Comparative Analysis of Morphological, Physiological, Anatomic and Biochemical Responses in Relatively Sensitive Zinnia elegans ‘Zinnita Scarlet’ and Relatively Tolerant Zinnia marylandica ‘Double Zahara Fire Improved’ under Saline Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Experimental Design and Treatments
2.3. Plant Growth Parameters
2.4. Physiological Parameters
2.4.1. Ion Leakage
2.4.2. Loss of Turgidity
2.4.3. Minimum Fluorescence
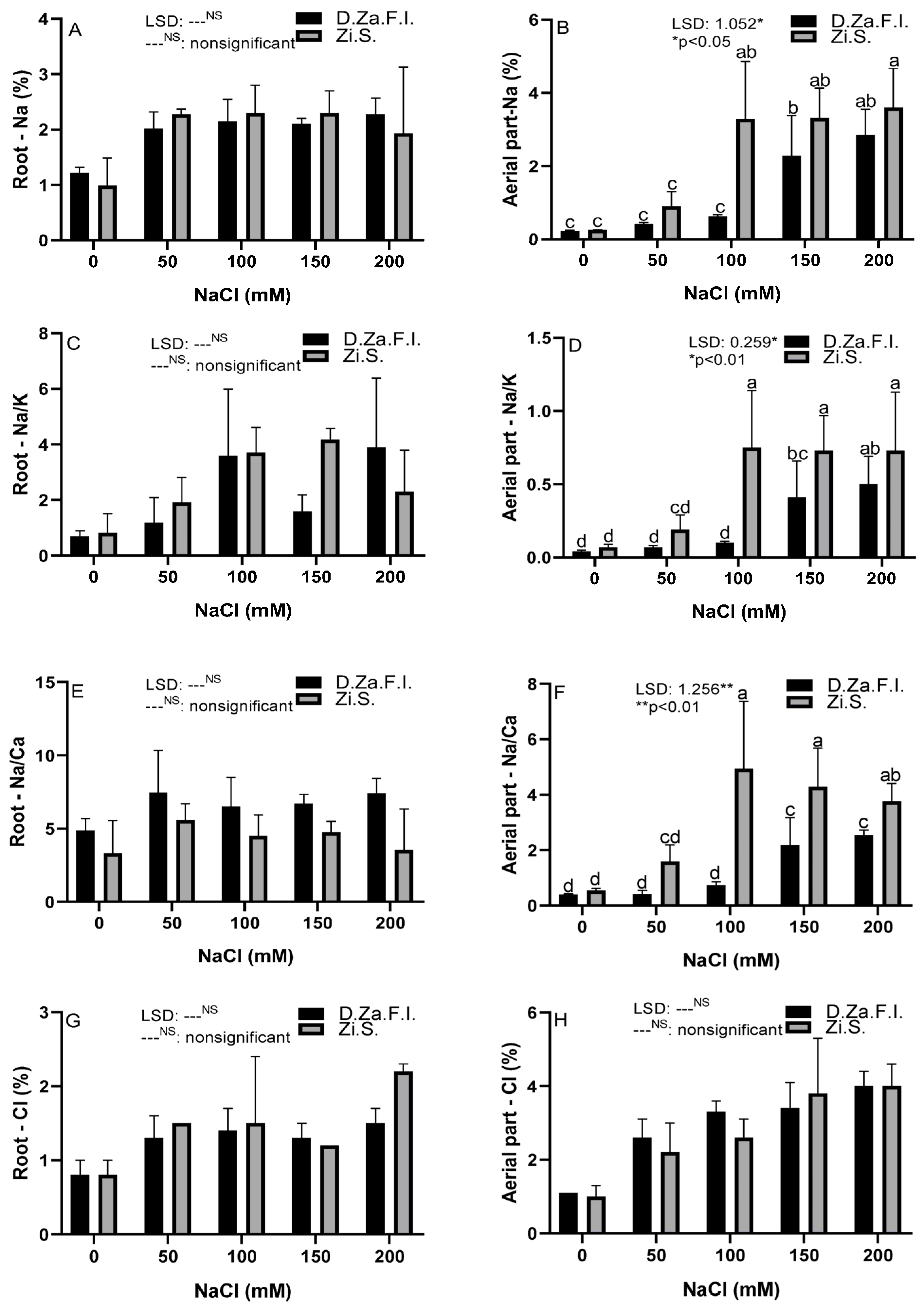
2.4.4. Ion Concentration Analysis
2.5. Anatomical Parameters
2.5.1. Preparation for Stomatal Examination
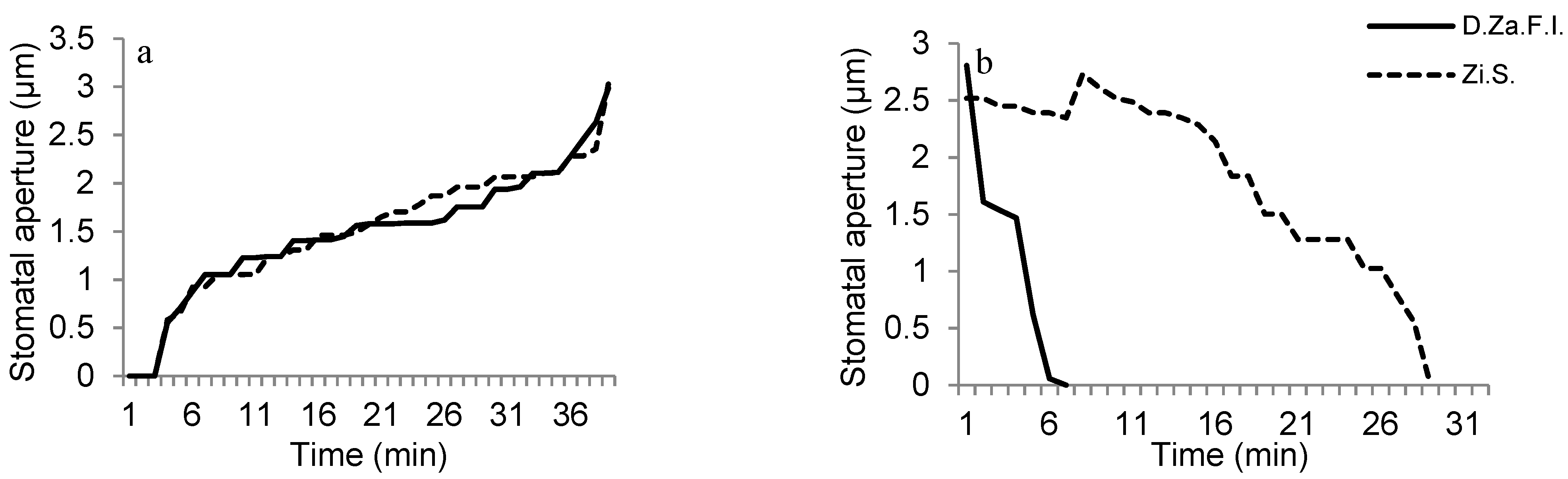
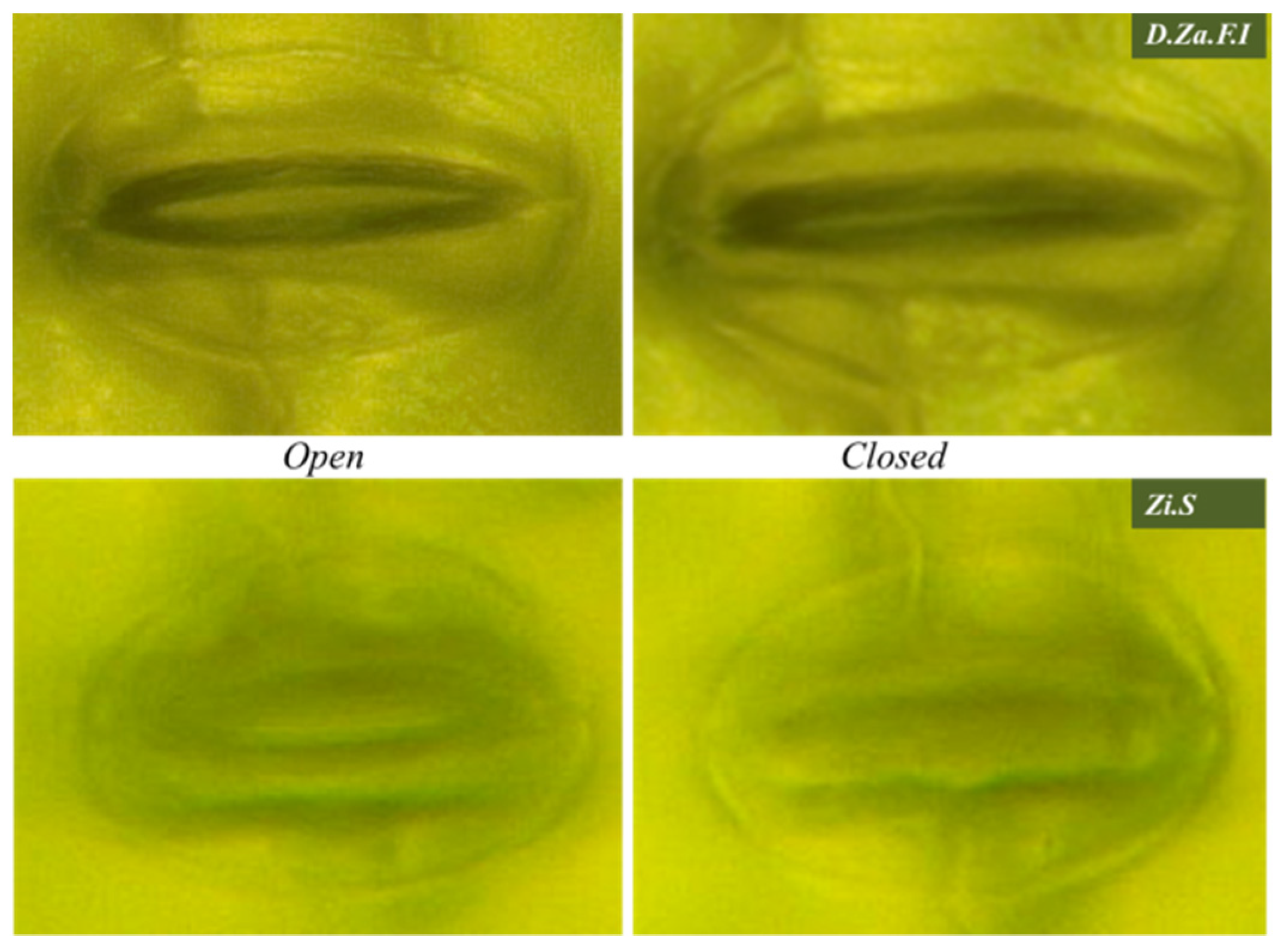
2.5.2. Investigation of Stoma Behaviors against Light Application
2.5.3. Investigation of Stoma Behaviors against ABA Perfusion
2.5.4. Preparation for Leaf Cross-Section Examinations
2.6. Biochemical Parameters
2.6.1. Proline Content
2.6.2. Photosynthetic Pigment and Total Carotenoid Content
2.7. Statistical Analysis
3. Results
3.1. Morphological Parameters
3.2. Anatomical Parameters
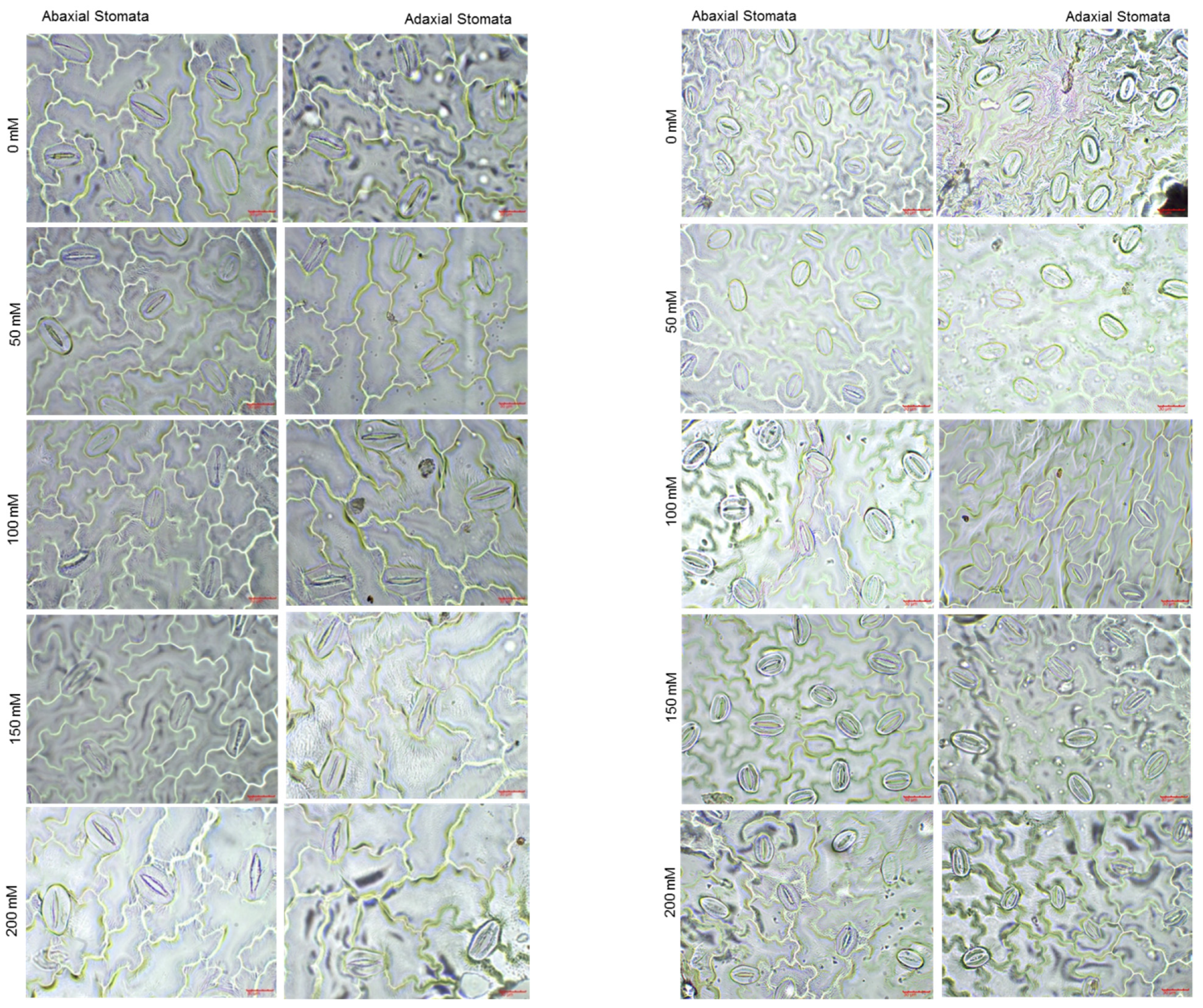
3.2.1. Leaf Stomatal Parameters on Abaxial and Adaxial Epiderma
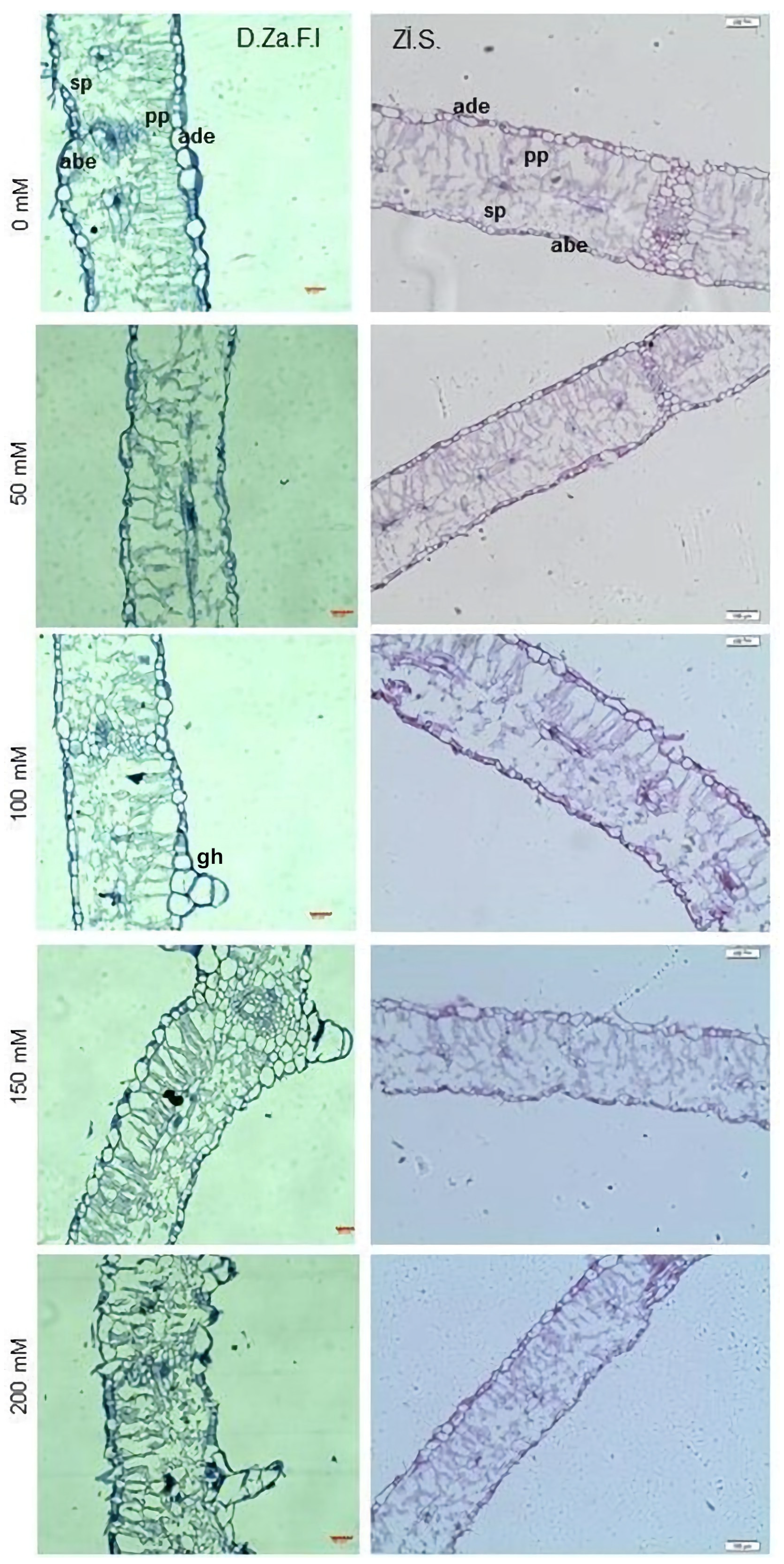
3.2.2. Leaf Cross-Sections
3.3. Physiological and Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2018, 212, 12–22. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl– ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Hewage, K.A.H.; Yang, J.; Wang, D.; Hao, G.; Yang, G.; Zhu, J. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef] [PubMed]
- Akcin, T.A.; Akcin, A.; Yalcın, E. Anatomical changes induced by salinity stress in Salicornia freitagii (Amaranthaceae). Braz. J. Bot. 2017, 40, 1013–1018. [Google Scholar] [CrossRef]
- Souza, P.U.; Lima, L.K.S.; Soares, T.L.; de Jesus, O.N.; Filho, M.A.C.; Girardi, E.A. Biometric, physiological and anatomical responses of Passiflora spp. to controlled water deficit. Sci. Hortic. 2018, 229, 77–90. [Google Scholar] [CrossRef]
- Çavuşoğlu, K.; Kılıç, S.; Kabar, K. Some morphological and anatomical observations during alleviation of salinity (NaCI) stress on seed germination and seedling growth of barley by polyamines. Acta Physiol. Plant. 2007, 29, 551–557. [Google Scholar] [CrossRef]
- Boughalleb, F.; Denden, M.; Ben Tiba, B. Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiol. Plant. 2009, 31, 947–960. [Google Scholar] [CrossRef]
- Kılıc, S.; Cavusoglu, K.; Kabar, K. Effects of 24-epibrassinolide on salinity stress induced inhibition of seed germination, seedling growth and leaf anatomy of barley. Suleyman Demirel Univ. Fac. Art. Sci. J. Sci. 2007, 2, 41–52. [Google Scholar]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; Rehman, H.U.; Bilal, H.M. Synergistic consequences of salinity and potassium deficiency in quinoa: Linking with stomatal patterning, ionic relations and oxidative metabolism. Plant Physiol. Biochem. 2020, 159, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of Abscisic Acid in the Drought Stress Tolerance of Plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Stimart, D.; Boyle, T. Zinnia. In Flower Breeding and Genetics; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherland, 2007; pp. 337–357. [Google Scholar] [CrossRef]
- Marković, M.; Šoštarić, J.; Kojić, A.; Popović, B.; Bubalo, A.; Bošnjak, D.; Stanisavljević, A. Zinnia (Zinnia elegans L.) and Periwinkle (Catharanthus roseus (L.) G. Don) Responses to Salinity Stress. Water 2022, 14, 1066. [Google Scholar] [CrossRef]
- Yasemín, S.; Güzel Değer, A.; Çevík, S.; Koksal, N. Benchmarking of the effects of salinity on antioxidant enzymes ac-tivities, lipid peroxidation and H2O2 levels in the leaves of two Zinnia species. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Doğa Derg. 2021, 24, 31–39. [Google Scholar] [CrossRef]
- Yasemin, S.; Değer, A.G.; Köksal, N. The effects of salt stress in Zinnia (Zinnia sp.) cultivars during seed germination and at the early stages of seedling growth. Turk. J. Agric. Res. 2020, 7, 253–265. [Google Scholar]
