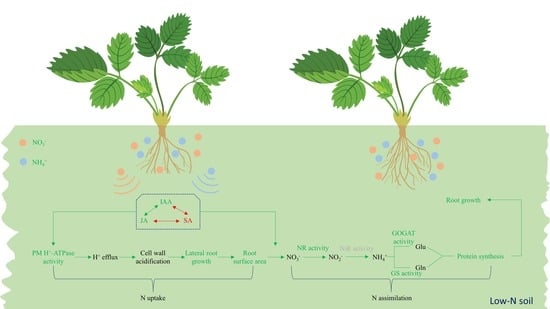
Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation Conditions
2.2. Biomass Determination
2.3. Root Morphology Examination
2.4. Lateral Root Density Determination
2.5. Enzyme Activity Assay
2.6. Soluble Protein Content Determination
2.7. Total N Content Measurement
2.8. PM H+-ATPase Activity Assay
2.9. Determination of Plant Hormones
2.10. Statistical Analysis
3. Results
3.1. Biomass of Strawberry Roots
3.2. Morphology of Strawberry Roots
3.3. Lateral Root Density
3.4. Hormone Contents in Strawberry Roots
3.5. PM H+-ATPase Activity
3.6. Enzyme Activity Related to Nitrogen Assimilation
3.7. Soluble Protein Content in Strawberry Roots
3.8. Total Nitrogen Content in Strawberry Roots
4. Discussion
4.1. Root Architecture Changes for Better N Uptake
4.2. Improving N Utilization via Enzyme Activities Changes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Urban, A.; Rogowski, P.; Wasilewska-Debowska, W.; Romanowska, E. Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis. Plants 2021, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.Y.; Qian, Q.F.; Ye, Z.L.; Zeng, J.B.; Han, Z.G.; Zhang, G.P. Metabolic Analysis of Two Contrasting Wild Barley Genotypes Grown Hydroponically Reveals Adaptive Strategies in Response to Low Nitrogen Stress. J. Plant Physiol. 2016, 206, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.D.M. Nitrogen Use Efficiency of Crop Plants: Physiological Constraints Upon Nitrogen Absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar] [CrossRef]
- Undurraga, S.F.; Ibarra-Henriquez, C.; Fredes, I.; Alvarez, J.M.; Gutierrez, R.A. Nitrate Signaling and Early Responses in Arabidopsis Roots. J. Exp. Bot. 2017, 68, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Wang, H.Y.; Lei, Q.L.; Luo, J.F.; Lindsey, S.; Zhang, J.Z.; Zhai, L.M.; Wu, S.X.; Zhang, J.S.; Liu, X.X.; et al. Optimizing the Nitrogen Application Rate for Maize and Wheat Based on Yield and Environment on the Northern China Plain. Sci. Total Environ. 2018, 618, 1173–1183. [Google Scholar] [CrossRef]
- Forde, B.G. Local and Long-Range Signaling Pathways Regulating Plant Responses to Nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef]
- Krouk, G.; Ruffel, S.; Gutierrez, R.A.; Gojon, A.; Crawford, N.M.; Coruzzil, G.M.; Lacombe, B. A Framework Integrating Plant Growth with Hormones and Nutrients. Trends Plant Sci. 2011, 16, 178–182. [Google Scholar] [CrossRef]
- Wang, X.; Shen, J.; Liao, H. Acquisition or Utilization, Which Is More Critical for Enhancing Phosphorus Efficiency in Modern Crops? Plant Sci. 2010, 179, 302–306. [Google Scholar] [CrossRef]
- Gaudin, A.C.M.; McClymont, S.A.; Holmes, B.M.; Lyons, E.; Raizada, M.N. Novel Temporal, Fine-Scale and Growth Variation Phenotypes in Roots of Adult-Stage Maize (Zea Mays L.) in Response to Low Nitrogen Stress. Plant Cell Environ. 2011, 34, 2122–2137. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Chen, F.; Zhang, F.; Ren, T.; Zhuang, Z.; Mi, G. Mapping Qtls for Root Traits under Different Nitrate Levels at the Seedling Stage in Maize (Zea Mays L.). Plant Soil 2008, 305, 253–265. [Google Scholar] [CrossRef]
- Kirk, J.G.D.; Du, L.V. Changes in Rice Root Architecture, Porosity, and Oxygen and Proton Release under Phosphorus Deficiency. New Phytol. 1997, 135, 191–200. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, P.X.; Cai, X.T.; Mao, J.L.; Miao, Z.Q.; Xiang, C.B. Integration of Jasmonic Acid and Ethylene into Auxin Signaling in Root Development. Front. Plant Sci. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Genes Controlling Expression of Defense Responses in Arabidopsis—2001 Status. Curr. Opin. Plant Biol. 2001, 4, 301–308. [Google Scholar] [CrossRef]
