Mycorrhization Enhances Vegetative Growth, Leaf Gas Exchange, and Root Development of Micropropagated Philodendron bipinnatifidum Schott ex Endl. Plantlets during Acclimatization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Arbuscular Mycorrhizal Fungi (AMF) Treatment
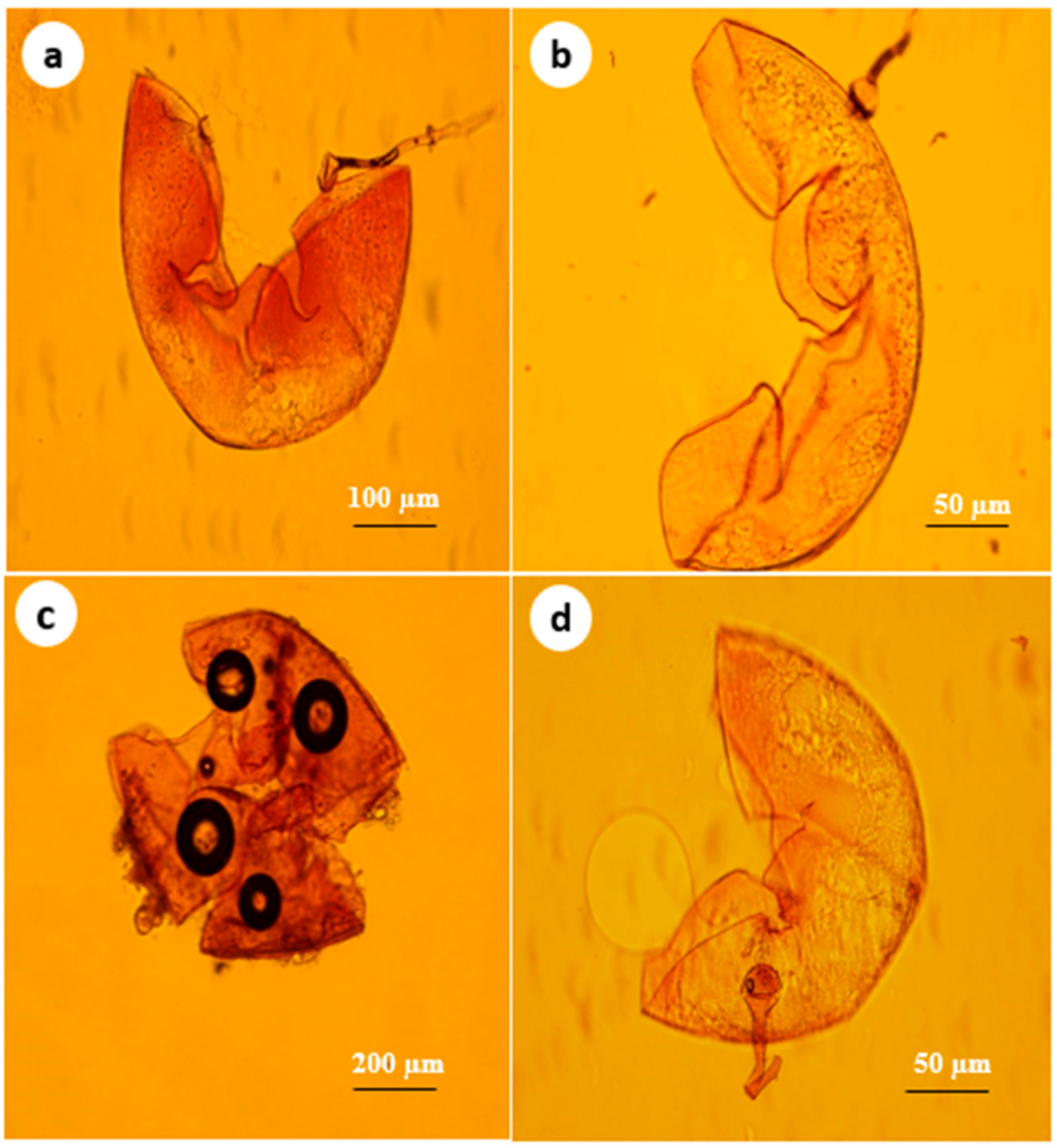
2.2. Collection of Soil Samples and Identification of Arbuscular Mycorrhizal Fungi (AMF)
2.3. AMF Colonization with Transplanted Philodendron Plants
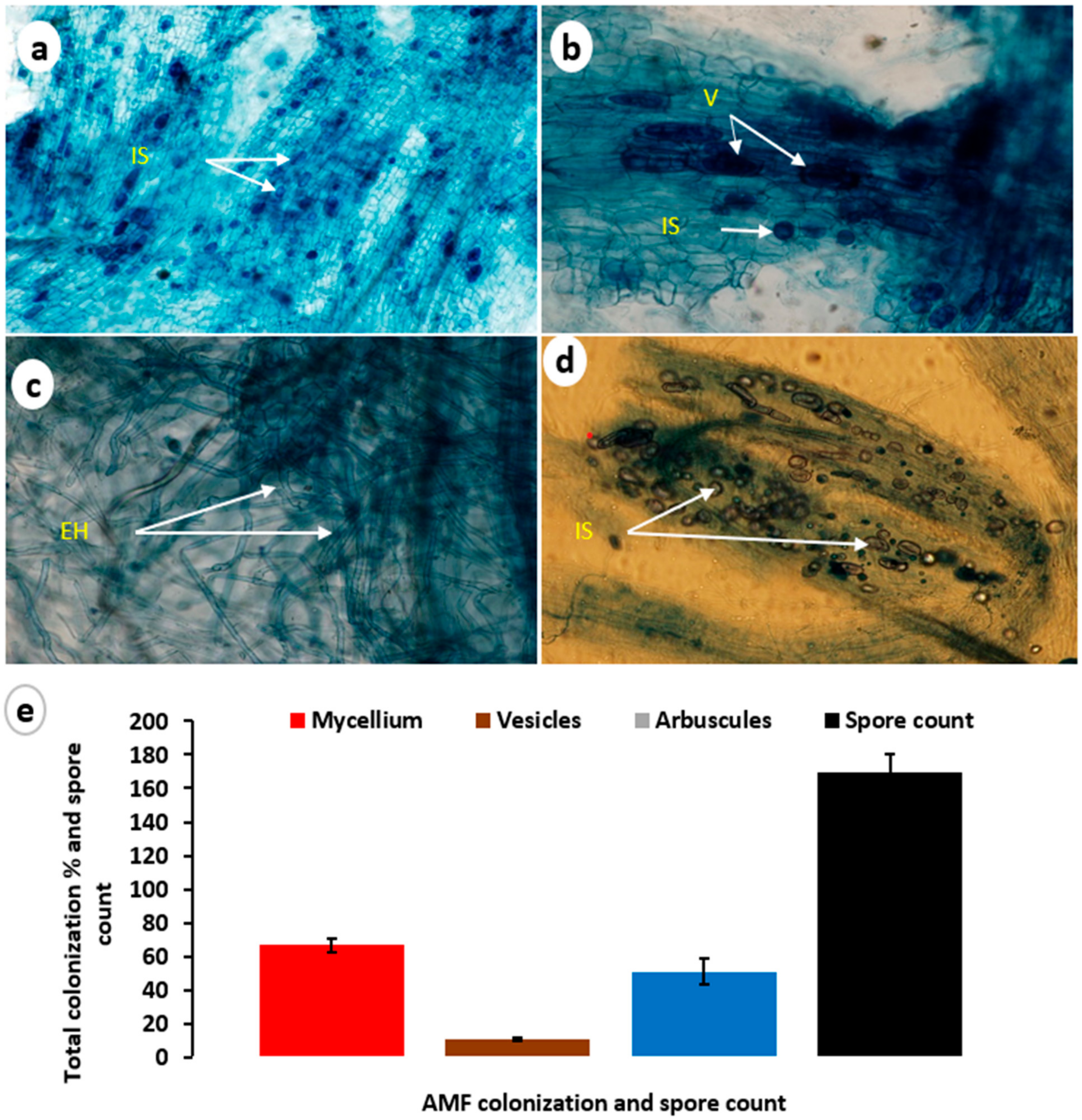
2.4. Estimation of Symbiotic Development and Spore Count
2.5. Leaf Gas Exchange Parameters
2.6. Measurements of Vegetative Parameters
2.7. Measurements Leaf Pigments
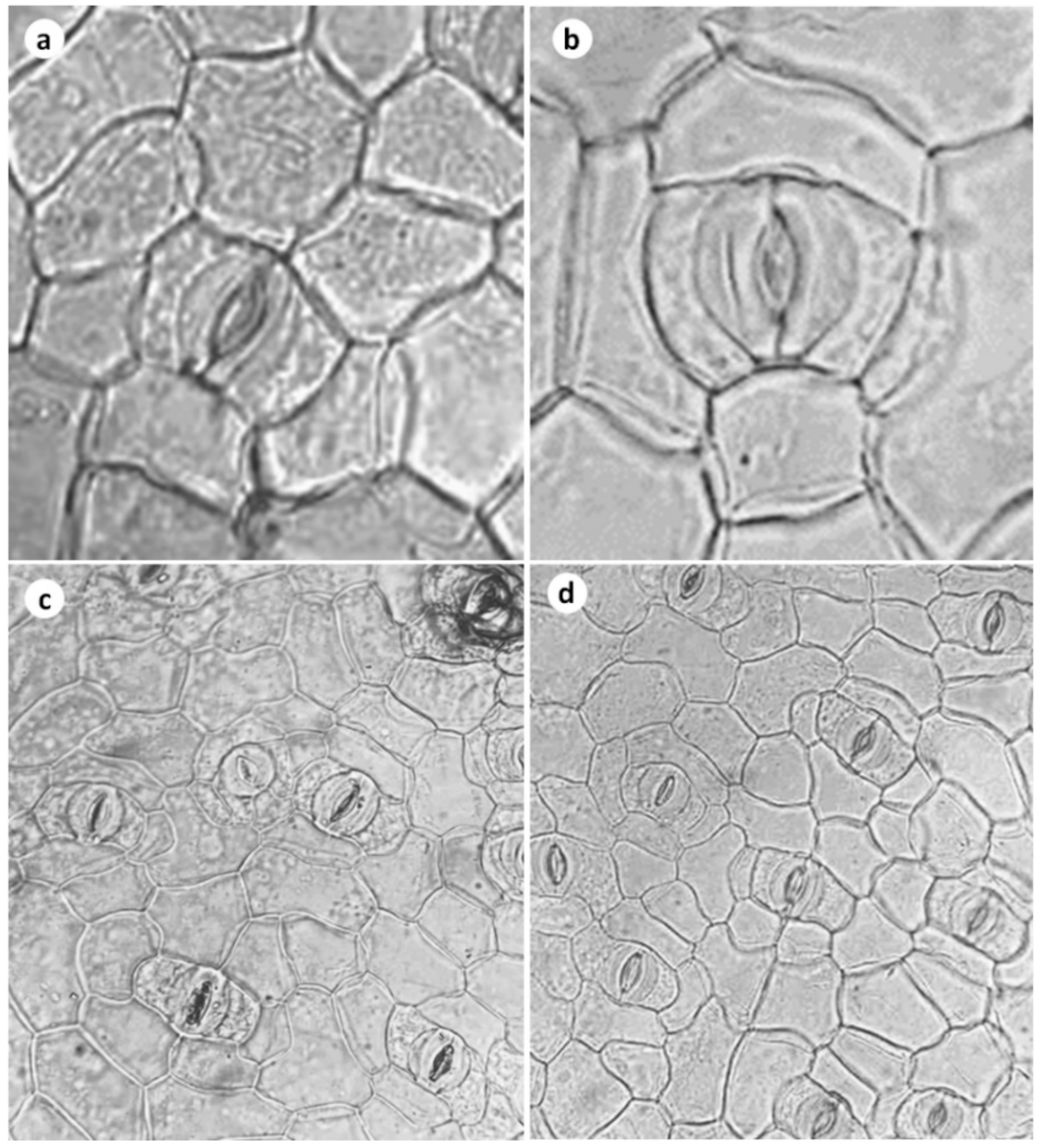
2.8. Microscopic Observations of Stomata
2.9. Measurement of the Root Growth Parameters
2.10. Experimental Design and Data Analysis
3. Results
3.1. Mycorrhizal Colonization
3.2. Plant Growth
3.3. Stomatal Frequency, Stomatal Conductance, Leaf Gas Exchange, and Transpiration Rate
3.4. Chlorophyll and Carotenoid Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz-Perez, J.C.; Shackel, K.A.; Sutter, E.G. Effect of in vitro formed roots and acclimatization on water status and gas exchange of tissue-cultured apple shoots. J. Am. Soc. Hortic. Sci. 1995, 120, 435–440. [Google Scholar] [CrossRef]