- Macherla, K.; McAvoy, R.J. The Effect of Salinity on the Growth and Nutrient Status of Zinnia Grown Under Short- and Long-cycle Subirrigation Management. Hortscience 2017, 52, 770–773. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Arum, L.S.; Ko, C.H.; Muneer, S.; Jeong, B.R. Silicon-mediated enhancement of physiological and biochemical characteristics of Zinnia elegans ‘Dreamland Yellow’ grown under salinity stress. Hortic. Environ. Biotechnol. 2015, 56, 721–731. [Google Scholar] [CrossRef]
- Escalona, A.; Salas-Sanjuán, M.C.; Santos, C.D.; Guzmán, M. The effect of water salinity on growth and ionic con-centration and relation in plant tissues in Zinnia elegans and Tagetes erecta for use in urban landscasping. ITEA 2014, 110, 325–334. [Google Scholar]
- Bizhani, S.; Jowkar, A.; Abdolmaleki, M. Growth and antioxidant response of Zinnia elegans under salt stress conditions. Tech. J. Eng. Appl. Sci. 2013, 3, 1285–1292. [Google Scholar]
- Niu, G.; Wang, M.; Rodriguez, D.; Zhang, D. Response of Zinnia Plants to Saline Water Irrigation. Hortscience 2012, 47, 793–797. [Google Scholar] [CrossRef]
- Villarino, G.H.; Mattson, N.S. Assessing Tolerance to Sodium Chloride Salinity in Fourteen Floriculture Species. Horttechnology 2011, 21, 539–545. [Google Scholar] [CrossRef]
- Zivdar, S.; Khaleghi, E.; Dehkordi, F.S. Effect of salinity and temperature on seed germination indices of Zinnia elegans L. J. Appl. Hortic. 2011, 13, 48–51. [Google Scholar] [CrossRef]
- Carter, C.T.; Grieve, C.M. Growth and Nutrition of Two Cultivars of Zinnia elegans Under Saline Conditions. Hortscience 2010, 45, 1058–1063. [Google Scholar] [CrossRef]
- Yasemin, S. The Changes on Morphological Anatomic, Physiological and Biochemical Features of Zinnia (Zinnia sp.) Species under Salt Stress. Ph.D. Thesis, Cukurova University, Sarıçam/Adana, Turkey, 2020; 248p. [Google Scholar]
- Arora, R.; Pitchay, D.S.; Bearce, B.C. Water-stress-induced heat tolerance in geranium leaf tissues: A possible linkage through stress proteins? Physiol. Plant. 1998, 103, 24–34. [Google Scholar] [CrossRef]
- Kesici, M.; Gulen, H.; Ergin, S.; Turhan, E.; Ipek, A.; Koksal, N. Heat-stress Tolerance of Some Strawberry (Fragaria × ananassa) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 244. [Google Scholar] [CrossRef]
- Padhi, B.; Chauhan, G.; Kandoi, D.; Stirbet, A.; Tripathy, B.; Govindjee, G. A comparison of chlorophyll fluorescence transient measurements, using Handy PEA and FluorPen fluorometers. Photosynthetica 2021, 59, 399–408. [Google Scholar] [CrossRef]
- Çimen, B. Rootstock influences on photosynthetic performance of young ‘Interdonato’ trees grown in calcareous soil. Akad. Ziraat Derg. 2019, 8, 185–194. [Google Scholar] [CrossRef]
- Torun, A.A.; Erdem, N.; Kacar, Y.; Serçe, S. Screening of Wild Strawberry Genotypes against Iron Deficiency under Greenhouse Conditions. Not. Bot. Horti Agrobot. 2013, 41, 560–566. [Google Scholar] [CrossRef]
- Incesu, M.; Cimen, B.; Yesiloglu, T.; Yilmaz, B. Growth and Photosynthetic Response of Two Persimmon Rootstocks (Diospyros kaki and D. virginiana) under Different Salinity Levels. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 386–391. [Google Scholar] [CrossRef]
- Guzel Deger, A.; Scherzer, S.; Nuhkat, M.; Kedzierska, J.; Kollist, H.; Brosche, M.; Unyayar, S.; Boudsocq, M.; Hedrich, R.; Roelfsema, M.R.G. Guard cell SLAC 1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 2015, 208, 162–173. [Google Scholar] [CrossRef]
- Roelfsema, M.R.G.; Levchenko, V.; Hedrich, R. ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J. 2004, 37, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Åström, H.; Metsovuori, E.; Saarinen, T.; Lundell, R.; Hänninen, H. Morphological characteristics and photosynthetic capacity of Fragaria vesca L. winter and summer leaves. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 215, 33–39. [Google Scholar] [CrossRef]
