Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview
Abstract
1. Introduction
2. Anthocyanins in Fruits
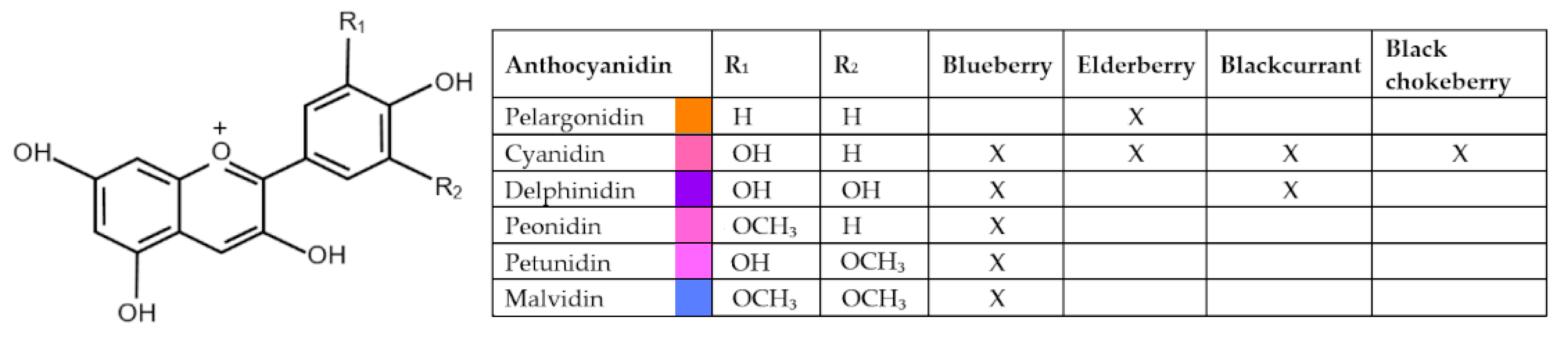
2.1. Ericaceae
2.2. Caprifoliaceae
2.3. Grossulariaceae
2.4. Rosaceae
3. Viability of Anthocyanins in an Antimicrobial and Antiviral Role
4. Effects of Processing and Extraction on Anthocyanins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results; United Nations Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022; UN DESA/POP/2022/TR/No. 3; 54p. [Google Scholar]
- Bacigalupo, I.; Mayer, F.; Lacorte, E.; Di Pucchio, A.; Marzolini, F.; Canevelli, M.; Di Fiandra, T.; Vanacore, N. A Systematic Review and Meta-Analysis on the Prevalence of Dementia in Europe: Estimates from the Highest-Quality Studies Adopting the DSM IV Diagnostic Criteria. J. Alzheimer’s Dis. 2018, 66, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef] [PubMed]
- Liubertas, T.; Kairaitis, R.; Stasiule, L.; Capkauskiene, S.; Stasiulis, A.; Viskelis, P.; Viškelis, J.; Urbonaviciene, D. The Influence of Amaranth (Amaranthus Hypochondriacus) Dietary Nitrates on the Aerobic Capacity of Physically Active Young Persons. J. Int. Soc. Sports Nutr. 2020, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- BCC Research Staff. Nutraceuticals: Global Markets to 2023; BBC: London, UK, 2018. [Google Scholar]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant Nutraceuticals as Antimicrobial Agents in Food Preservation: Terpenoids, Polyphenols and Thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Jakobek, L.; Drenjančević, M.; Jukić, V.; Šeruga, M. Phenolic Acids, Flavonols, Anthocyanins and Antiradical Activity of “Nero”, “Viking”, “Galicianka” and Wild Chokeberries. Sci. Hortic. 2012, 147, 56–63. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of Fruits and Vegetables - Their Occurrence, Analysis and Role in Human Nutrition. J. Fruit Ornam. Plant Res. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Viskelis, P.; Rubinskienė, M.; Bobinaitė, R.; Dambrauskienė, E. Bioactive Compounds and Antioxidant Activity of Small Fruits in Lithuania. J. Food Agric. Environ. 2010, 8, 259–263. [Google Scholar]
- Achterfeldt, S.; Traka, M.; Martin, C.; Vauzour, D.; Kroon, P.A. Do Anthocyanins in Purple Tomatoes Reduce the Risk of Cardiovascular Disease? Proc. Nutr. Soc. 2015, 78, 591. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Müller, D.; Schantz, M.; Richling, E. High Performance Liquid Chromatography Analysis of Anthocyanins in Bilberries (Vaccinium myrtillus L.), Blueberries (Vaccinium corymbosum L.), and Corresponding Juices. J. Food Sci. 2012, 77, C340–C345. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of Phenolic Compounds from Bilberry (Vaccinium myrtillus L.) Press Residue: Effects on Phenolic Composition and Cell Proliferation. LWT-Food Sci. Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Sitohy, M.; Mosa, B.; Ismaiel, A.; Enan, G.; Osman, A. Antimicrobial Activity and Chemical Constitution of the Crude, Phenolic-Rich Extracts of Hibiscus Sabdariffa, Brassica Oleracea and Beta Vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef] [PubMed]
- Sharonova, N.; Nikitin, E.; Terenzhev, D.; Lyubina, A.; Amerhanova, S.; Bushmeleva, K.; Rakhmaeva, A.; Fitsev, I.; Sinyashin, K. Comparative Assessment of the Phytochemical Composition and Biological Activity of Extracts of Flowering Plants of Centaurea cyanus L., Centaurea jacea L. and Centaurea scabiosa L. Plants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viskelis, P.; Bobinas, Č.; Mieželienė, A.; Alenčikienė, G.; Venskutonis, P.R. Raspberry Marc Extracts Increase Antioxidative Potential, Ellagic Acid, Ellagitannin and Anthocyanin Concentrations in Fruit Purees. LWT-Food Sci. Technol. 2016, 66, 460–467. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Isaak, C.K.; Petkau, J.C.O.K.; Debnath, S.C.; Siow, Y.L. Manitoba Lingonberry (Vaccinium vitis-idaea ) Bioactivities in Ischemia-Reperfusion Injury. J. Agric. Food Chem. 2015, 63, 5660–5669. [Google Scholar] [CrossRef]