- Estrada-Luna, A.; Davies, F.T., Jr. Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscisic acid, and growth of micropropgated chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. J. Plant Physiol. 2003, 160, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Wetzstein, H.Y.; Sommer, H.E. Leaf anatomy of tissue cultured Liquidambar styraciflua (Hemamelidacae) during acclimatization. Am. J. Bot. 1982, 69, 1579–1586. [Google Scholar] [CrossRef]
- Ziv, M.; Schwarz, A.; Fleminger, D. Malfunctioning stomata in vitreous leaves of carnation (Dianthus caryophyllus) plants propagated in vitro; implications for hardening. Plant Sci. 1987, 52, 127–134. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant-water relations of apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Kozai, T. Acclimatization of micropropagated plants. In Biotechnology in Agriculture and Forestry, High-Tech and Micropropagation I; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 17, pp. 127–141. [Google Scholar]
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation Technology and Applications; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 71–93. [Google Scholar]
- Quatrini, P.; Gentile, M.; Carimi, F.; Pasquale, F.D.; Puglia, M. Effect of native arbuscular mycorrhizal fungi and Glomus mosseae on acclimatization and development of micropropgated Citrus limon (L.) Burm. J. Hortic. Sci. Biotechnol. 2003, 78, 39–45. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolra, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Yano-Melo, A.M.; Saggin, O.J., Jr.; Lima-Filho, J.M.; Melo, N.F.; Maia, L.C. Effect of arbuscular mycorrhizal fungi on the acclimatization of micropropagated banana plantlets. Mycorrhiza 1999, 9, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.R.; de Melo, N.F.; Yano-Melo, A.M. Acclimatization of micropropagated plants Etlingera elatior (Jack) R.M. Sm. Inoculated with arbuscular mycorrhizal fungi. S. Afr. J. Bot. 2017, 113, 164–169. [Google Scholar] [CrossRef]
- Joshee, N.; Mentreddy, S.R.; Yadav, A.K. Mycorrhizal fungi and growth and development of micropropagated Scutellaria integrifolia plants. Ind. Crop. Prod. 2007, 25, 169–177. [Google Scholar] [CrossRef]
- Yadav, K.; Aggarwal, A.; Singh, N. Arbuscular mycorrhizal fungi induced acclimatization and growth enhancement of Glycyrrhiza glabra L.: A potential medicinal plant. Agric. Res. 2013, 2, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.P.D.S.; Queiroz, M.D.S.; Andrade, M.M.D.; Alberton, O.; Goncalves, J.; Gazim, Z.C.; Magalhaes, H.M. Substrate-associated mycorrhizal fungi promote changes in terpene composition, antioxidant activity, and enzymes in Curcuma longa L. acclimatized plants. Rhizosphere 2020, 13, 100191. [Google Scholar] [CrossRef]
