5]. Therefore, this calls for studies to explore strategies for promoting nutrient uptake by rootstocks to enhance fruit tree growth.
Gamma amino butyric acid (GABA) is a four-carbon, non-protein amino acid compound that is widely distributed in animals, plants, and microorganisms [6]. As a natural active substance, GABA participates in diverse physiological activities of plants and plays an essential role in plant growth and development [7], signaling [8], and stress response [9]. Studies have revealed that applying exogenous GABA can promote plant growth and increase the contents of endogenous GABA, amino acids, and endogenous hormones by upregulating the expression of several critical genes for phytohormone synthesis [10,11]. Xie et al., (2020) reported that the accumulation of endogenous GABA in poplar affects the levels of other endogenous hormones and inhibits the formation of adventitious roots [12]. However, GABA in high levels inhibits cell elongation and leads to the decreased expression of genes encoding secretory proteins in the GABA catabolic mutant pop2 of Arabidopsis [13]. Under salt stress, GABA regulates the metabolism of amino acids and organic acids in plant roots [14]. In addition, exogenous GABA can promote increased amino acid synthase activity in barley and increase the content of free amino acids in barley [15]. Regarding nutrient uptake, GABA may modulate the metabolism of free amino acids by regulating the aluminum-activated malate transporter (ALMT) to mediate nitrogen (N) metabolism and uptake in Arabidopsis [16].
Loquat [Eriobotrya japonica (Thunb.) Lindl.] is a self-pollinating evergreen fruit tree, and is propagated by seeds or grafting. The fruits of loquat are rich in vitamins, phenols, carotenoids, triterpenoids, and flavonoids, and thus have high food and medicinal values [17,18]. There are some local varieties or cultivars in Sichuan Province, China, including ‘Dawuxing’, ‘No. 1 of Longquan’, and ‘No. 6 of Zaozhong’, which are an important economic source for fruit farmers [18]. However, Wu (2020) revealed that the loquat tree also suffers from slow growth, a shallow root system, and difficulty surviving during production [4]. Herein, we hypothesized that applying GABA to loquat seedlings might promote their growth and nutrient uptake. Therefore, we explored the effects of GABA on the growth and nutrient uptake of loquat seedlings. The aim of this study was to determine the optimal concentration of GABA to promote growth and nutrient uptake in loquat seedlings, with the overarching goal of providing a reference for loquat production.
2. Materials and Methods
2.1. Materials
The seeds of loquat were collected from a seven-year-old ‘Dawuxing’ (cultivar variety) loquat tree. The seedlings of loquat were nurtured in the Chongzhou Modern Agriculture Research and Development Base of Sichuan Agricultural University, Chongzhou, Chengdu, Sichuan, China. Annual seedlings were collected when they grew to 20 cm in height.
Fluvo-aquic soil was collected from the farmland around the Chengdu Campus of Sichuan Agricultural University, Chengdu, Sichuan, China. The soil had the following basic physicochemical properties: pH value (7.42), alkaline hydrolysis N concentration (60.13 mg kg−1), available phosphorus (P) concentration (16.13 mg kg−1), and available potassium (K) concentration (51.03 mg kg−1). These were determined in accordance with Bao (2000) [19].
GABA was purchased from Shanghai Yuanye Biotechnology Co., Ltd., Shangshai, China. Its chemical name is 4-amino butyric acid, with CAS No. of 56-12-2.
2.2. Experimental Design
The study was conducted in a greenhouse at the Chengdu Campus of Sichuan Agricultural University. Briefly, the Fluvo-aquic soil was air-dried, crushed, and passed through a 5-mm sieve. Next, 3.0 kg soil was put into a plastic pot (20 cm diameter and 18 cm depth), and three uniform-height loquat seedlings were transplanted in each pot with even distribution. Then, different concentrations (0, 0.5, 1.0, 1.5, and 2.0 g L−1) of GABA [15,20,21] were sprayed on both sides of loquat seedling leaves until the solution started dripping. For each pot, 25 mL of GABA solution was sprayed. The GABA solution was re-sprayed again 15 days later. Notably, each treatment was conducted in triplicate (three pots per repetition with a total of 45 pots), and the pots were placed in a completely random design. The loquat seedlings were watered daily.
2.3. Determination of Indicators
We collected the fourth mature leaf of each plant 45 days after the first treatment to determine the content of photosynthetic pigments (chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid). The photosynthetic pigments were extracted using the acetone-ethanol (1:1) extraction method, followed by determining the absorbencies at 663, 645, 653, and 470 nm, using a spectrophotometer. The contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid were calculated according to Hao et al., (2004) [22] and Li et al., (2022) [23], and the chlorophyll a/b = chlorophyll a content/chlorophyll b content. Next, the roots and shoots were harvested separately, dried at 80 °C to measure the biomass (dry weight) using an electronic balance. Ground dried samples were digested using sulfuric acid/hydrogen peroxide (5:1, v/v) at 200 °C. The digestion solutions were used to determine the total N content via the Kjeldahl method, the total P content via the Mo-Sb anti-colorimetry, and the total K content via flame photometry [19]. The pot soil was collected, air-dried, and passed through a 1.0-mm sieve for chemical analysis. We determined the soil pH value using a pH meter [19]. Finally, the concentration of alkali-hydrolyzable N was determined by the alkali diffusion method, whereas the concentration of soil available P was determined by Mo-Sb anti-colorimetry. In addition, flame photometry was applied to determine the concentration of soil available K [19].
2.4. Statistical Analysis
All statistical analyses were performed using the SPSS 20.0.0 software (IBM, Chicago, IL, USA). All data were normalized and subjected to a homogeneity test, followed by performing one-way analysis of variance (ANOVA) and Duncan’s multiple range test (P < 0.05) to compare differences among groups. The regression relationship between the GABA concentration and biomass, total N content, total P content, or total K content was analyzed by regression analysis, and the polynomial regression was checked as the best fit regression curves. Pearson’s correlation analysis was performed to explore the relationships among the indicators. Grey relational analysis was used to analyze the relationships of the shoot N, P, or K content with other indicators according to the method deccribed by Zhang et al., (2023) [24] and Lin et al., (2023) [25].