- Chehri, A.; Yarani, R.; Yousefi, Z.; Shakouri, S.K.; Ostadrahimi, A.; Mobasseri, M.; Araj-Khodaei, M. Phytochemical and Pharmacological Anti-Diabetic Properties of Bilberries (Vaccinium myrtillus), Recommendations for Future Studies. Prim. Care Diabetes 2022, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, S.H.; Kang, B.S. Radioprotective Effects of Delphinidin on Normal Human Lung Cells against Proton Beam Exposure. Nutr. Res. Pract. 2018, 12, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.V.; Sisodia, R. Evaluation of the Free Radical Scavenging Activity and Radioprotective Efficacy of Grewia asiatica Fruit. J. Radiol. Prot. 2009, 29, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Pedisić, S.; Dragović-Uzelac, V.; Levaj, B.; Škevin, D. Effect of Maturity and Geographical Region on Anthocyanin Content of Sour Cherries (Prunus cerasus var. marasca). Food Technol. Biotechnol. 2010, 48, 86–93. [Google Scholar]
- Viskelis, P.; Rubinskienė, M.; Jasutienė, I.; Šarkinas, A.; Daubaras, R.; Česonienė, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait.) and Their Press Cakes. J. Food Sci. 2009, 74, C157–C161. [Google Scholar] [CrossRef]
- Rimpapa, Z.; Toromanović, J.; Tahirović, I.; Sapcanin, A.; Sofić, E. Total Content of Phenols and Anthocyanins in Edible Fruits from Bosnia. Bosn. J. Basic Med. Sci. 2007, 7, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Phytochemical Properties and Antioxidant Activities of Extracts from Wild Blueberries and Lingonberries. Plant Foods Hum. Nutr. 2017, 72, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. [Google Scholar] [CrossRef]
- Benvenuti, S.; Brighenti, V.; Pellati, F. High-Performance Liquid Chromatography for the Analytical Characterization of Anthocyanins in Vaccinium myrtillus L. (Bilberry) Fruit and Food Products. Anal. Bioanal. Chem. 2018, 410, 3559–3571. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Toledo, T. Wild Berries and Related Wild Small Fruits as Traditional Healthy Foods. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian Bilberries (Vaccinium myrtillus L.) and Blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit Physical Features, Phenolic Compounds Profile and Inhibition Activities of Cranberry Cultivars (Vaccinium macrocarpon) Compared to Wild-Grown Cranberry (Vaccinium oxycoccus). Plant Foods Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Vvedenskaya, I.O.; Vorsa, N. Flavonoid Composition over Fruit Development and Maturation in American Cranberry, Vaccinium macrocarpon Ait. Plant Sci. 2004, 167, 1043–1054. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Lingonberry (Vaccinium vitis-idaea L.) Grown in the Pacific Northwest of North America: Anthocyanin and Free Amino Acid Composition. J. Funct. Foods 2012, 4, 213–218. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.-L. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Haskap Berries (Lonicera caerulea L.) Using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.B.K.H.; Rupasinghe, H.P.V. Polyphenols Composition and Anti-Diabetic Properties in Vitro of Haskap (Lonicera caerulea L.) Berries in Relation to Cultivar and Harvesting Date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Khattab, R.; Brooks, M.S.-L.; Ghanem, A. Phenolic Analyses of Haskap Berries (Lonicera caerulea L.): Spectrophotometry Versus High Performance Liquid Chromatography. Int. J. Food Prop. 2016, 19, 1708–1725. [Google Scholar] [CrossRef]