- Karabıyık, Ş.; Gundesli, M.A.; Eti, S.; Kafkas, S.; Güney, M.; Zarıfıkhosroshahı, M.; Kafkas, N. Detection of bud abscission of pistachio via histological analysis. Acta Hortic. 2018, 1219, 193–198. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw-Hill Publishing Company, Ltd.: London, UK, 1940; pp. viii+523. [Google Scholar]
- Pérez-Clemente, R.M.; Montoliu, A.; Zandalinas, S.I.; de Ollas, C.; Gómez-Cadenas, A. Carrizo citrange Plants Do Not Require the Presence of Roots to Modulate the Response to Osmotic Stress. Sci. World J. 2012, 2012, 795396. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Transac. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, G.; Perez, C.; Pemberton, H.B.; Altland, J. Responses of Marigold Cultivars to Saline Water Irrigation. Horttechnology 2018, 28, 166–171. [Google Scholar] [CrossRef]
- Oliveira, F.I.F.; de Medeiros, W.J.F.; de Lacerda, C.F.; Neves, A.L.R.; Oliveira, D.R. Saline water irrigation managements on growth of ornamental plants. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 739–745. [Google Scholar] [CrossRef]
- Carvalho, D.R.; Vasconcelos, M.W.; Lee, S.; Vreugdenhil, D.; Heuvelink, E.; Carvalho, S.M. Moderate salinity improves stomatal functioning in rose plants grown at high relative air humidity. Environ. Exp. Bot. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Kumar, D.; Al Hassan, M.; Vicente, O.; Agrawal, V.; Boscaiu, M. Mechanisms of Response to Salt Stress in Oleander (Nerium oleander L.). Bull. UASVM Hortic. 2016, 73, 249. [Google Scholar] [CrossRef]
- Salachna, P.; Piechocki, R. Effects of sodium chloride on growth and mineral nutrition of purpletop vervain. J. Ecol. Eng. 2016, 17, 148–152. [Google Scholar] [CrossRef]
- Li, X.; Wan, S.; Kang, Y.; Chen, X.; Chu, L. Chinese rose (Rosa chinensis) growth and ion accumulation under irrigation with waters of different salt contents. Agric. Water Manag. 2015, 163, 180–189. [Google Scholar] [CrossRef]
- Zapryanova, N.; Atanassova, B. Effects of Salt Stress on Growth and Flowering of Ornamental Annual Species. Biotechnol. Biotechnol. Equip. 2009, 23, 177–179. [Google Scholar] [CrossRef]
- Navarro, A.; Bañon, S.; Olmos, E.; Sánchez-Blanco, M. Effects of sodium chloride on water potential components, hydraulic conductivity, gas exchange and leaf ultrastructure of Arbutus unedo plants. Plant Sci. 2007, 172, 473–480. [Google Scholar] [CrossRef]
- Fernández-García, N.; Olmos, E.; Bardisi, E.; la Garma, J.G.-D.; López-Berenguer, C.; Rubio-Asensio, J.S. Intrinsic water use efficiency controls the adaptation to high salinity in a semi-arid adapted plant, henna (Lawsonia inermis L.). J. Plant Physiol. 2014, 171, 64–75. [Google Scholar] [CrossRef]
- Acosta-Motos, J.-R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sánchez-Blanco, M.J.; Hernández, J.A. NaCl-induced physiological and biochemical adaptative mechanisms in the ornamental Myrtus communis L. plants. J. Plant Physiol. 2015, 183, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bellot, M.J.; Nortes, P.A.; Ortuño, M.F.; Romero-Trigueros, C.; Fernández-García, N.; Sánchez-Blanco, M.J. Influence of arbuscular mycorrizal fungi and treated wastewater on water relations and leaf structure alterations of Viburnum tinus L. plants during both saline and recovery periods. J. Plant Physiol. 2015, 188, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Ashraf, M.; Naz, N. Anatomical adaptations to salinity in cogon grass [Imperata cylindrica (L.) Raeuschel] from the Salt Range, Pakistan. Plant Soil 2009, 322, 229–238. [Google Scholar] [CrossRef]
- Li, S.; Hamani, A.K.M.; Zhang, Y.; Liang, Y.; Gao, Y.; Duan, A. Coordination of leaf hydraulic, anatomical, and economical traits in tomato seedlings acclimation to long-term drought. BMC Plant Biol. 2021, 21, 536. [Google Scholar] [CrossRef]