- Turner, J.G.; Ellis, C.; Devoto, A. The Jasmonate Signal Pathway. Plant Cell 2002, 14, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, J.Q.; Zhai, Q.Z.; Zhou, W.K.; Qi, L.L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.L.; Qi, J.; et al. The Basic Helix-Loop-Helix Transcription Factor MYC2 Directly Represses PLETHORA Expression During Jasmonate-Mediated Modulation of the Root Stem Cell Niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis Erf109 Mediates Cross-Talk between Jasmonic Acid and Auxin Biosynthesis During Lateral Root Formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Zhao, F.; Chen, Y.Q.; Pan, Y.; Sun, L.J.; Bao, N.; Zhang, T.; Cui, C.X.; Qiu, Z.Z.; Zhang, Y.J.; et al. Jasmonate-Mediated Wound Signalling Promotes Plant Regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef]
- Conesa, C.M.; Saez, A.; Navarro-Neila, S.; de Lorenzo, L.; Hunt, A.G.; Sepulveda, E.B.; Baigorri, R.; Garcia-Mina, J.M.; Zamarreno, A.M.; Sacristan, S.; et al. Alternative Polyadenylation and Salicylic Acid Modulate Root Responses to Low Nitrogen Availability. Plants 2020, 9, 251. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic Acid Affects Root Meristem Patterning Via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct Signalling Pathways and Transcriptome Response Signatures Differentiate Ammonium- and Nitrate-Supplied Plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef]
- Ishiyama, K.; Kojima, S.; Takahashi, H.; Hayakawa, T.; Yamaya, T. Cell Type Distinct Accumulations of Mrna and Protein for Nadh-Dependent Glutamate Synthase in Rice Roots in Response to the Supply of Nh4+. Plant Physiol. Biochem. 2003, 41, 643–647. [Google Scholar] [CrossRef]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-Atpase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of Auxin-Induced Cell Elongation Is Alive and Well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Rober-Kleber, N.; Albrechtova, J.T.P.; Fleig, S.; Huck, N.; Michalke, W.; Wagner, E.; Speth, V.; Neuhaus, G.; Fischer-Iglesias, C. Plasma Membrane H+-Atpase Is Involved in Auxin-Mediated Cell Elongation During Wheat Embryo Development. Plant Physiol. 2003, 131, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Duby, G.; Boutry, M. The Plant Plasma Membrane Proton Pump Atpase: A Highly Regulated P-Type Atpase with Multiple Physiological Roles. Pflug. Arch. Eur. J. Physiol. 2009, 457, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.M. Changes in the Concentration of Leaf Nitrogen over the Season Affect the Diagnosis of Deficiency or Sufficiency in Strawberries in the Subtropics. Agriculture 2018, 8, 126. [Google Scholar] [CrossRef]
- Nestby, R.; Lieten, F.; Pivot, D.; Lacroix, C.R.; Tagliavini, M. Influence of Mineral Nutrients on Strawberry Fruit Quality and Their Accumulation in Plant Organs: A Review. Int. J. Fruit Sci. 2005, 5, 139–156. [Google Scholar] [CrossRef]
- Yoshida, Y.; Goto, T.; Hirai, M.; Masuda, M. Anthocyanin Accumulation in Strawberry Fruits as Affected by Nitrogen Nutrition; International Society for Horticultural Science: Leuven, Belgium, 2002; pp. 357–360. [Google Scholar]
- Ojeda-Real, L.A.; Lobit, P.; Cárdenas-Navarro, R.; Grageda-Cabrera, O.; Farías-Rodríguez, R.; Valencia-Cantero, E.; Macías-Rodríguez, L. Effect of Nitrogen Fertilization on Quality Markers of Strawberry (Fragaria× Ananassa Duch. Cv. Aromas). J. Sci. Food Agric. 2009, 89, 935–939. [Google Scholar] [CrossRef]