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera caerulea L. Berries, Cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef]
- Deineka, V.I.; Sorokopudov, V.N.; Deineka, L.A.; Shaposhnik, E.I.; Kol’tsov, S.V. Anthocyans from Fruit of Some Plants of the Caprifoliaceae Family. Chem. Nat. Compd. 2005, 41, 162–164. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of Anthocyanin Profile of Four Elderberry Species and Interspecific Hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-Products a Source of Anthocyanins and Antioxidant Polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Dimkova, S.; Svetla, M.; Koleva, L. Content of Antioxidants in Sambucus ebulus L. and in Different Plum Cultivars Prunus domestica L. Journal of Mountain Agriculture on the Balkans. J. Mt. Agric. Balk. 2017, 20, 326–334. [Google Scholar]
- Feng, C.; Su, S.; Wang, L.; Wu, J.; Tang, Z.; Xu, Y.; Shu, Q.; Wang, L. Antioxidant Capacities and Anthocyanin Characteristics of the Black–Red Wild Berries Obtained in Northeast China. Food Chem. 2016, 204, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2006, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Ruusunen, V.; Laaksonen, O.; Tahvonen, R.; Hellsten, J.; Kallio, H. Compositional Differences of Phenolic Compounds between Black Currant (Ribes nigrum L.) Cultivars and Their Response to Latitude and Weather Conditions. J. Agric. Food Chem. 2012, 60, 6581–6593. [Google Scholar] [CrossRef]
- Sasnauskas, A.; Viskelis, P.; Rubinskienė, M.; Rugienius, R.; Bobinas, C. Productivity and small fruit quality of blackcurrant cultivars. Acta Hortic. 2014, 289–293. [Google Scholar] [CrossRef]
- Viskelis, P.; Anisimovienė, N.; Rubinskienė, M.; Jankovska, E.; Sasnauskas, A. Physical Properties, Anthocyanins and Antioxidant Activity of Blackcurrant Berries of Different Maturities. J. Food Agric. Environ. 2010, 8, 159–162. [Google Scholar]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of Total Phenolics, Anthocyanins, Ellagic Acid and Radical Scavenging Capacity in Various Raspberry (Rubus Spp.) Cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- Mazur, S.P.; Nes, A.; Wold, A.-B.; Remberg, S.F.; Aaby, K. Quality and Chemical Composition of Ten Red Raspberry (Rubus idaeus L.) Genotypes during Three Harvest Seasons. Food Chem. 2014, 160, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.; Moga, M. Rubus Fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef]
- Scalzo, J.; Currie, A.; Stephens, J.; Alspach, P.; McGhie, T. The Anthocyanin Composition of Different Vaccinium, Ribes and Rubus Genotypes. BioFactors 2008, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, Organic Acids, Phenolic Composition and Antioxidant Activity of Sweet Cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized Extraction of Anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ Cherries. Food Chem. 2018, 256, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S. Evaluation of Chemical Constituents and Antioxidant Activity of Sweet Cherry (Prunus avium L.) Cultivars: Chemical Constituents of Sweet Cherry. Int. J. Food Sci. Technol. 2011, 46, 2530–2537. [Google Scholar] [CrossRef]