- Becker, V.I.; Goessling, J.W.; Duarte, B.; Caçador, I.; Liu, F.; Rosenqvist, E.; Jacobsen, S.-E. Combined effects of soil salinity and high temperature on photosynthesis and growth of quinoa plants (Chenopodium quinoa). Funct. Plant Biol. 2017, 44, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2014, 22, 4056–4075. [Google Scholar] [CrossRef]
- Breś, W.; Bandurska, H.; Kupska, A.; Niedziela, J.; Frąszczak, B. Responses of pelargonium (Pelargonium × hortorum L.H. Bailey) to long-term salinity stress induced by treatment with different NaCl doses. Acta Physiol. Plant. 2015, 38, 26. [Google Scholar] [CrossRef]
- Veatch-Blohm, M.E.; Malinowski, M.; Keefer, D. Leaf water status, osmotic adjustment and carbon assimilation in colored calla lilies in response to saline irrigation. Sci. Hortic. 2012, 144, 65–73. [Google Scholar] [CrossRef]
- Trivellini, A.; Gordillo, B.; Rodríguez-Pulido, F.J.; Borghesi, E.; Ferrante, A.; Vernieri, P.; Quijada-Morín, N.; González-Miret, M.L.; Heredia, F.J. Effect of Salt Stress in the Regulation of Anthocyanins and Color of Hibiscus Flowers by Digital Image Analysis. J. Agric. Food Chem. 2014, 62, 6966–6974. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Hernández, J.A.; Álvarez, S.; Barba-Espín, G.; Sánchez-Blanco, M.J. Long-term resistance mechanisms and irrigation critical threshold showed by Eugenia myrtifolia plants in response to saline reclaimed water and relief capacity. Plant Physiol. Biochem. 2017, 111, 244–256. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological adaptations of two halophytes to salt stress: Photosynthesis, PS II photochemistry and anti-oxidant feedback—Implications for resilience in climate change. Plant Physiol. Biochem. 2013, 67, 178–188. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting Responses of Photosynthesis to Salt Stress in the Glycophyte Arabidopsis and the Halophyte Thellungiella: Role of the Plastid Terminal Oxidase as an Alternative Electron Sink. Plant Physiol. 2008, 149, 1154–1165. [Google Scholar] [CrossRef]
- Parida, A.; Das, A.B.; Das, P. NaCl stress causes changes in photosynthetic pigments, proteins, and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J. Plant Biol. 2002, 45, 28–36. [Google Scholar] [CrossRef]
- Wu, S.; Sun, Y.; Niu, G.; Pantoja, G.L.G.; Rocha, A.C. Responses of Six Lamiaceae Landscape Species to Saline Water Irrigation. J. Environ. Hortic. 2016, 34, 30–35. [Google Scholar] [CrossRef]
- Miralles, J.; Franco, J.; Sánchez-Blanco, M.; Bañón, S. Effects of pot-in-pot production system on water consumption, stem diameter variations and photochemical efficiency of spindle tree irrigated with saline water. Agric. Water Manag. 2016, 170, 167–175. [Google Scholar] [CrossRef]
- Cai, X.; Niu, G.; Starman, T.; Hall, C. Response of six garden roses (Rosa × hybrida L.) to salt stress. Sci. Hortic. 2014, 168, 27–32. [Google Scholar] [CrossRef]
- Bañón, S.; Miralles, J.; Ochoa, J.; Franco, J.; Sánchez-Blanco, M. Effects of diluted and undiluted treated wastewater on the growth, physiological aspects and visual quality of potted lantana and polygala plants. Sci. Hortic. 2011, 129, 869–876. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Zehra, A.; Petrik, P.; Kurjak, D. Suffer or Survive: Decoding Salt-Sensitivity of Lemongrass and Its Implication on Essential Oil Productivity. Front. Plant Sci. 2022, 13, 903954. [Google Scholar] [CrossRef]
- Vernieri, P.; Trivellini, A.; Malorgio, F.; Ferrante, A.; Serra, G. Effect of salt spray on six ornamental species. Acta Hortic. 2010, 881, 463–468. [Google Scholar] [CrossRef]
- Eom, S.H.; Setter, T.; DiTommaso, A.; Weston, L. Differential Growth Response to Salt Stress Among Selected Ornamentals. J. Plant Nutr. 2007, 30, 1109–1126. [Google Scholar] [CrossRef]
- Lee, M.K.; Van Iersel, M.W. Sodium Chloride Effects on Growth, Morphology, and Physiology of Chrysanthemum (Chrysanthemum × morifolium). Hortscience 2008, 43, 1888–1891. [Google Scholar] [CrossRef]