3. Results
3.1. Effects of GABA on the Biomass (Dry Weight) of Loquat Seedlings
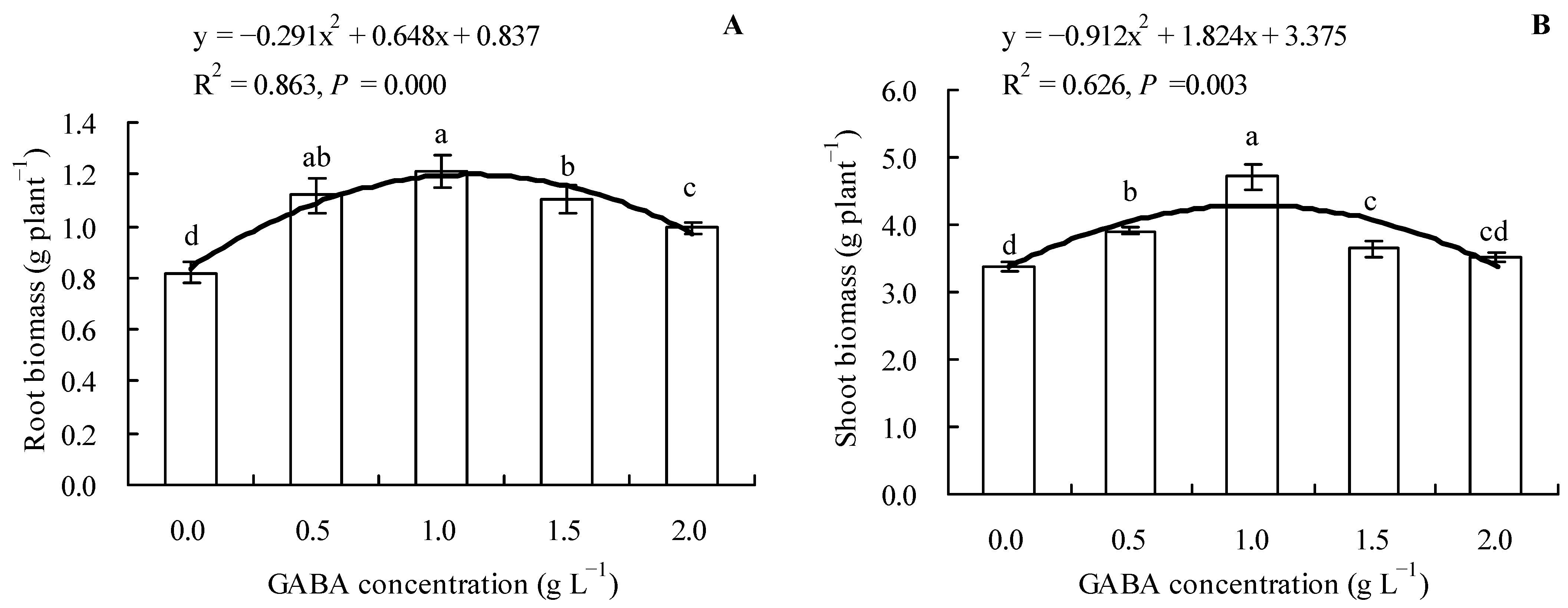
Results showed that loquat seedlings’ root and shoot biomass exhibited an increasing trend when the GABA concentration was not higher than 1.0 g L−1 and a decreasing trend when the GABA concentration was higher than 1.0 g L−1 (Figure 1A,B). Compared to the control, 0.5, 1.0, 1.5, and 2.0 g L−1 of GABA increased the root biomass of loquat seedlings by 36.46, 47.56, 34.76, and 21.10%, respectively, whereas 0.5, 1.0, and 1.5 g L−1 of GABA increased the shoot biomass by 15.78, 39.46, and 7.90%, respectively. In addition, GABA concentration exhibited a quadratic polynomial regression relationship with both the root and shoot biomasses.
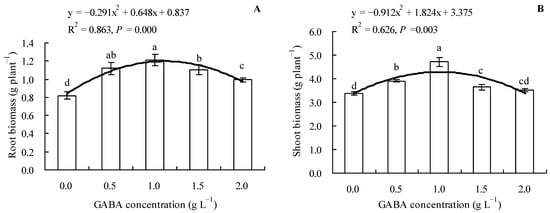
Figure 1.
Biomass (dry weight) of loquat seedlings under the influence of gamma amino butyric acid. (A): Root biomass; (B): shoot biomass. Values are means (±SD) of three replicates. Different lowercase letters indicate significant differences among the treatments (Duncan’s Multiple Range Test, P < 0.05). GABA = gamma amino butyric acid.
3.2. Effects of GABA on Photosynthetic Pigment Content in the Leaves of Loquat Seedlings
Totals of 0.5, 1.0, and 1.5 g L−1 of GABA increased the chlorophyll a, chlorophyll b, and total chlorophyll contents in loquat seedling leaves (Table 1). Compared to the control, 0.5, 1.0, and 1.5 g L−1 of GABA increased the total chlorophyll content by 27.29, 23.63, and 14.66%, respectively. With regard to the chlorophyll a/b, 0.5 and 1.5 g L−1 of GABA decreased the chlorophyll a/b of loquat seedling leaves, whereas 1.0 and 2.0 g L−1 of GABA had no significant effect. Besides, 0.5, 1.0, and 1.5 g L−1 of GABA increased the carotenoid content in loquat seedling leaves by 53.66, 65.85, 26.83, and 21.95%, respectively, compared to the control.

Table 1.
Photosynthetic pigment content in loquat seedling leaves under the influence of gamma amino butyric acid.