- Šimunić, V.; Kovač, S.; Gašo-Sokač, D.; Pfannhauser, W.; Murkovic, M. Determination of Anthocyanins in Four Croatian Cultivars of Sour Cherries (Prunus cerasus). Eur. Food Res. Technol. 2005, 220, 575–578. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, J.; Ma, Y.; Sun, X.; Li, D.; Li, L.; Bai, H.; Xin, G.; Meng, X. Composition and Antioxidant Activity of Anthocyanins from Aronia melanocarpa Cultivated in Haicheng, Liaoning, China. Food Biosci. 2019, 30, 100413. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant Activity of High Purity Blueberry Anthocyanins and the Effects on Human Intestinal Microbiota. LWT 2020, 117, 108621. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Bobinas, Č.; Viškelis, P.; Venskutonis, P.R. Total Phenolics, Anthocyanins, Ellagitannins Content and Radical Scavenging Activity of Raspberries During Ripening. Rural. Dev. 2013, 6, 44–48. [Google Scholar]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry Anthocyanins: Isolation, Identification and Antioxidant Activities: Berry Anthocyanins. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Aloud, B.M.; Raj, P.; McCallum, J.; Kirby, C.; Louis, X.L.; Jahan, F.; Yu, L.; Hiebert, B.; Duhamel, T.A.; Wigle, J.T.; et al. Cyanidin 3- O -Glucoside Prevents the Development of Maladaptive Cardiac Hypertrophy and Diastolic Heart Dysfunction in 20-Week-Old Spontaneously Hypertensive Rats. Food Funct. 2018, 9, 3466–3480. [Google Scholar] [CrossRef]
- Shi, M.; Mathai, M.L.; Xu, G.; McAinch, A.J.; Su, X.Q. The Effects of Supplementation with Blueberry, Cyanidin-3-O-β-Glucoside, Yoghurt and Its Peptides on Obesity and Related Comorbidities in a Diet-Induced Obese Mouse Model. J. Funct. Foods 2019, 56, 92–101. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, A.M.; De Leo, M.; Braca, A. Phenolics of Arbutus Unedo L. (Ericaceae) Fruits: Identification of Anthocyanins and Gallic Acid Derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Burdulis, D.; Šarkinas, A.; Jasutiene, I.; Stackevičiene, E.; Nikolajevas, L.; Janulis, V. Comparative Study of Anthocyanin Composition, Antimicrobial and Antioxidant Activity in Bilberry (Vaccinium myrtillus L.) and Blueberry (Vaccinium corymbosum L.) Fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar]
- Werner, R.D.C.; & Merz, A.D.B. European Medicines Agency (EMA)—Committee on Herbal Medicinal Products (HMPC) Assessment Report on Vaccinium myrtillus L. Fructus Recens and Vaccinium myrtillus L., Fructus Siccus. HMPC Monogr. 2015, 555161, 1–83. [Google Scholar]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In Vitro Antioxidant Properties and Anthocyanin Compositions of Elderberry Extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Onofrei, C.; Turturică, M.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. The Kinetics of Thermal Degradation of Polyphenolic Compounds from Elderberry (Sambucus nigra L.) Extract. Food Sci. Technol. Int. 2018, 24, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Pączkowski, C.; Szakiel, A. Triterpenoid Profile of Fruit and Leaf Cuticular Waxes of Edible Honeysuckle Lonicera caerulea Var. Kamtschatica. Acta Soc. Bot. Pol. 2017, 86. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kucharska, A.Z. Effect of Pre-Treatment of Blue Honeysuckle Berries on Bioactive Iridoid Content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue Honeysuckle (Lonicera caerulea Subsp. Edulis (Turcz. Ex Herder) Hultén.) Berries and Changes in Their Ingredients across Different Locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, Á.A.; Oszmiański, J.; Golis, T. Variability of Phytochemical Properties and Content of Bioactive Compounds in Lonicera caerulea L. Var. Kamtschatica Berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef] [PubMed]
- Senters, A.E.; Soltis, D.E. Phylogenetic Relationships in Ribes (Grossulariaceae) Inferred from ITS Sequence Data. TAXON 2003, 52, 51–66. [Google Scholar] [CrossRef]
- Eurostat Regional Yearbook 2014: Agriculture. Available online: Https://Ec.Europa.Eu/Eurostat/Documents/3217494/5786409/KS-HA-14-001-11-EN.PDF.Pdf/2def3682-3ceb-4bdd-9cea-5e1a61bab98b?T=1414777838000 (accessed on 16 February 2023).