- Bahadoran, M.; Salehi, H. Growth and flowering of two tuberose (Polianthes tuberosa L.) cultivars under deficit irrigation by saline water. J. Agric. Sci. Technol. 2015, 17, 415–426. [Google Scholar]
- Cantabella, D.; Piqueras, A.; Acosta-Motos, J.R.; Bernal-Vicente, A.; Hernández, J.A.; Díaz-Vivancos, P. Salt-tolerance mechanisms induced in Stevia rebaudiana Bertoni: Effects on mineral nutrition, antioxidative metabolism and steviol glycoside content. Plant Physiol. Biochem. 2017, 115, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; McGarvey, D.; Truscott, T. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Falk, J.; Munné-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Wang, L.-Y.; Kong, F.-Y.; Deng, Y.-S.; Li, B.; Meng, Q.-W. Constitutive accumulation of zeaxanthin in tomato alleviates salt stress-induced photoinhibition and photooxidation. Physiol. Plant. 2012, 146, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2014, 35, 425–437. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Li, W.; Meng, R.; Liu, Y.; Chen, S.; Jiang, J.; Wang, L.; Zhao, S.; Wang, Z.; Fang, W.; Chen, F.; et al. Heterografted chrysanthemums enhance salt stress tolerance by integrating reactive oxygen species, soluble sugar, and proline. Hortic. Res. 2022, 9, uhac073. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Pestana, M.; Correia, P.J.; Lao, M.T. Lavandula multifida response to salinity: Growth, nutrient uptake, and physiological changes. J. Plant Nutr. Soil Sci. 2016, 180, 96–104. [Google Scholar] [CrossRef]
- Naidoo, G. Effects of salinity and nitrogen on growth and water relations in the mangrove, Avicennia marina (forsk.) vierh. New Phytol. 1987, 107, 317–325. [Google Scholar] [CrossRef]
- Abdelgadir, E.M.; Oka, M.; Fujiyama, H. Characteristics of Nitrate of Vegetables and Ornamentals; Savvas, D., Passam, H., Eds.; Embryo Publications: Athens, Greece, 2005; pp. 211–261. [Google Scholar]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Kirnak, H.; Higgs, D. Enhancement of growth and normal growth parameters by foliar application of potassium and phosphorus in tomato cultivars grown at high (NaCl) salinity. J. Plant Nutr. 2001, 24, 357–367. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; p. 849. [Google Scholar]
- Simó, M.; Nieves-Cordones, M.; Nieves, M. Differences in growth and ornamental parameters between young Chamaerops humilis L. and Washingtonia robusta H. Wendl palm trees in response to salinity. J. Hortic. Sci. Biotechnol. 2010, 85, 7–11. [Google Scholar] [CrossRef]
- Navarro, A.; Elia, A.; Conversa, G.; Campi, P.; Mastrorilli, M. Potted mycorrhizal carnation plants and saline stress: Growth, quality and nutritional plant responses. Sci. Hortic. 2012, 140, 131–139. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Pestana, M.; Correia, P.J.; Lao, M.T. Tolerance mechanisms of three potted ornamental plants grown under moderate salinity. Sci. Hortic. 2016, 201, 84–91. [Google Scholar] [CrossRef]
- Jampeetong, A.; Brix, H. Effects of NaCl salinity on growth, morphology, photosynthesis and proline accumulation of Salvinia natans. Aquat. Bot. 2009, 91, 181–186. [Google Scholar] [CrossRef]
- Cassaniti, C.; Leonardi, C.; Flowers, T.J. The effects of sodium chloride on ornamental shrubs. Sci. Hortic. 2009, 122, 586–593. [Google Scholar] [CrossRef]
- Cassaniti, C.; Romano, D.; Hop, M.E.C.M.; Flowers, T.J. Growing floricultural crops with brackish water. Environ. Exp. Bot. 2013, 92, 165–175. [Google Scholar] [CrossRef]
- Picchioni, G.; Graham, C. Salinity, growth, and ion uptake selectivity of container-grown Crataegus opaca. Sci. Hortic. 2001, 90, 151–166. [Google Scholar] [CrossRef]
- Colmer, T.D.; Munns, R.; Flowers, T.J. Improving salt tolerance of wheat and barley: Future prospects. Aust. J. Exp. Agric. 2005, 45, 1425–1443. [Google Scholar] [CrossRef]
- Boursier, P.; Läuchli, A. Growth Responses and Mineral Nutrient Relations of Salt-Stressed Sorghum. Crop. Sci. 1990, 30, 1226–1233. [Google Scholar] [CrossRef]