- Campos, M.A.D.S.; Silva, F.S.B.; Yano-Melo, A.M.; Melo, N.F.D.; Maia, L.C. Application of arbuscular mycorrhizal fungi during the acclimatization of Alpinia purpurata to induce tolerance to Meloidogyne arenaria. Plant Pathol. J. 2017, 33, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Fortuna, P.; Citernesi, A.S.; Morini, S.; Viaglano, C.; Giovannetti, M. Influence of arbuscular mycorrhizae and phosphate fertilization on shoot apical growth of micropropagated apple and plum rootstocks. Tree Physiol. 1996, 16, 757–763. [Google Scholar] [CrossRef]
- Guillemin, J.P.; Gianinazzi, S.; Gianinazzi-Pearson, V.; Marchal, J. Contribution of arbuscular mycorrhizas of biological protection of micropropagated pineapple (Ananas comosus (L.) Merr) against Phytophthora cinnamomi Rands. Agric. Food Sci. 1994, 3, 241–251. [Google Scholar] [CrossRef]
- Varma, A.; Schuepp, H. Mycorrhization of the commercially important micropropagated plants. Crit. Rev. Biotechnol. 1995, 15, 313–328. [Google Scholar] [CrossRef]
- Chen, F.C.; Wang, C.Y.; Fang, J.Y. Micropropagation of self-heading Philodendron via direct shoot regeneration. Sci. Hortic. 2012, 141, 23–29. [Google Scholar] [CrossRef]
- Alawaadh, A.A.; Dewir, Y.H.; Alwihibi, M.S.; Aldubai, A.A.; El-Hendawy, S.; Naidoo, Y. Micropropagation of lacy tree philodendron (Philodendron bipinnatifidum Schott ex Endl.). HortScience 2020, 55, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Grout, B.W.W.; Aston, M.J. Transplanting of cauliflower plants regenerated from meristem culture. II. Carbon dioxide fixation and development of photosynthetic ability. Hortic. Res. 1978, 17, 65–71. [Google Scholar]