3.3. Effects of GABA on Total N Content in Loquat Seedlings
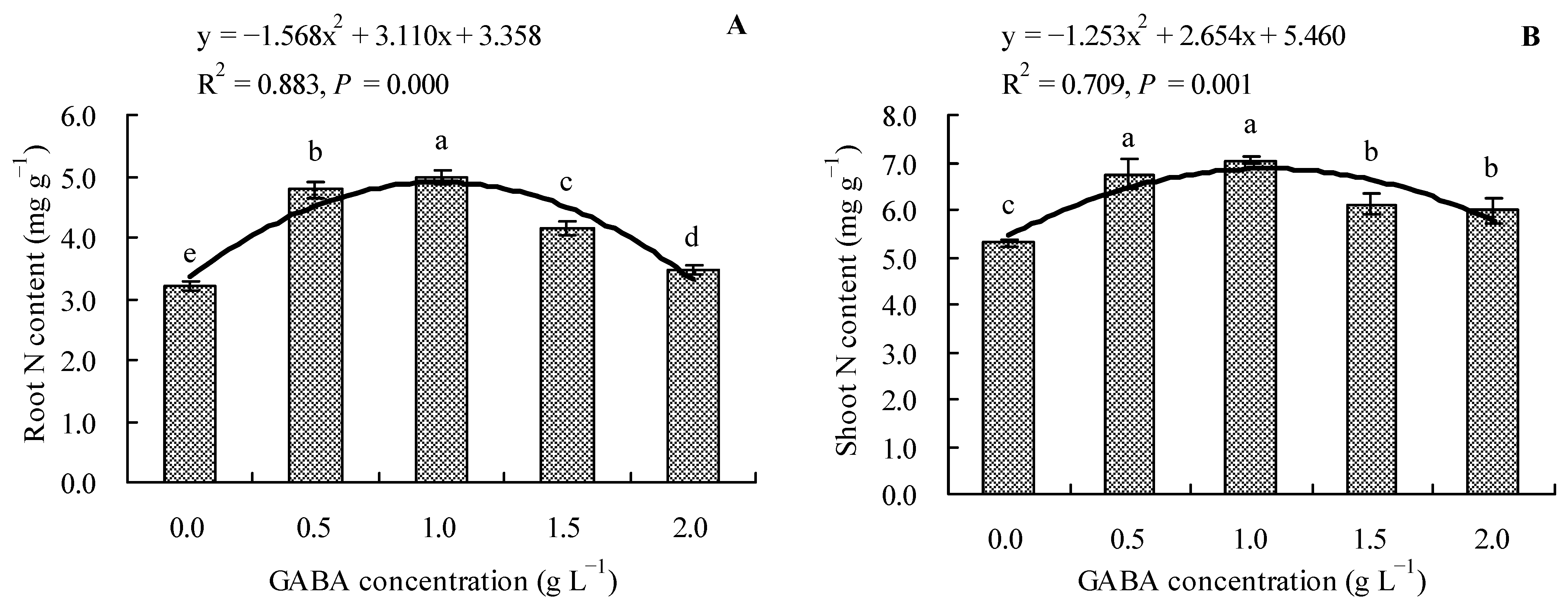
The results revealed that the GABA increased the total N contents in the roots and shoots of loquat seedlings (Figure 2A,B). Compared to the control, 0.5, 1.0, 1.5, and 2.0 g L−1 of GABA increased the total N content in roots by 48.57, 54.73, 28.61, and 7.96%, respectively, and the total N content in shoots by 27.30, 32.99, 15.41, and 12.93%, respectively. Moreover, it was evident that GABA concentration had a quadratic polynomial regression relationship with both the root total N content and shoot total N content.
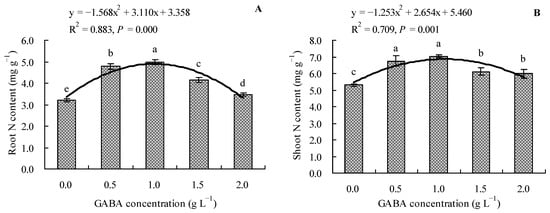
Figure 2.
Total N content in loquat seedlings under the influence of gamma amino butyric acid. (A): Root N content; (B): shoot N content. Values are means (±SD) of three replicates. Different lowercase letters indicate significant differences among the treatments (Duncan’s Multiple Range Test, P < 0.05). GABA = gamma amino butyric acid.
3.4. Effects of GABA on Total P Content in Loquat Seedlings
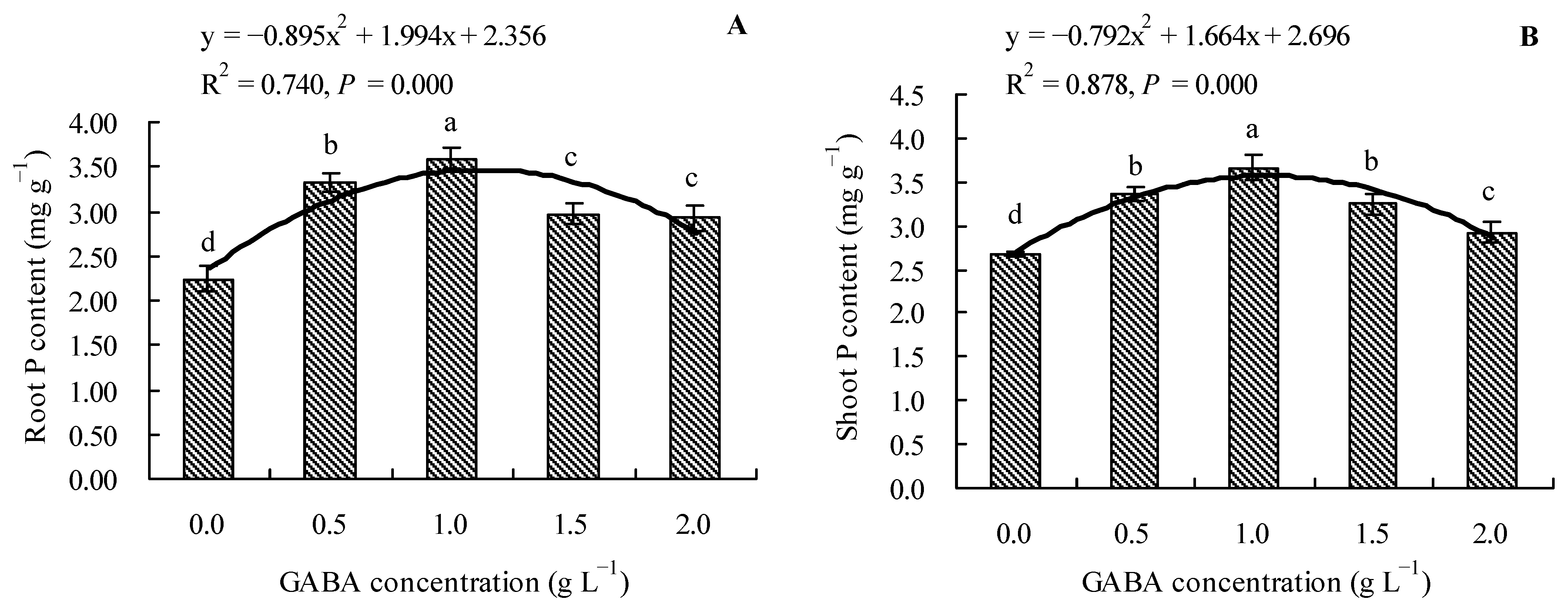
GABA increased the total P contents in roots and shoots of loquat seedlings (Figure 3A,B). Compared to the control, 0.5, 1.0, 1.5, and 2.0 g L−1 of GABA increased the total P content in roots by 48.33, 60.21, 32.47, and 30.68%, respectively, and increased the total P content in shoots by 26.12, 37.52, 21.99, and 9.61%, respectively. The results demonstrated that increasing the GABA concentration initially increased the total P content in the roots and shoots; however, the content then exhibited a decreasing trend. Moreover, GABA concentration exhibited a quadratic polynomial regression relationship with the total P contents in roots and shoots.