- Alfocea, F.P.; Balibrea, M.E.; Alarcón, J.J.; Bolarín, M.C. Composition of Xylem and Phloem Exudates in Relation to the Salt-tolerance of Domestic and Wild Tomato Species. J. Plant Physiol. 2000, 156, 367–374. [Google Scholar] [CrossRef]
- Adams, E.; Shin, R. Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 2014, 56, 231–249. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil 2011, 352, 353–362. [Google Scholar] [CrossRef]
- Carter, C.T.; Grieve, C.M.; Poss, J.A.; Suarez, D.L. Production and ion uptake of Celosia argentea irrigated with saline wastewaters. Sci. Hortic. 2005, 106, 381–394. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.; Grattan, S.; Shouse, P.; Lieth, J.; Zeng, L. Productivity and Mineral Nutrition of Limonium Species Irrigated with Saline Wastewaters. Hortscience 2005, 40, 654–658. [Google Scholar] [CrossRef]
- Carter, C.T.; Grieve, C.M. Mineral Nutrition, Growth, and Germination of Antirrhinum majus L. (Snapdragon) when Produced Under Increasingly Saline Conditions. Hortscience 2008, 43, 710–718. [Google Scholar] [CrossRef]
- Navarro, A.; Bañón, S.; Conejero, W.; Sánchez-Blanco, M. Ornamental characters, ion accumulation and water status in Arbutus unedo seedlings irrigated with saline water and subsequent relief and transplanting. Environ. Exp. Bot. 2008, 62, 364–370. [Google Scholar] [CrossRef]
- Grieve, C.; Poss, J.; Amrhein, C. Response of Matthiola incana to Irrigation with Saline Wastewaters. Hortscience 2006, 41, 119–123. [Google Scholar] [CrossRef]
- Niu, G.; Starman, T.; Byrne, D. Responses of Growth and Mineral Nutrition of Garden Roses to Saline Water Irrigation. Hortscience 2013, 48, 756–761. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. K+Nutrition and Na+Toxicity: The Basis of Cellular K+/Na+Ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Mansour, M.; Salama, K.; Al-Mutawa, M. Transport proteins and salt tolerance in plants. Plant Sci. 2003, 164, 891–900. [Google Scholar] [CrossRef]
- Zeng, L.; Poss, J.A.; Wilson, C.; Draz, A.-S.E.; Gregorio, G.B.; Grieve, C.M. Evaluation of salt tolerance in rice genotypes by physiological characters. Euphytica 2003, 129, 281–292. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L. Effects of Salinity and Sodicity on Plant Growth. Annu. Rev. Phytopathol. 1975, 13, 295–312. [Google Scholar] [CrossRef]
- Gadallah, M. Effects of Proline and Glycinebetaine on Vicia Faba Responses to Salt Stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Álvarez, S.; Hernández, J.A.; Sánchezblanco, M.J. Irrigation of Myrtus communis plants with reclaimed water: Morphological and physiological responses to different levels of salinity. J. Hortic. Sci. Biotechnol. 2014, 89, 487–494. [Google Scholar] [CrossRef]
- Koksal, N.; Kulahlioglu, I.; Ertargin, E.; Alkan-Torun, A. Relationship between salinity stress and ion uptake of hyacinth (Hyacinthus orientalis). Turk. J. Agric. Nat. Sci. 2014, 578–583. [Google Scholar]
- Koksal, N.; Alkan-Torun, A.; Kulahlioglu, I.; Ertargin, E.; Karalar, E. Ion uptake of marigold under saline growth conditions. Springerplus 2016, 5, 139. [Google Scholar] [CrossRef] [PubMed]
- Esteves, B.S.; Suzuki, M.S. Typha domingensis Pers. subject to interactions among water level and fire event in a tropical lagoon. Acta Limnol. Bras. 2008, 20, 227–234. [Google Scholar]
- Rout, N.P.; Shaw, B.P. Salt Tolerance in Aquatic Macrophytes: Ionic Relation and Interaction. Biol. Plant. 2001, 44, 95–99. [Google Scholar] [CrossRef]
- Marschner, H. Mineral nutrition of higher plants. J. Ecol. 1995, 76, 1250. [Google Scholar]

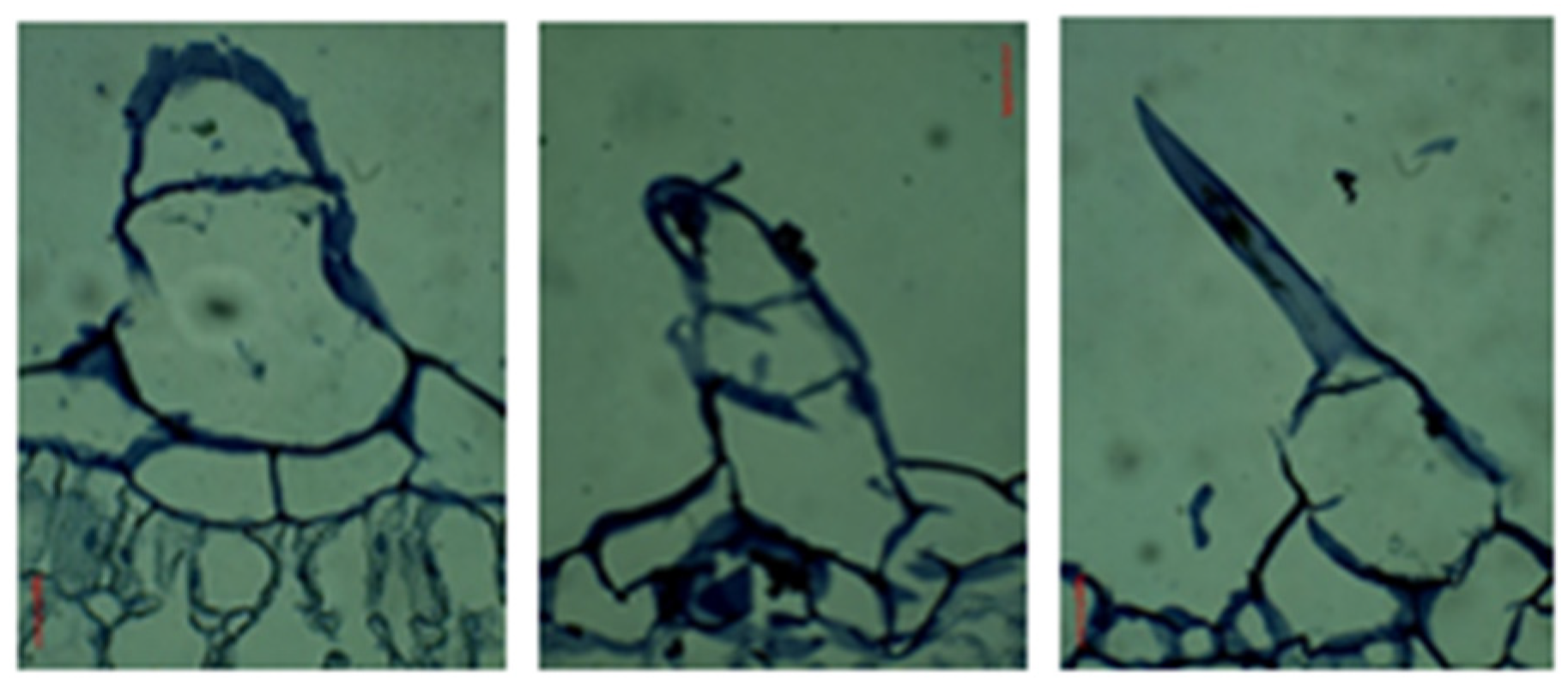

| Plant | NaCl (mM) | Shoot Lenght (cm) | Branch Number (Unit) | Branch Length (cm) | Stem Diameter (mm) | Root Collar Diameter (mm) | Leaf Width (mm) | Leaf Length (mm) | Root Fresh Weight (g) | Root Dry Weight (g) | Shoot Fresh Weight (g) | Shoot Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D.Za.F.I | 0 | 15.6 ± 1.4 | 14.9 ± 1.6 | 9.92 + 1.4 a | 5.22 ± 0.2 a | 6.16 ± 0.2 a | 30.4 ± 1.7 | 99.6 ± 6.3 | 6.66 ± 0.7 | 0.48 ± 0.06 | 30.7 ± 0.7 | 3.51 ± 1.0 |