- Grout, B.W.W.; Milan, S. Photosynthetic development of micropropagated strawberry plantlets following transplanting. Ann. Bot. 1985, 55, 129–131. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Ibrahim, A.; Murthy, H.N.; Aldubai, A.A.; Al-Ali, A.M.; Al-Aizari, A.A.; Migdadi, H.; Alsadon, A.; Al-Suhaibani, N.A. Integration of salicylic acid in in vitro rooting medium improves photosynthesis and growth during acclimatization of Philodendron bipinnatifidum Schott ex endl. Plantlets. Propag. Ornam. Plants 2020, 20, 103–108. [Google Scholar]
- John, T.S. A list of naturally infected Brazilian tropical plant species with vesicular-arbuscular mycorrhiza. Acta Amaz. 1980, 10, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gerdemann, J.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Schenck, N.C.; Perez, Y. Manual for Identification of VA Mycorrhizal Fungi; Synergistic Publication: Gainesville, FL, USA, 1990. [Google Scholar]
- Walker, C. Spore Extraction by Centrifugation-Sugar Flotation; Biological Research and Imaging Laboratory: Hampshire, UK, 1997. [Google Scholar]
- International Culture of Vesicular-Arbuscular Mycorrhizal Fungi (INAVAM). Available online: http://invam.caf.wvu.edu (accessed on 1 July 2022).