- Kun, C.; Yimin, C.; Hong, Z.; Tongyong, L.; Qing, X.; Lei, W. Effects of Different No3− Concentrations on Growth and Photosynthetic Characteristics in Strawberry Seedling. Chin. Agric. Sci. Bull. 2012, 28, 221–224. [Google Scholar]
- Placido, D.F.; Sandhu, J.; Sato, S.J.; Nersesian, N.; Quach, T.; Clemente, T.E.; Staswick, P.E.; Walia, H. The Lateral Root Density Gene Regulates Root Growth During Water Stress in Wheat. Plant Biotechnol. J. 2020, 18, 1955–1968. [Google Scholar] [CrossRef] [Green Version]
- Majláth, I.; Darko, E.; Palla, B.; Nagy, Z.; Janda, T.; Szalai, G. Reduced Light and Moderate Water Deficiency Sustain Nitrogen Assimilation and Sucrose Degradation at Low Temperature in Durum Wheat. J. Plant Physiol. 2016, 191, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Bowsher, C.G.; Emes, M.J.; Cammack, R.; Hucklesby, D.P. Purification and Properties of Nitrite Reductase from Roots of Pea (Pisum Sativum Cv. Meteor). Planta 1988, 175, 334–340. [Google Scholar] [CrossRef]
- Singh, R.P.; Srivastava, H.S. Increase in Glutamate Synthase Nadh Activity in Maize Zea-Mays Cultivar Ganga-Safed-2 Seedlings in Response to Nitrate and Ammonium Nitrogen. Physiol. Plant. 1986, 66, 413–416. [Google Scholar] [CrossRef]
- Yuefu, W.; Zhenwen, Y.; Shangxia, L.; Songlie, Y. Effect of Nitrogen Nutrition on the Change of Key Enzyme Activity During the Nitrogen Metabolism and Kernel Protein Content in Winter Wheat. Zuo Wu Xue Bao 2002, 28, 743–748. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Saez-Plaza, P.; Jose Navas, M.; Wybraniec, S.; Michalowski, T.; Garcia Asuero, A. An Overview of the Kjeldahl Method of Nitrogen Determination. Part Ii. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, L.; Ji, Y.; Fang, Y. Effects of Nacl Stress on Activity and Expression of Plasma Membrane H+-Atpase in Broussonetia Papyrifera. J. Beijing For. Univ. 2011, 33, 21–26. [Google Scholar]
- Yang, L.; Jon, C.-S.; Wang, L.; Zou, Y.; Liu, L.; Ri, H.-C.; Zhao, J.; Cui, M.; Shang, H.-B.; Li, D. Analysis of Multiple-Phytohormones During Fruit Development in Strawberry by Using Miniaturized Dispersive Solid-Phase Extraction Based on Ionic Liquid-Functionalized Carbon Fibers. J. Food Compos. Anal. 2022, 106, 104262. [Google Scholar] [CrossRef]
- van der Bom, F.J.; Williams, A.; Bell, M.J. Root Architecture for Improved Resource Capture: Trade-Offs in Complex Environments. J. Exp. Bot. 2020, 71, 5752–5763. [Google Scholar] [CrossRef]
- Kenobi, K.; Atkinson, J.A.; Wells, D.M.; Gaju, O.; De Silva, J.G.; Foulkes, M.J.; Dryden, I.L.; Wood, A.T.A.; Bennett, M.J. Linear Discriminant Analysis Reveals Differences in Root Architecture in Wheat Seedlings Related to Nitrogen Uptake Efficiency. J. Exp. Bot. 2017, 68, 4969–4981. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chen, F.; Yuan, L.; Zhang, F.; Mi, G. A Comprehensive Analysis of Root Morphological Changes and Nitrogen Allocation in Maize in Response to Low Nitrogen Stress. Plant Cell Environ. 2015, 38, 740–750. [Google Scholar] [CrossRef]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and Shoot Traits for Rice Varieties with Higher Grain Yield and Higher Nitrogen Use Efficiency at Lower Nitrogen Rates Application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Wu, Q.; Lai, N.; Yuan, L.; Zhang, F. Ideotype Root Architecture for Efficient Nitrogen Acquisition by Maize in Intensive Cropping Systems. Sci. China-Life Sci. 2010, 53, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bucio, J.; Cruz-Ramirez, A.; Herrera-Estrella, L. The Role of Nutrient Availability in Regulating Root Architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal Control of Nitrogen Acquisition: Roles of Auxin, Abscisic Acid, and Cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wiren, N. Local Auxin Biosynthesis Acts Downstream of Brassinosteroids to Trigger Root Foraging for Nitrogen. Nat. Commun. 2021, 12, 5437. [Google Scholar] [CrossRef]