| 50 | 12.7 ± 1.1 | 14.8 ± 0.9 | 9.72 ± 0.8 a | 4.97 ± 0.5 ab | 5.77 ± 0.3 ab | 28.2 ± 3.2 | 92.5 ± 6.0 | 6.51 ± 0.6 | 0.39 ± 0.02 | 22.1 ± 1.5 | 2.04 ± 0.2 | |
| 100 | 11.4 ± 0.8 | 13.1 ± 1.1 | 8.73 ± 0.4 b | 5.01 ± 0.2 ab | 5.71 ± 0.1 bc | 25.7 ± 3.0 | 84.5 ± 6.9 | 6.11 ± 0.5 | 0.38 ± 0.02 | 19.4 ± 3.1 | 2.14 ± 0.0 | |
| 150 | 10.3 ± 0.5 | 11.9 ± 1.3 | 6.82 ± 0.7 d | 4.44 ± 0.2 c | 5.33 ± 0.1 cd | 24.8 ± 2.5 | 80.0 ± 6.1 | 5.39 ± 0.5 | 0.31 ± 0.04 | 14.9 ± 1.1 | 1.57 ± 0.1 | |
| 200 | 9.9 ± 0.6 | 11.8 ± 0.9 | 6.30 ± 0.7 d | 4.64 ± 0.1 bc | 5.26 ± 0.3 d | 22.3 ± 2.2 | 78.2 ± 3.8 | 4.78 ± 0.8 | 0.28 ± 0.05 | 13.3 ± 2.6 | 1.49 ± 0.2 | |
| Zi.S | 0 | 14.9 ± 2.2 | 8.6 ± 2.2 | 9.48 ± 1.2 a | 5.21 ± 0.4 a | 5.63 ± 0.4 bcd | 41.4 ± 5.1 | 74.7 ± 9.8 | 7.90 ± 3.6 | 0.59 ± 0.24 | 24.8 ± 3.2 | 2.70 ± 0.4 |
| 50 | 13.4 ± 1.2 | 8.4 ± 1.3 | 7.53 ± 1.3 c | 4.82 ± 0.5 abc | 4.71 ± 0.1 e | 39.2 ± 4.1 | 67.1 ± 7.1 | 5.62 ± 2.3 | 0.36 ± 0.11 | 20.0 ± 2.0 | 2.07 ± 0.3 | |
| 100 | 12.8 ± 3.1 | 7.0 ± 1.1 | 6.46 ± 1.0 d | 4.82 ± 0.2 abc | 4.49 ± 0.2 e | 36.0 ± 5.8 | 63.4 ± 7.4 | 3.88 ± 1.6 | 0.30 ± 0.10 | 12.4 ± 4.3 | 1.59 ± 0.3 | |
| 150 | 10.2 ± 1.3 | 7.0 ± 1.2 | 5.06 ± 0.9 e | 4.74 ± 0.1 bc | 4.43 ± 0.1 e | 33.8 ± 3.6 | 60.9 ± 6.7 | 3.99 ± 0.5 | 0.28 ± 0.05 | 12.0 ± 0.7 | 1.55 ± 0.1 | |
| 200 | 10.1 ± 1.0 | 6.7 ± 1.3 | 4.49 ± 0.6 e | 3.64 ± 0.1 d | 3.39 ± 0.1 f | 33.3 ± 5.3 | 58.1 ± 6.6 | 2.23 ± 1.0 | 0.16 ± 0.07 | 8.6 ± 1.4 | 1.15 ± 0.1 | |
| LSD | ---NS | ---NS | 0.708 ** | 0.445 ** | 0.404 ** | ---NS | ---NS | ---NS | ---NS | ---NS | ---NS | |
| Cultivar | NaCl (mM) | Abaxial Stomatal Parameters | Adaxial Stomatal Parameters | ||||
|---|---|---|---|---|---|---|---|
| Width (µm) | Length (µm) | Density (Unit) | Width (µm) | Length (µm) | Density (unit) | ||
| D.Za.F.I | 0 | 27.0 ± 3.0 a | 46.3 ± 4.4 a | 147 ± 32 c | 25.4 ± 2.4 b | 46.7 ± 3.4 b | 85 ± 24 d |
| 50 | 24.2 ± 2.0 bc | 44.4 ± 3.9 ab | 135 ± 30 c | 22.3 ± 1.8 c | 46.5 ± 2.8 b | 71 ± 11 de | |
| 100 | 24.1 ± 1.7 bc | 43.1 ± 4.6 b | 141 ± 17 c | 28.5 ± 3.9 a | 52.6 ± 3.4 a | 66 ± 10 e | |
| 150 | 24.3 ± 2.0 bc | 43.3 ± 5.6 b | 139 ± 18 c | 28.0 ± 3.2 a | 51.0 ± 3.5 a | 62 ± 15 e | |
| 200 | 24.9 ± 2.4 b | 44.6 ± 5.3 ab | 88 ± 16 d | 25.3 ± 3.7 b | 48.6 ± 3.9 b | 64 ± 14 e | |
| Zi.S | 0 | 19.7 ± 2.2 d | 32.1 ± 2.9 d | 227 ± 34 a | 17.9 ± 1.6 d | 30.4 ± 2.1 ef | 175 ± 19 a |
| 50 | 20.2 ± 2.1 d | 32.4 ± 3.7 d | 238 ± 36 a | 20.5 ± 1.7 c | 32.5 ± 2.2 de | 173 ± 25 a | |
| 100 | 22.8 ± 2.8 c | 36.4 ± 2.4 c | 177 ± 9 b | 18.6 ± 2.1 d | 33.5 ± 2.9 cd | 150 ± 16 b | |
| 150 | 19.8 ± 1.9 d | 30.3 ± 1.9 de | 179 ± 20 b | 21.2 ± 3.0 c | 35.5 ± 5.2 c | 152 ± 28 b | |
| 200 | 19.5 ± 2.7 d | 28.3 ± 3.5 e | 182 ± 25 b | 17.9 ± 2.0 d | 29.4 ± 2.0 f | 124 ± 17 c | |
| LSD | 1.612 *** | 2.802 *** | 23.811 *** | 1.314 *** | 2.290 ** | 17.590 * | |
| Cultivar | NaCl (mM) | LT (µm) | LAbEC (µm) | LPL (µm) | WSPC (µm) | LAdEC (µm) |
|---|---|---|---|---|---|---|
| D.Za.F.I | 0 | 272.8 ± 21 c | 25.5 ± 5 | 73.0 ± 5 b | 17.4 ± 4 de | 15.6 ± 3 cd |
| 50 | 270.5 ± 12 c | 24.8 ± 6 | 73.0 ± 8 b | 15.7 ± 2 ef | 15.1 ± 3 d | |
| 100 | 292.1 ± 23 b | 33.5 ± 8 | 57.8 ± 7 d | 14.2 ± 4 f | 19.8 ± 6 a | |
| 150 | 246.2 ± 12 d | 28.1 ± 5 | 54.0 ± 5 e | 14.4 ± 2 f | 18.1 ± 3 abc | |
| 200 | 247.0 ± 26 d | 27.6 ± 6 | 54.4 ± 8 de | 14.5 ± 3 f | 18.3 ± 5 ab | |
| Zi.S | 0 | 359.7 ± 22 a | 20.5 ± 3 | 84.1 ± 7 a | 22.0 ± 3 ab | 11.3 ± 6 e |
| 50 | 303.1 ± 10 b | 23.9 ± 4 | 72.2 ± 4 b | 20.0 ± 3 bc | 18.2 ± 7 ab | |
| 100 | 303.4 ± 36 b | 33.1 ± 5 | 82.2 ± 4 a | 23.9 ± 5 a | 17.2 ± 5 bcd | |
| 150 | 302.5 ± 57 b | 28.5 ± 5 | 80.5 ± 7 a | 19.2 ± 3 cd | 15.7 ± 8 bcd | |
| 200 | 255.6 ± 33 cd | 25.0 ± 4 | 67.5 ± 4 c | 17.6 ± 4 de | 15.2 ± 7 d | |
| LSD | 17.996 *** | ---NS | 3.826 *** | 2.180 *** | 2.611 ** | |