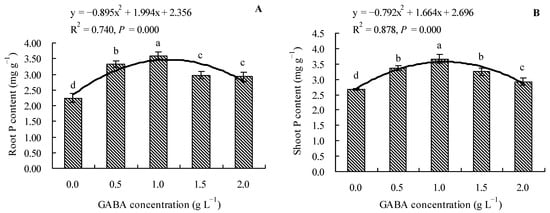
Figure 3.
Total P content in loquat seedlings under the influence of gamma amino butyric acid. (A): Root P content; (B): shoot P content. Values are means (±SD) of three replicates. Different lowercase letters indicate significant differences among the treatments (Duncan’s Multiple Range Test, P < 0.05). GABA = gamma amino butyric acid.
3.5. Effects of GABA on Total K Content in Loquat Seedlings
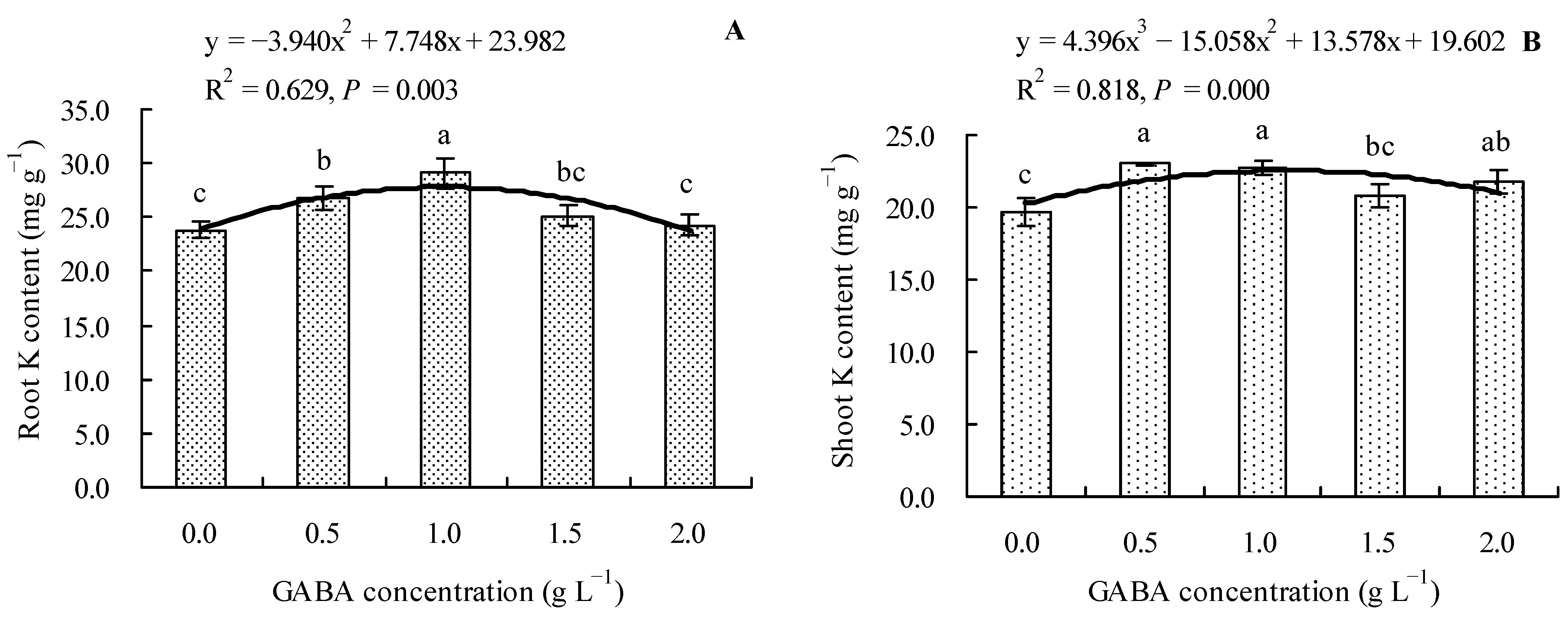
We found that 0.5 and 1.0 g L−1 of GABA increased the total K content in roots of loquat seedlings by 12.30 and 21.99%, respectively, compared to the control. On the other hand, 1.5 and 2.0 g L−1 of GABA had no significant effect on the total K content in roots (Figure 4A). Compared to the control, 0.5, 1.0, and 2.0 g L−1 of GABA increased the total K content in the shoots of loquat seedlings by 17.16, 15.94, and 10.69%, respectively, whereas 1.5 g L−1 of GABA had no significant effect (Figure 4B). GABA concentration also had a polynomial regression relationship with the total K contents in roots and shoots.
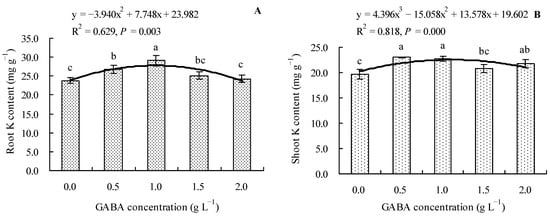
Figure 4.
Total K content in loquat seedlings under the influence of gamma amino butyric acid. (A): Root K content; (B): shoot K content. Values are means (±SD) of three replicates. Different lowercase letters indicate significant differences among the treatments (Duncan’s Multiple Range Test, P < 0.05). GABA = gamma amino butyric acid.
3.6. Effects of GABA on Soil pH Value and Available Nutrient Concentration
Table 2 shows that GABA decreased the soil pH value. The results indicated that the soil pH value had a decreasing trend when the GABA concentration was not higher than 1.0 g L−1 and an increasing trend when the GABA concentration was higher than 1.0 g L−1. We also found that 0.5, 1.0, 1.5, and 2.0 g L−1 of GABA increased the soil alkali-hydrolyzable N concentration by 10.92, 13.74, 12.46, and 5.55%, respectively, compared to the control. In terms of the available P concentration in the soil, only 1.0 g L−1 GABA increased the soil’s available P concentration, whereas the other concentrations exhibited no significant effects. Furthermore, only 1.5 g L−1 of GABA decreased available K concentration in the soil, whereas the other concentrations had no significant effects.

Table 2.