- Schüssler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Gener. In The Glomeromycota; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2010. [Google Scholar]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Evaluation of VA infection levels in root systems. Research for estimation methods having a functional significance. Physiol. Genet. Asp. Mycorrhizae 1986, 1, 217–221. [Google Scholar]
- Al-Qarawi, A.; Mridha, M.; Alghamdi, O. Diversity of structural colonization and spore population of arbuscular mycorrhizal fungi in some plants from Riyadh, Saudi Arabia. J. Pure Appl. Microbiol. 2012, 6, 1119–1125. [Google Scholar]
- Dewir, Y.H.; Alsadon, A.; Ibrahim, A.; El-Mahrouk, M. Effects of growing substrate, mode of nutrient supply, and Saffron corm size on flowering, growth, photosynthetic competence, and cormlet formation in hydroponics. HortTechnology 2022, 32, 234–240. [Google Scholar] [CrossRef]
- Lichtenethaler, H.K. Biosynthesis, accumulation and emission of carotenoids, α-tocopherol, plastoquinone and isoprene in leaves under high photosynthetic irradiance. Photosynth. Res. 2007, 92, 163–179. [Google Scholar] [CrossRef]
- Cotton, R. Cytotaxonomy of the Genus Vulpia; The University of Manchester: Manchester, UK, 1974. [Google Scholar]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Smith, F.A.; Smith, S.E. What is the significance of the arbuscular mycorrhizal colonization of many economically important crop plants? Plant Soil 2011, 348, 63–79. [Google Scholar] [CrossRef]
- Azcon-Aguilar, C.; Barea, J.M. Applying mycorrhiza biotechnology to horticulture: Significance and potentials. Sci. Hortic. 1997, 68, 1–24. [Google Scholar] [CrossRef]
- Sato, A.Y.; Nannetti, D.D.C.; Pinto, J.E.B.P.; Siqueira, J.O.; Blank, M.D.F.A. Application of arbuscular mycorrhiza to micropropagated Heliconia and Gerbera plants during acclimatization period. Hortic. Bras. 1999, 17, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Yadav, K.; Aggarwal, A.; Singh, N. Arbuscular mycorrhizal fungi (AMF) induced acclimatization, growth enhancement and colchicine content of micropropagated Gloriosa superba L. plantlets. Ind. Crop. Prod. 2013, 45, 88–93. [Google Scholar] [CrossRef]
- Koffi, M.C.; Declerck, S. In vitro mycorrhization of banana (Musa acuminata) plantlets improves their growth during acclimatization. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 265–273. [Google Scholar] [CrossRef]
- Wen, S.S.; Cheng, F.Y.; Zhong, Y.; Wang, X.; Li, L.Z.; Zhang, Y.X.; Qiu, J.M. Efficient protocols for the micropropagation of tree peony (Paeonia suffruticosa “Jin Pao Hong”, P. suffruticosa “Wu Long Peng Sheng”, and P. × lemoninei “High Noon’) and application of arbuscular mycorrhizal fungi to improve plantlet establishment. Sci. Hortic. 2016, 201, 10–17. [Google Scholar] [CrossRef]