- Meier, M.; Liu, Y.; Lay-Pruitt, K.S.; Takahashi, H.; von Wiren, N. Auxin-Mediated Root Branching Is Determined by the Form of Available Nitrogen. Nat. Plants 2020, 6, 1136–1145. [Google Scholar] [CrossRef]
- Liu, Y.; von Wiren, N. Integration of Nutrient and Water Availabilities Via Auxin into the Root Developmental Program. Curr. Opin. Plant Biol. 2022, 65, 102117. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Wang, P.; Chen, F.; Yuan, L.; Mi, G. Low Nitrogen Induces Root Elongation Via Auxin-Induced Acid Growth and Auxin-Regulated Target of Rapamycin (Tor) Pathway in Maize. J. Plant Physiol. 2020, 254, 153281. [Google Scholar] [CrossRef]
- Wang, S.C.; Ichii, M.; Taketa, S.; Xu, L.L.; Xia, K.; Zhou, X. Lateral Root Formation in Rice (Oryza Sativa): Promotion Effect of Jasmonic Acid. J. Plant Physiol. 2002, 159, 827–832. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Ye, S.; Jiang, H.; Chen, Q.; Liu, F.; Zhou, W.; Chen, R.; Li, X.; Tietz, O.; et al. Arabidopsis Asa1 Is Important for Jasmonate-Mediated Regulation of Auxin Biosynthesis and Transport During Lateral Root Formation. Plant Cell 2009, 21, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin Biosynthesis by the Yucca Flavin Monooxygenases Controls the Formation of Floral Organs and Vascular Tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentrich, M.; Boettcher, C.; Duechting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The Jasmonic Acid Signaling Pathway Is Linked to Auxin Homeostasis through the Modulation of Yucca8 and Yucca9 Gene Expression. Plant J. 2013, 74, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.P.; Zhang, C.L.; Zheng, H.H.; Sun, M.; Zhang, F.; Zhang, M.Y.; Cui, F.H.; Lv, D.P.; Liu, L.J.; Guo, S.Y.; et al. Antagonistic Interaction between Auxin and Sa Signaling Pathways Regulates Bacterial Infection through Lateral Root in Arabidopsis. Cell Rep. 2020, 32, 108060. [Google Scholar] [CrossRef]
- Echevarria-Machado, I.; Escobedo-Gm, R.M.; Larque-Saavedra, A. Responses of Transformed Catharanthus Roseus Roots to Ferntomolar Concentrations of Salicylic Acid. Plant Physiol. Biochem. 2007, 45, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Kitakura, S.; Vanneste, S.; Robert, S.; Lofke, C.; Teichmann, T.; Tanaka, H.; Friml, J. Clathrin Mediates Endocytosis and Polar Distribution of Pin Auxin Transporters in Arabidopsis. Plant Cell 2011, 23, 1920–1931. [Google Scholar] [CrossRef]
- Llorente, F.; Muskett, P.; Sanchez-Vallet, A.; Lopez, G.; Ramos, B.; Sanchez-Rodriguez, C.; Jorda, L.; Parker, J.; Molina, A. Repression of the Auxin Response Pathway Increases Arabidopsis Susceptibility to Necrotrophic Fungi. Mol. Plant 2008, 1, 496–509. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic Acid and Jasmonic Acid Crosstalk in Plant Immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.B.A.; Liu, S.D.; Zhang, S.P.; Ge, C.W.; Shen, Q.; Ma, H.J.; Zhang, X.M.; Dong, H.L.; Zhao, X.H.; et al. Nitrogen Stress Inhibits Root Growth by Regulating Cell Wall and Hormone Changes in Cotton (Gossypium Hirsutum L.). J. Agron. Crop Sci. 2021, 207, 1006–1023. [Google Scholar] [CrossRef]