Soil pH value and available nutrient concentration under the influence of gamma amino butyric acid.
3.7. Correlation and Grey Relational Analyses
Correlation analysis showed that both the root and shoot biomass had a highly-significant (P < 0.01) or significant (0.01 ≤ P < 0.05) positive correlation with the chlorophyll a content, chlorophyll b content, carotenoid content, root total N content, shoot total N content, root total P content, shoot total P content, root total K content, shoot total K content, soil alkali-hydrolyzable N concentration, soil available P concentration, and soil available K concentration. However, the root and shoot biomass exhibited a highly-significant (P < 0.01) negative correlation with the soil pH value (Table 3). The total N content in both the roots and shoots exhibited a highly-significant (P < 0.01) or significant (0.01 ≤ P < 0.05) positive correlation with the chlorophyll a content, chlorophyll b content, carotenoid content, root total P content, shoot total P content, root total K content, shoot total K content, soil alkali-hydrolyzable N concentration, and available P concentration in the soil. On the other hand, it had a highly-significant (P < 0.01) negative correlation with the soil pH value. In addition, the shoot total N content had a significant (0.01 ≤ P < 0.05) positive correlation with the available K concentration in the soil. The total P content in both the roots and shoots showed a highly-significant (P < 0.01) positive correlation with the chlorophyll a content, chlorophyll b content, carotenoid content, root total K content, shoot total K content, soil alkali-hydrolyzable N concentration, soil available P concentration, and soil available K concentration. In contrast, it exhibited a highly-significant (P < 0.01) negative correlation with the soil pH value. Moreover, the total K content in roots and shoots had a highly-significant (P < 0.01) or significant (0.01 ≤ P < 0.05) positive correlation with the chlorophyll a content, chlorophyll b content, carotenoid content, and soil alkali-hydrolyzable N concentration. However, it had a highly-significant (P < 0.01) or significant (0.01 ≤ P < 0.05) negative correlation with the soil pH value. Finally, the root total K content also had a highly-significant (P < 0.01) or significant (0.01 ≤ P < 0.05) positive correlation with the soil available P concentration and soil available K concentration.

Table 3.
Correlations among the different indicators of loquat seedlings.
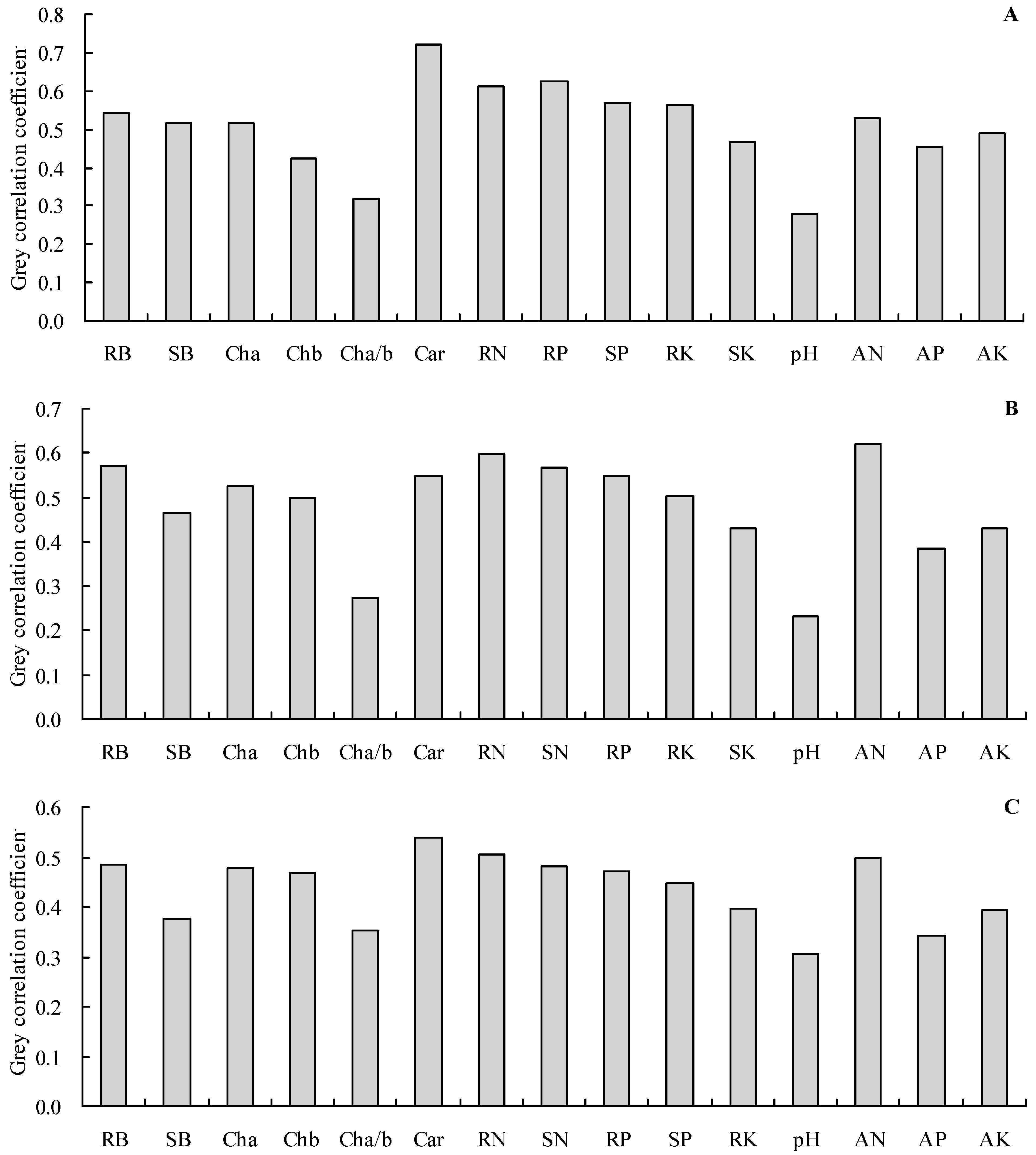
Grey relational analysis was applied to further analyze the grey relationships of the shoot total N, P, and K contents with the other indicators (Figure 5A–C). The results showed that the total N, P, and K contents in the shoots had a grey correlation with all of the other indicators (the grey correlation coefficients were higher than 0.20). The carotenoid content, root total P content, and root total N content were the top three most closely associated with the shoot total N content (Figure 5A). The soil alkali-hydrolyzable N concentration, root total N content, and root biomass were the top three most closely associated with the shoot total P content (Figure 5B). Furthermore, the carotenoid content, root total N content, and soil alkali-hydrolyzable N concentration were the top three most closely associated with the shoot total P content (Figure 5C).