- Munck, I.A.; Tanguay, P.; Weimer, J.; Villani, S.M.; Cox, K.D. Impact of White Pine Blister Rust on Resistant Cultivated Ribes and Neighboring Eastern White Pine in New Hampshire. Plant Dis. 2015, 99, 1374–1382. [Google Scholar] [CrossRef]
- Lee, S.G.; Vance, T.M.; Nam, T.-G.; Kim, D.-O.; Koo, S.I.; Chun, O.K. Contribution of Anthocyanin Composition to Total Antioxidant Capacity of Berries. Plant Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin Composition and Antioxidant Activity of the Crowberry (Empetrum Nigrum ) and Other Berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef]
- Rachtan-Janicka, J.; Ponder, A.; Hallmann, E. The Effect of Organic and Conventional Cultivations on Antioxidants Content in Blackcurrant (Ribes nigrum L.) Species. Appl. Sci. 2021, 11, 5113. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black Currant Polyphenols: Their Storage Stability and Microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E.; Kwolek, M.; Średnicka-Tober, D.; Kazimierczak, R. Genetic Differentiation in Anthocyanin Content among Berry Fruits. Curr. Issues Mol. Biol. 2021, 43, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, J.; Tadic, V.; Dimitrijevic, S.; Stamenic, M.; Petrovic, S.; Zizovic, I. Antioxidant Properties of the Anthocyanin-Containing Ultrasonic Extract from Blackberry Cultivar “Čačanska Bestrna". Ind. Crops Prod. 2014, 53, 274–281. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Health Benefits of Prunus avium Plant Parts: An Unexplored Source Rich in Phenolic Compounds. Food Rev. Int. 2022, 38, 118–146. [Google Scholar] [CrossRef]
- Tavares, L.; Figueira, I.; Macedo, D.; McDougall, G.J.; Leitão, M.C.; Vieira, H.L.A.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective Effect of Blackberry (Rubus Sp.) Polyphenols Is Potentiated after Simulated Gastrointestinal Digestion. Food Chem. 2012, 131, 1443–1452. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: In Vitro and in Vivo Evidences and Possible Mechanisms of Action: A Re-View. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- de Ancos, B.; Gonzalez, E.; Cano, M.P. Differentiation of Raspberry Varieties According to Anthocyanin Composition. Z. Lebensm. Forsch. A 1999, 208, 33–38. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Characterization of Phenolic Compounds of Thorny and Thornless Blackberries. J. Agric. Food Chem. 2015, 63, 3012–3021. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Demir, N. Phenolic Compounds, Volatiles, and Sensory Characteristics of Twelve Sweet Cherry (Prunus avium L.) Cultivars Grown in Turkey. J. Food Sci. 2016, 81, C7–C18. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical Characterisation of Four Cherry Species (Prunus Spp.) Grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Usenik, V.; Stampar, F.; Petkovsek, M.M.; Kastelec, D. The Effect of Fruit Size and Fruit Colour on Chemical Composition in ‘Kordia’ Sweet Cherry (Prunus avium L.). J. Food Compos. Anal. 2015, 38, 121–130. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Villani, F.; Mecucci, F.; Walker, R.P.; Famiani, F.; Battistelli, A. Quality and Nutritional Compounds of Prunus cerasus L. Var. Austera Fruit Grown in Central Italy. HortScience 2019, 54, 1005–1012. [Google Scholar] [CrossRef]
- Mihailović, N.R.; Ćirić, A.R.; Cvijović, M.R.; Joksović, L.G.; Mihailović, V.B.; Srećković, N.Z. Analysis of Wild Raspberries (Rubus idaeus L.): Optimization of the Ultrasonic-Assisted Extraction of Phenolics and a New Insight in Phenolics Bioacces-Sibility. Plant Foods Hum. Nutr. 2019, 74, 399–404. [Google Scholar] [CrossRef]
- Blando, F.; Scardino, A.P.; De Bellis, L.; Nicoletti, I.; Giovinazzo, G. Characterization of in Vitro Anthocyanin-Producing Sour Cherry (Prunus cerasus L.) Callus Cultures. Food Res. Int. 2005, 38, 937–942. [Google Scholar] [CrossRef]