- Allu, A.D.; Brotman, Y.; Xue, G.P.; Balazadeh, S. Transcription Factor Anac032 Modulates Ja/Sa Signalling in Response to Pseudomonas Syringae Infection. Embo Rep. 2016, 17, 1578–1589. [Google Scholar] [CrossRef]
- Ndamukong, I.; Al Abdallat, A.; Thurow, C.; Fode, B.; Zander, M.; Weigel, R.; Gatz, C. Sa-Inducible Arabidopsis Glutaredoxin Interacts with Tga Factors and Suppresses Ja-Responsive Pdf1.2 Transcription. Plant J. 2007, 50, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Spivey, N.W.; Zeng, W.Q.; Liu, P.P.; Fu, Z.Q.; Klessig, D.F.; He, S.Y.; Dong, X.N. Coronatine Promotes Pseudomonas Syringae Virulence in Plants by Activating a Signaling Cascade That Inhibits Salicylic Acid Accumulation. Cell Host Microbe 2012, 11, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Obroucheva, N.V.; Lityagina, S.V.; Sinkevich, I.A. Activation and Activity of Plasma Membrane H+-Atpase: Key Events in Germinating Vicia Faba Seeds. Seed Sci. Res. 2021, 31, 76–82. [Google Scholar] [CrossRef]
- Anderson, C.T.; Kieber, J.J. Dynamic Construction, Perception, and Remodeling of Plant Cell Walls. Annu. Rev. Plant Biol. 2020, 71, 39–69. [Google Scholar] [CrossRef]
- Sperandio, M.V.L.; Santos, L.A.; Tavares, O.C.H.; Fernandes, M.S.; de Freitas Lima, M.; de Souza, S.R. Silencing the Oryza Sativa Plasma Membrane H+-Atpase Isoform OsA2 Affects Grain Yield and Shoot Growth and Decreases Nitrogen Concentration. J. Plant Physiol. 2020, 251, 153220. [Google Scholar] [CrossRef]
- Sperandio, M.V.L.; Santos, L.A.; Bucher, C.A.; Fernandes, M.S.; de Souza, S.R. Isoforms of Plasma Membrane H+-Atpase in Rice Root and Shoot Are Differentially Induced by Starvation and Resupply of No3− or Nh4+. Plant Sci. 2011, 180, 251–258. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A Trigger for Change in Plant Development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Hu, L.; Zhang, Y.; Zhang, B.; Xia, H.; Du, W.; Fan, S.; Kong, L. Low-Nitrogen Stress Stimulates Lateral Root Initiation and Nitrogen Assimilation in Wheat: Roles of Phytohormone Signaling. J. Plant Growth Regul. 2021, 40, 436–450. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Wei, M.; Ge, Y.; Hou, J.; Cheng, Y.; Chen, J. Methyl Jasmonate Maintained Antioxidative Ability of Ginger Rhizomes by Regulating Antioxidant Enzymes and Energy Metabolism. Sci. Hortic. 2019, 256, 108578. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, C.; Guo, Z.; Zhang, Q.; Li, S.; Zhang, X.; Gong, J.; Shen, Y. Herbivore Exposure Alters Ion Fluxes and Improves Salt Tolerance in a Desert Shrub. Plant Cell Environ. 2020, 43, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Abdoli, S. Improving Atpase and Ppase Activities, Nutrient Uptake and Growth of Salt Stressed Ajowan Plants by Salicylic Acid and Iron-Oxide Nanoparticles. Plant Cell Rep. 2021, 40, 559–573. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Liu, H.; Huang, W. Salicylic Acid or Heat Acclimation Pre-Treatment Enhances the Plasma Membrane-Associated Atpase Activities in Young Grape Plants under Heat Shock. Sci. Hortic. 2008, 119, 21–27. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, H.T.; Pan, Q.H.; Yang, H.R.; Zhan, J.C.; Huang, W.D. The Plasma Membrane H+-Atpase Is Related to the Development of Salicylic Acid-Induced Thermotolerance in Pea Leaves. Planta 2009, 229, 1087–1098. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. A Central Role for the Nitrate Transporter Nrt2.1 in the Integrated Morphological and Physiological Responses of the Root System to Nitrogen Limitation in Arabidopsis. Plant Physiol. 2006, 140, 909–921. [Google Scholar] [CrossRef]