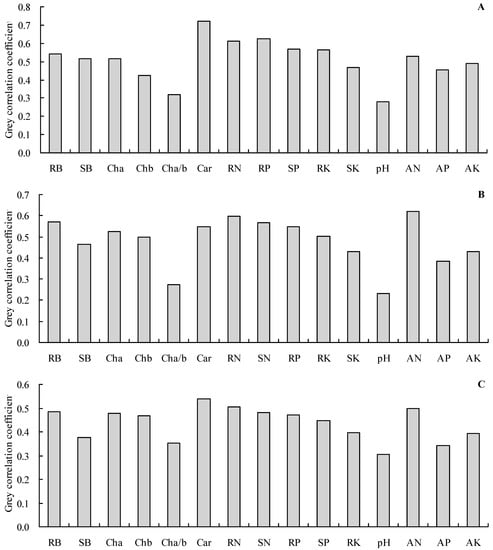
Figure 5.
Grey correlation coefficients. (A): grey correlation coefficient of the biomass, photosynthetic pigment content, root N content, P content, and K content with the shoot N content; (B): grey correlation coefficient of the biomass, photosynthetic pigment content, N content, root P content, and K content with the shoot P content; (C): grey correlation coefficient of the biomass, photosynthetic pigment content, N content, P content, and root K content with the shoot K content. RB = root biomass; SB = shoot biomass; Cha = chlorophyll a content; Chb = chlorophyll b content; Cha/b = chlorophyll a/b; Car = carotenoid content; RN = root total N content; SN = shoot total N content; RP = root total P content; SP = shoot total P content; RK = root total K content; SK = shoot total K content; pH = soil pH value; AN = alkali-hydrolyzable N concentration; AP = available P concentration; AK = available K concentration.
4. Discussion
The metabolic branch of GABA participates in numerous physiological and metabolic processes in plants, including protein synthesis, and thereby plays an essential role in plant growth and development [26]. Exogenous GABA improves the growth parameters, including the plant height, biomass, and root structure, of Malus hupehensis (Pamp.) Rehd., thus alleviating salinity stress [21]. Li et al., (2016) also found that GABA increased the seed germination rate and biomass of wheat under salt stress [27]. Exogenous GABA can increase the transcription levels of genes associated with indole acetic acid (IAA) biosynthesis and promote plant growth [28]. In this study, GABA increased the biomass of loquat seedlings, and GABA concentration exhibited a quadratic polynomial regression relationship with the biomass. These results suggest that GABA promotes the growth of loquat seedlings, which may be attributed to the fact that GABA stimulates cell division and elongation, ultimately increasing plants’ biomass [29]. In contrast, we also found that higher concentrations of GABA had no significant effect on the biomass, which may be associated with the defective cell elongation caused by excessive accumulation of GABA in plants’ reproductive and nutritional tissues [14].
Photosynthesis directly affects plant biomass [30]. Applying exogenous GABA under salt stress could directly or indirectly affect the activity of photosynthetic reaction centers in lettuce (Lactuca sativa L.) by regulating other processes that interact with photosynthesis [31]. Xiang et al., (2016) reported that GABA can regulate ABA levels in plants to maintain a high degree of stomatal opening in leaves, and they also found that the shunting effect of GABA can promote the tricarboxylic acid cycle (TCA) and ensure the operation of the photosynthetic electron transport chain [32]. Moreover, applying exogenous GABA under flooding conditions could alleviate the reduction in chlorophyll content in maize and prevent disruption of the chloroplast morphology and leaf ultrastructure, thereby improving the photosynthetic assimilation capacity of maize [33]. Under hypoxic stress, exogenous GABA increases the photosynthetic pigment content and promotes photosynthesis in melon seedlings [34]. In this study, GABA increased the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid in loquat seedling leaves, and decreased the chlorophyll a/b to some extent, which is consistent with previous studies [20,34]. Collectively, these results suggest that GABA could improve the photosynthesis of loquat seedlings, which may be associated with the ability of GABA to enhance the degree of binding between chlorophyll and chloroplast proteins and reduce the exocytosis of chlorophyll molecules [35].
The physicochemical properties of soil can influence the nutrient uptake by plants, and affect plant growth and development [36]. The soil applied by amino acid organic fertilizer can increase soil ammonium N, nitrate N, and available P concentrations [37]. In this study, GABA application decreased the soil pH value and increased the soil alkali-hydrolyzable N concentration. Notably, only 1.0 g L−1 GABA increased the soil available P concentration. We also found that GABA decreased or had no significant effects on the available K concentration in the soil. These results imply that GABA could affect the soil pH value and available nutrient concentration in the soil by affecting the root secretions of plants. This may be because GABA metabolism is the main pathway for succinate production in plant roots [38]. We speculate that the exogenous GABA promoted more organic acids-based root secretions of the loquat seedlings, which decreased the soil pH value and further affected the concentration of soil available nutrients. The appropriate concentration of GABA can be used as an N source to promote plant growth and increase NH4+ content in plants while, at the same time, decreasing the contents of NO3− and NO2− in plants [39]. The previous study reported that GABA can affect the activity of plant N metabolizing enzymes under stress conditions and promote the uptake of N by plants [40]. Carillo (2018) reported that the shunt pathway of GABA can replenish the content of succinate, an intermediate of the TCA reaction, thereby maintaining a normal TCA cycle [41]. The pathway can also increase the organic acid contents in plant roots and stems and reduce the acidity of cells, ultimately regulating the uptake of mineral elements by plants [42]. Under Cd stress, GABA increased the contents of K+ and Mg2+ in Monoraphidium sp. [43]. Under low oxygen stress, GABA promoted K+, Ca2+, Mg2+, and Zn2+ uptake in melon and maintained the balance of mineral element uptake [44]. In addition, Zhou (2004) revealed that GABA promotes K+ uptake and increases the K+/Na+ ratio of maize seedlings under salt stress [45]. In the present study, applying exogenous GABA (all concentrations) increased the N and P contents in loquat seedlings, and only 0.5 and 1.0 g L−1 of GABA increased the K content. These results indicate that GABA could promote the nutrient uptake of loquat seedlings, which is almost consistent with previous studies [40,43,44,45]. This effect may be attributed to GABA affecting the production of organic acids-based root secretions of loquat seedlings. Furthermore, correlation analysis showed that the total N, P, and K contents were negatively correlated with the soil pH value, which further suggests that GABA may promote the secretion of organic acids by the roots of loquat seedlings to decrease the soil pH value and activate soil nutrients. However, further studies should be conducted to elucidate how GABA regulates root secretion. Correlation analysis also showed that the total N and P contents in shoots of loquat seedlings were positively correlated with the alkali-hydrolyzable N concentration, P concentration, root biomass, shoot biomass, chlorophyll a content, chlorophyll b content, carotenoid content, root total N content, root total P content, root total K content, and shoot total K content. In addition, the total K content in shoots was positively correlated with the alkali-hydrolyzable N concentration, root biomass, shoot biomass, chlorophyll a content, chlorophyll b content, carotenoid content, root total N content, root total P content, and root total K content. Grey relational analysis showed that the carotenoid content, root total P content, and root total N content were the top three indicators that were closely associated with the shoot total N content. The soil alkali-hydrolyzable N concentration, root total N content, and root biomass were the top three indicators that were closely associated with the shoot total P content. Moreover, the top three indicators that were closely associated with the shoot total P content were the carotenoid content, root total N content, and soil alkali-hydrolyzable N concentration. Altogether, these results suggest that GABA promotes the nutrient uptake of loquat seedlings by increasing their carotenoid content, root biomass, and soil alkali-hydrolyzable N concentration, which can be used in loquat production.