| Plant | NaCl (mM) | P | K | Ca | Mg | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|
| D.Za.F.I. | 0 | 0.59 ± 0.10 | 1.86 ± 0.6 | 0.25 ± 0.02 | 1.36 ± 0.2 | 278.2 ± 72 | 40.1 ± 2.5 | 24.4 ± 5 | 117.0 ± 19 |
| 50 | 0.61 ± 0.12 | 2.16 ± 1.0 | 0.29 ± 0.09 | 1.12 ± 0.2 | 270.2 ± 76 | 41.5 ± 1.5 | 24.4 ± 2 | 166.2 ± 40 | |
| 100 | 0.70 ± 0.05 | 0.97 ± 0.9 | 0.34 ± 0.06 | 1.05 ± 0.4 | 252.7 ± 6 | 41.1 ± 1.2 | 28.8 ± 11 | 150.8 ± 65 | |
| 150 | 0.79 ± 0.01 | 1.42 ± 0.4 | 0.32 ± 0.05 | 0.88 ± 0.2 | 165.8 ± 18 | 38.2 ± 2.9 | 23.0 ± 6 | 132.3 ± 14 | |
| 200 | 0.61 ± 0.11 | 0.75 ± 0.4 | 0.31 ± 0.06 | 0.92 ± 0.3 | 215.4 ± 42 | 42.3 ± 0.5 | 28.5 ± 12 | 163.7 ± 54 | |
| Zi.S. | 0 | 0.55 ± 0.15 | 1.51 ± 0.5 | 0.34 ± 0.08 | 1.12 ± 0.3 | 237.8 ± 90 | 44.7 ± 7 | 63.7 ± 23 | 86.9 ± 15 |
| 50 | 0.55 ± 0.02 | 1.45 ± 0.9 | 0.42 ± 0.09 | 1.30 ± 0.1 | 277.3 ± 63 | 56.9 ± 24 | 68.2 ± 32 | 86.8 ± 13 | |
| 100 | 0.49 ± 0.15 | 0.62 ± 0.0 | 0.53 ± 0.07 | 0.83 ± 0.2 | 260.6 ± 110 | 45.5 ± 8 | 57.2 ± 28 | 94.1 ± 24 | |
| 150 | 0.52 ± 0.25 | 1.28 ± 0.9 | 0.50 ± 0.14 | 0.77 ± 0.3 | 255.2 ± 74 | 48.4 ± 7 | 57.9 ± 24 | 95.4 ± 6 | |
| 200 | 0.39 ± 0.19 | 0.85 ± 0.1 | 0.70 ± 0.30 | 0.45 ± 0.3 | 191.6 ± 52 | 59.9 ± 13 | 43.8 ± 25 | 84.6 ± 11 | |
| LSD | ---NS | ---NS | ---NS | ---NS | ---NS | ---NS | ---NS | ---NS | |
| Plant | NaCl (mM) | N | P | K | Ca | Mg | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| D.Za.F.I. | 0 | 2.68 ± 0.37 bc | 0.77 ± 0.03 | 5.30 ± 0.9 | 0.57 ± 0.03 | 1.54 ± 0.03 | 203.9 ± 31 | 46.1 ± 5 | 204.3 ± 15 | 174.2 ± 15 |
| 50 | 2.88 ± 0.60 bc | 0.79 ± 0.01 | 6.19 ± 0.4 | 1.03 ± 0.30 | 1.82 ± 0.11 | 188.1 ± 27 | 43.1 ± 1 | 206.1 ± 41 | 180.9 ± 18 | |
| 100 | 3.33 ± 0.78 ab | 0.73 ± 0.05 | 6.12 ± 0.1 | 0.86 ± 0.13 | 1.74 ± 0.15 | 162.9 ± 14 | 42.6 ± 6 | 226.9 ± 17 | 168.2 ± 27 | |
| 150 | 3.94 ± 0.24 ab | 0.76 ± 0.03 | 5.84 ± 0.8 | 1.05 ± 0.11 | 1.87 ± 0.15 | 149.9 ± 11 | 39.9 ± 2 | 239.2 ± 11 | 179.6 ± 27 | |
| 200 | 4.29 ± 0.64 a | 0.71 ± 0.03 | 5.84 ± 0.7 | 1.11 ± 0.20 | 1.91 ± 0.36 | 232.6 ± 10 | 42.4 ± 2 | 273.8 ± 10 | 157.1 ± 23 | |
| Zi.S. | 0 | 3.84 ± 0.36 ab | 1.01 ± 0.07 | 4.10 ± 1.4 | 0.46 ± 0.07 | 1.91 ± 0.17 | 144.0 ± 19 | 38.7 ± 3 | 223.3 ± 36 | 200.4 ± 65 |
| 50 | 4.48 ± 0.79 a | 1.14 ± 0.09 | 4.96 ± 0.6 | 0.55 ± 0.12 | 1.91 ± 0.39 | 143.3 ± 6 | 38.7 ± 4 | 209.7 ± 49 | 142.6 ± 60 | |
| 100 | 4.46 ± 0.07 a | 1.21 ± 0.11 | 4.43 ± 0.6 | 0.67 ± 0.06 | 1.99 ± 0.20 | 151.1 ± 18 | 44.2 ± 5 | 298.4 ± 50 | 165.4 ± 69 | |
| 150 | 1.87 ± 1.99 c | 1.05 ± 0.16 | 4.58 ± 0.5 | 0.79 ± 0.08 | 1.94 ± 0.10 | 144.3 ± 6 | 38.9 ± 3 | 272.5 ± 18 | 225.1 ± 12 | |
| 200 | 0.30 ± 0.29 d | 1.09 ± 0.17 | 4.97 ± 1.9 | 0.95 ± 0.19 | 1.87 ± 0.37 | 138.9 ± 8 | 38.7 ± 2 | 241.6 ± 23 | 201.8 ± 54 | |
| LSD | 1.361 *** | ---NS | ---NS | ---NS | ---NS | ---NS | ---NS | ---NS | ---NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasemin, S.; Koksal, N. Comparative Analysis of Morphological, Physiological, Anatomic and Biochemical Responses in Relatively Sensitive Zinnia elegans ‘Zinnita Scarlet’ and Relatively Tolerant Zinnia marylandica ‘Double Zahara Fire Improved’ under Saline Conditions. Horticulturae 2023, 9, 247. https://doi.org/10.3390/horticulturae9020247
Yasemin S, Koksal N. Comparative Analysis of Morphological, Physiological, Anatomic and Biochemical Responses in Relatively Sensitive Zinnia elegans ‘Zinnita Scarlet’ and Relatively Tolerant Zinnia marylandica ‘Double Zahara Fire Improved’ under Saline Conditions. Horticulturae. 2023; 9(2):247. https://doi.org/10.3390/horticulturae9020247
Chicago/Turabian StyleYasemin, Sara, and Nezihe Koksal. 2023. "Comparative Analysis of Morphological, Physiological, Anatomic and Biochemical Responses in Relatively Sensitive Zinnia elegans ‘Zinnita Scarlet’ and Relatively Tolerant Zinnia marylandica ‘Double Zahara Fire Improved’ under Saline Conditions" Horticulturae 9, no. 2: 247. https://doi.org/10.3390/horticulturae9020247