- Bavaresco, L.; Fogher, C. Lime-induced chlorosis of grape-vine as affected by rootstock and root infection with arbuscular mycorrhiza and Pseudomonas fluorescence. Vitis 1996, 35, 119–123. [Google Scholar]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Salomon, Y.; Dietrich, L.; Sevanto, S.; Holtta, T.; Dannoura, M.; Epron, D. Drought impacts on tree phloem: From cell-level responses to ecological significance. Tree Physiol. 2019, 39, 173–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth promoting microorganisms. Front. Plant Sci. 2017, 8, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massonnet, C.; Costes, E.; Rambal, S.; Dreyer, E.; Regnard, J.L. Stomatal regulation of photosynthesis in apple leaves: Evidence for different water-use strategies between two cultivars. Ann. Bot. 2007, 100, 1347–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Growth Parameters | Non-AMF | AMF Treated |
|---|---|---|
| Number of stomata (mm2) | 78.93 ± 9.874 | 136.68 ± 5.775 ** |
| Aperture length (μm) | 11.00 ± 1.354 | 11.5 ± 0.2886 NS |
| Aperture width (μm) | 8.25 ± 0.6292 | 9.5 ± 0.2887 NS |
| Net CO2 assimilation (μmol CO2·m−2·s−1) | 7.1905 ± 0.3865 | 9.2673 ± 0.4032 *** |
| Stomatal conductance (mol H2O·m−2·s−1) | 0.0239 ± 0.0007 | 0.0492 ± 0.0029 *** |
| Transpiration rate (mol H2O·m−2·s−1) | 0.8784 ± 0.0234 | 1.6324 ± 0.0901 *** |
| Chlorophyll a (mg·g−1 FW) | 1.379 ± 0.0024 | 1.512 ± 0.0130 ** |
| Chlorophyll b (mg·g−1 FW) | 0.571 ± 0.0088 | 0.598 ± 0.0096 NS |
| Chlorophyll a+b (mg·g−1 FW) | 1.950 ± 0.0112 | 2.110 ± 0.0226 ** |
| Chlorophyll a/b ratio | 2.414 ± 0.2751 | 2.529 ± 0.0188 NS |
| Carotenoids (mg·g−1 FW) | 0.423 ± 0.0004 | 0.533 ± 0.0070 *** |
| Number of leaves/plants | 7.00 ± 0.408 | 10.67 ± 0.624 ** |
| Leaf area/plant (cm2) | 31.11 ± 3.058 | 75.63 ± 1.207 *** |
| Plant height (cm) | 12.13 ± 0.409 | 14.17 ± 0.118 ** |
| Shoot fresh weight/plant (g) | 1.776 ± 0.081 | 3.307 ± 0.047 *** |
| Shoot dry weight/plant (g) | 0.148 ± 0.013 | 0.315 ± 0.004 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewir, Y.H.; Habib, M.M.; AlQarawi, A.A.; Alshahrani, T.S.; Alaizari, A.A.; Malik, J.A.; Alwahibi, M.S.; Murthy, H.N. Mycorrhization Enhances Vegetative Growth, Leaf Gas Exchange, and Root Development of Micropropagated Philodendron bipinnatifidum Schott ex Endl. Plantlets during Acclimatization. Horticulturae 2023, 9, 276. https://doi.org/10.3390/horticulturae9020276
Dewir YH, Habib MM, AlQarawi AA, Alshahrani TS, Alaizari AA, Malik JA, Alwahibi MS, Murthy HN. Mycorrhization Enhances Vegetative Growth, Leaf Gas Exchange, and Root Development of Micropropagated Philodendron bipinnatifidum Schott ex Endl. Plantlets during Acclimatization. Horticulturae. 2023; 9(2):276. https://doi.org/10.3390/horticulturae9020276
Chicago/Turabian StyleDewir, Yaser Hassan, Muhammad M. Habib, AbdulAziz A. AlQarawi, Thobayet S. Alshahrani, Ahmed Ali Alaizari, Jahangir A. Malik, Mona S. Alwahibi, and Hosakatte Niranjana Murthy. 2023. "Mycorrhization Enhances Vegetative Growth, Leaf Gas Exchange, and Root Development of Micropropagated Philodendron bipinnatifidum Schott ex Endl. Plantlets during Acclimatization" Horticulturae 9, no. 2: 276. https://doi.org/10.3390/horticulturae9020276
APA StyleDewir, Y. H., Habib, M. M., AlQarawi, A. A., Alshahrani, T. S., Alaizari, A. A., Malik, J. A., Alwahibi, M. S., & Murthy, H. N. (2023). Mycorrhization Enhances Vegetative Growth, Leaf Gas Exchange, and Root Development of Micropropagated Philodendron bipinnatifidum Schott ex Endl. Plantlets during Acclimatization. Horticulturae, 9(2), 276. https://doi.org/10.3390/horticulturae9020276