5. Conclusions
This study has revealed that spraying GABA promoted the growth of loquat seedlings by increasing their biomass and photosynthetic pigment contents. GABA, in different concentrations, can also promote the uptakes of N and P in loquat seedlings, whereas 0.5 and 1.0 g L−1 of GABA promotes K uptake. GABA concentration had a polynomial regression relationship with the biomass and three nutrient (N, P, and K) contents. Furthermore, we found that the carotenoid content, root biomass, and soil alkali-hydrolyzable N concentration were the indicators that were most closely associated with the contents of N, P, and K in shoots. Therefore, GABA can be used in loquat production. Future studies should explore how GABA promotes nutrient uptake in loquat.
Author Contributions
Conceptualization, Q.D. and Y.W.; investigation, Z.Y., Y.X., P.S., X.L. (Xinyu Li), J.Z., H.X., D.L., X.L. (Xian Luo) and H.Z.; data curation, L.L.; writing—original draft preparation, Z.Y.; writing—review and editing, Q.D.; supervision, Q.D. and Y.W.; funding acquisition, Q.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the “14th Five-Year Plan” Breeding Research Project of Sichuan Science and Technology (2021YFYZ0023-07) and Sichuan Fruit Innovation Team Project of National Modernized Agricultural Industry Technology System (sccxtd-2022-04).
Data Availability Statement
Data will be made available on genuine request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goldstein, G.; Bucci, S.J.; Scholz, F.G. Why do trees adjust water relations and hydraulic architecture in response to nutrient availability? Tree Physiol. 2013, 33, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Tuteja, N. NPKS uptake, sensing, and signaling and miRNAs in plant nutrient stress. Protoplasma 2016, 253, 767–786. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Pan, H.L.; Pan, T.F.; Tang, H.R.; Wang, X.R.; Pan, D.M. Research progress on the interaction between scion and rootstock in fruit trees. Acta Hortic. Sin. 2017, 44, 1645–1657. [Google Scholar]
- Wu, T.R. Preliminary Study on the Potential of Different Loquat (Eriobotrya japonica) Resources as Rootstocks; Southwest University: Chongqing, China, 2020. [Google Scholar]
- Chen, X.; Li, N.; Liu, C.; Wang, H.; Li, Y.; Xie, Y.; Ma, F.; Liang, J.; Li, C. Exogenous GABA improves the resistance of apple seedlings to long-term drought stress by enhancing GABA shunt and secondary cell wall biosynthesis. Tree Physiol. 2022, 42, 2563–2577. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, X.J.; Hu, Y.; Ji, Y.L.; Bai, T.; Zhu, M.X.; Zhang, Y.H. Metabolism and enrichment mechanism of γ-aminobutyric acid in plants. J. Anhui Agric. Sci. 2020, 48, 9–12. [Google Scholar]
- Uzma Jalil, S.; Khan, M.I.R.; Ansari, M.I. Role of GABA transaminase in the regulation of development and senescence in Arabidopsis thaliana. Curr. Plant Biol. 2019, 19, 100119. [Google Scholar] [CrossRef]
- Fromm, H. GABA signaling in plants: Targeting the missing pieces of the puzzle. J. Exp. Bot. 2020, 71, 6238–6245. [Google Scholar] [CrossRef] [PubMed]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging roles of γ aminobutyric acid (GABA) gated channels in plant stress tolerance. Plants 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.B.A.; Ashrafuzzaman, M.U.P.M.; Ismail, M.R.U.P.; Shahidullah, M.S.U.P.; Prodhan, A.K.M.A. Influence of foliar applied GABA on growth and yield contributing characters of white gourd (Benincasa hispida). Int. J. Agric. Biol. 2010, 12, 373–376. [Google Scholar]
- Hijaz, F.; Nehela, Y.; Killiny, N. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta 2018, 248, 909–918. [Google Scholar] [CrossRef]
- Xie, T.; Ji, J.; Chen, W.; Yue, J.; Du, C.; Sun, J.; Chen, L.; Jiang, Z.; Shi, S. GABA negatively regulates adventitious root development in poplar. J. Exp. Bot. 2020, 71, 1459–1474. [Google Scholar] [CrossRef]
- Renault, H.; Amrani, A.E.; Palanivelu, R.; Updegraff, E.P.; Yu, A.; Renou, J.; Preuss, D.; Bouchereau, A.; Deleu, C. GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 894–908. [Google Scholar] [CrossRef]
- Renault, H. Phylogeny and bioinformatics shed light on GABA functions in plants. Plant Signal Behav. 2013, 8, e24274. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Batushansky, A.; Kirma, M.; Grillich, N.; Pham, P.A.; Rentsch, D.; Galili, G.; Fernie, A.R.; Fait, A. The transporter GAT1 plays an important role in GABA-mediated carbon-nitrogen interactions in Arabidopsis. Front. Plant Sci. 2015, 6, 785. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Response of Melatonin at Different Concentrations to Loquat Seedlings under Cold Stress; Southwest University: Chongqing, China, 2020. [Google Scholar]
- Liu, Y.; Zhang, W.; Xu, C.; Li, X. Biological activities of extracts from loquat (Eriobotrya japonica Lindl.): A review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis; Chinese Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Fan, H.; Zhang, Z.Y.; Tan, Y.; Zhou, X.F.; Xu, H.W. Effect of external GABA on agronomic traits and photosynthetic characteristics of maize seedlings. Shandong Agric. Sci. 2022, 54, 67–72, 86. [Google Scholar]