- Sun, X.; Jia, X.; Huo, L.Q.; Che, R.M.; Gong, X.Q.; Wang, P.; Ma, F.W. Mdatg18a Overexpression Improves Tolerance to Nitrogen Deficiency and Regulates Anthocyanin Accumulation through Increased Autophagy in Transgenic Apple. Plant Cell Environ. 2018, 41, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Hu, B.; Chu, C.C. Nitrogen Assimilation in Plants: Current Status and Future Prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.T.; Shao, X.Q.; Li, J.X.; Ahammed, G.J.; Yao, Y.L.; Ding, J.; Hu, Z.J.; Yu, J.Q.; Shi, K. Nitrogen Forms and Metabolism Affect Plant Defence to Foliar and Root Pathogens in Tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef]
- Li, Y.T.; Han, D.X.; Sommerfeld, M.; Hu, Q.A. Photosynthetic Carbon Partitioning and Lipid Production in the Oleaginous Microalga Pseudochlorococcum Sp (Chlorophyceae) under Nitrogen-Limited Conditions. Bioresour. Technol. 2011, 102, 123–129. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Ma, H.T.; Hu, B.; Zhao, H.Y.; Wang, J.; Rennenberg, H.; Shi, X.J.; Zhang, Y.Q. Nitrogen Fertilization Stimulates Nitrogen Assimilation and Modifies Nitrogen Partitioning in the Spring Shoot Leaves of Citrus (Citrus Reticulata Blanco) Trees. J. Plant Physiol. 2021, 267, 153556. [Google Scholar] [CrossRef]
- Zhou, W.W.; Liang, X.; Li, K.J.; Dai, P.B.; Li, J.L.; Liang, B.; Sun, C.L.; Lin, X.Y. Metabolomics Analysis Reveals Potential Mechanisms of Phenolic Accumulation in Lettuce (Lactuca Sativa L.) Induced by Low Nitrogen Supply. Plant Physiol. Biochem. 2021, 158, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Li, Z.; Wang, L.; Dai, T.; Kang, Q.; Niu, D. Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism. Toxics 2020, 8, 1. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, R.; Singh, A.; Prasad, S.M. Role of Oxylipin on Luffa Seedlings Exposed to Nacl and Uv-B Stresses: An Insight into Mechanism. Plant Physiol. Biochem. 2021, 167, 691–704. [Google Scholar] [CrossRef]
- Hayat, S.; Fariduddin, Q.; Ali, B.; Ahmad, A. Effect of Salicylic Acid on Growth and Enzyme Activities of Wheat Seedlings. Acta Agron. Hung. 2005, 53, 433–437. [Google Scholar] [CrossRef]
- Gautam, S.; Singh, P.K. Salicylic Acid-Induced Salinity Tolerance in Corn Grown under Nacl Stress. Acta Physiol. Plant. 2009, 31, 1185–1190. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of Exogenous Salicylic Acid under Changing Environment: A Review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, T.; Zhang, J.; Lei, W.; Zhao, L.; Wang, S.; Shi, M.; Wei, M. Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks. Horticulturae 2023, 9, 249. https://doi.org/10.3390/horticulturae9020249
Zhang W, Zhang T, Zhang J, Lei W, Zhao L, Wang S, Shi M, Wei M. Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks. Horticulturae. 2023; 9(2):249. https://doi.org/10.3390/horticulturae9020249
Chicago/Turabian StyleZhang, Wenjie, Ting Zhang, Jia Zhang, Weiwei Lei, Lin Zhao, Shuai Wang, Mengyun Shi, and Meng Wei. 2023. "Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks" Horticulturae 9, no. 2: 249. https://doi.org/10.3390/horticulturae9020249
APA StyleZhang, W., Zhang, T., Zhang, J., Lei, W., Zhao, L., Wang, S., Shi, M., & Wei, M. (2023). Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks. Horticulturae, 9(2), 249. https://doi.org/10.3390/horticulturae9020249