- Li, Y.; Liu, B.; Peng, Y.; Liu, C.; Zhang, X.; Zhang, Z.; Liang, W.; Ma, F.; Li, C. Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci. Hortic. 2020, 261, 108982. [Google Scholar] [CrossRef]
- Hao, Z.B.; Cang, J.; Xu, Z. Plant Physiology Experiment; Harbin Institute of Technology Press: Harbin, China, 2004. [Google Scholar]
- Li, Z.; Fan, R.; Peng, X.; Shu, J.; Liu, L.; Wang, J.; Lin, L. Salicylic acid alleviates selenium stress and promotes selenium uptake of grapevine. Physiol. Mol. Biol. Plants 2022, 28, 625–635. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Y.; Liu, Y.; Liu, Q.; Zhang, L.; Li, Z.; Xu, Y.; Lin, L.; Wang, L. Effects of mutual intercropping on cadmium accumulation of Solanum photeinocarpum Nakamura et Odashima and its post-grafting generations. Int. J. Phytoremediat. 2023, 25, 350–358. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Tang, Y.; Wang, X.; Deng, Q.; Liao, M. 24-epibrassinolide promotes selenium uptake in grapevine under selenium stress. Sci. Hortic. 2023, 308, 111564. [Google Scholar] [CrossRef]
- Shelp, B.J.; Mullen, R.T.; Waller, J.C. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 2012, 17, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Guo, S.J.; Yang, X.H.; Meng, Q.W.; Wei, X.J. Exogenous gamma-aminobutyric acid increases salt tolerance of wheat by improving photosynthesis and enhancing activities of antioxidant enzymes. Biol. Plant 2016, 60, 123–131. [Google Scholar] [CrossRef]
- Guo, Z.; Du, N.; Li, Y.; Zheng, S.; Shen, S.; Piao, F. Gamma-aminobutyric acid enhances tolerance to iron deficiency by stimulating auxin signaling in cucumber (Cucumis sativus L.). Ecotoxicol. Environ. Saf. 2020, 192, 110285. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R.; Riaz, H.N.; Anwar, S.; Parveen, N.; Khalilzadeh, R.; Hussain, I.; Mahmood, S. Morpho-physiological changes in carrots by foliar γ-aminobutyric acid under drought stress. Rev. Bras. Dotânica 2020, 44, 57–68. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, W.Z.; Kang, H.M.; Nuosu, N. Effects of light intensities and water conditions on growth and photosynthetic characteristics of Nyssa yun-nanensis seedlings. J. Northeast. For. Univ. 2018, 46, 16–23. [Google Scholar]
- Kalhor, M.S.; Aliniaeifard, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef]
- Xiang, L.; Hu, L.; Xu, W.; Zhen, A.; Zhang, L.; Hu, X.; Shi, H. Exogenous γ-aminobutyric acid improves the structure and function of photosystem II in muskmelon seedlings exposed to salinity-alkalinity stress. PLoS ONE 2016, 11, e164847. [Google Scholar] [CrossRef]
- Salah, A.; Zhan, M.; Cao, C.; Han, Y.; Ling, L.; Liu, Z.; Li, P.; Ye, M.; Jiang, Y. γ-aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci. Rep. 2019, 9, 484. [Google Scholar] [CrossRef]
- Xia, Q.P.; Gao, H.B.; Li, J.R. Effects of gamma-aminobutyric acid on the photosynthesis and chlorophyll fluorescence parameters of muskmelon seedlings under hypoxia stress. Chin. J. Appl. Ecol. 2011, 22, 999–1006. [Google Scholar]
- Xiang, L.X. The Study of Exogenous γ-aminobutyric Acid Improves Structure and Function of Photosynthetic Apparatus in Muskmelon Seedlings Exposed to Salinity-Alkalinity Stress; Northwest A & F University: Yangling, China, 2016. [Google Scholar]
- Wang, J.; Xu, S.; Yan, T.; Ma, W.J.; Yan, Q.L. Effects of soil nutrients on seedling growth of major tree species in montane region of eastern Liaoning Province, China. Chin. J. Ecol. 2017, 36, 3148–3159. [Google Scholar]
- Lv, N.N.; Shen, Z.Z.; Wang, D.S.; Liu, H.J.; Xue, C.; Li, R.; Shen, Q.R. Effects of amino acid organic fertilizer on cucumber yield and soil biological characters. J. Nanjing Agric. Univ. 2018, 41, 456–464. [Google Scholar]
- Renault, H.; El, A.A.; Berger, A.; Mouille, G.; Soubigou-Taconnat, L.; Bouchereau, A.; Deleu, C. Gamma-aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ. 2013, 36, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, Z.; Wu, X.; Lv, G.; Ma, W.; Zhang, Y.; Gao, H. Gamma-aminobutyric acid (GABA) modulates nitrate concentrations and metabolism in the leaves of pakchoi (Brassica campestris ssp. chinensis Makino) treated with a nitrogen-rich solution. Plant Mol. Biol. Report. 2018, 36, 530–542. [Google Scholar] [CrossRef]
- Wang, Y.C. Mechanism of Aminobutyric acid (GABA) Regulating Maize Seed Germination and Seedling Growth under Salt Stress; Northeast Agricultural University: Harbin, China, 2016. [Google Scholar]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, X.X.; Jia, D.C.; Zhou, D.Y.; Peng, S.M.; Wang, Y.S. Progress in research on kiwifruit rootstocks. North. Hortic. 2022, 46, 125–133. [Google Scholar]
- Zhao, Y.; Song, X.; Zhong, D.B.; Yu, L.; Yu, X. Gamma-aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Bioresour. Technol. 2020, 297, 122500. [Google Scholar] [CrossRef]
- Song, S.L.; Li, J.R.; Gao, H.B.; Li, Q.Y.; Yang, L.W.; Gong, R.J. Effects of exogenous γ-aminobutyric acid on inorganic nitrogen metabolism and mineral elements contents of melon seedling under hypoxia stress. Acta Hortic. Sin. 2012, 39, 695–704. [Google Scholar]
- Zhou, X. Physiological Effects of Salt Stress-Induced GABA Accumulation in Maize Seedlings; China Agricultural University: Beijing, China, 2004. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).