- Jang, Y.; Koh, E. Sustainable Water Extraction of Anthocyanins in Aronia (Aronia melanocarpa L.) Using Conventional and Ultrasonic-Assisted Method. Korean J. Food Sci. Technol. 2021, 53, 527–534. [Google Scholar] [CrossRef]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Tănasie, C.; Boc, D.; Ienaşcu, I.; Ordodi, V. Anti-Hyperglycemic Effect of Bilberry, Blackberry and Mulberry Ultrasonic Extracts on Diabetic Rats. Plant Foods Hum. Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New Understanding of the Damage of SARS-CoV-2 Infection Outside the Respiratory System. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef]
- Vaillancourt, M.; Jorth, P. The Unrecognized Threat of Secondary Bacterial Infections with COVID-19. mBio 2020, 11, e01806-20. [Google Scholar] [CrossRef]
- Bottan, N.; Hoffmann, B.; Vera-Cossio, D. The Unequal Impact of the Coronavirus Pandemic: Evidence from Seventeen Developing Countries. PLoS ONE 2020, 15, e0239797. [Google Scholar] [CrossRef]
- Akinnusi, P.A.; Olubode, S.O.; Salaudeen, W.A. Molecular Binding Studies of Anthocyanins with Multiple Antiviral Activities against SARS-CoV-2. Bull. Natl. Res. Cent. 2022, 46, 102. [Google Scholar] [CrossRef]
- Messaoudi, O.; Gouzi, H.; El-Hoshoudy, A.N.; Benaceur, F.; Patel, C.; Goswami, D.; Boukerouis, D.; Bendahou, M. Berries Anthocyanins as Potential SARS-CoV–2 Inhibitors Targeting the Viral Attachment and Replication; Molecular Docking Simulation. Egypt. J. Pet. 2021, 30, 33–43. [Google Scholar] [CrossRef]
- Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-Glucoside and Peonidin-3-O-Glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway. Nutrients 2022, 14, 2738. [Google Scholar] [CrossRef]
- Raudsepp, P.; Anton, D.; Roasto, M.; Meremäe, K.; Pedastsaar, P.; Mäesaar, M.; Raal, A.; Laikoja, K.; Püssa, T. The Antioxidative and Antimicrobial Properties of the Blue Honeysuckle (Lonicera caerulea L.), Siberian Rhubarb (Rheum Rhaponticum L.) and Some Other Plants, Compared to Ascorbic Acid and Sodium Nitrite. Food Control 2013, 31, 129–135. [Google Scholar] [CrossRef]
- Junqueira-Gonçalves, M.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G. Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni Molinae Turcz.) Fruits. Assessment of Antioxidant and Antibacterial Activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Salamon, I.; Şimşek Sezer, E.N.; Kryvtsova, M.; Labun, P. Antiproliferative and Antimicrobial Activity of Anthocyanins from Berry Fruits after Their Isolation and Freeze-Drying. Appl. Sci. 2021, 11, 2096. [Google Scholar] [CrossRef]
- Puupponen-Pimia; Nohynek, L.; Meier, C.; Kahkonen, M.; Hopia, A.; Oksman-Caldentey, K. Antimicrobial Properties of Phenolic Compounds from Berries. J. Appl. Microbiol. 2001, 1500, 494–507. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Šarkinas, A.; Venskutonis, P.R. Phytochemical composition, antioxidant and antimicrobial properties of raspberry fruit, pulp, and marc extracts. CyTA-J. Food 2013, 11, 334–342. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action Against Severe Human Pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-O-Galactoside: A Natural Compound with Multiple Health Benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Kerbstadt, S.; Eliasson, L.; Mustafa, A.; Ahrné, L. Effect of Novel Drying Techniques on the Extraction of Anthocyanins from Bilberry Press Cake Using Supercritical Carbon Dioxide. Innov. Food Sci. Emerg. Technol. 2015, 29, 209–214. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Kraujalis, P.; Tamkutė, L.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Recovery of Bioactive Substances from Rowanberry Pomace by Consecutive Extraction with Supercritical Carbon Dioxide and Pressurized Solvents. J. Ind. Eng. Chem. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Urbonavičienė, D.; Bobinaitė, R.; Trumbeckaitė, S.; Raudonė, L.; Janulis, V.; Bobinas, Č.; Viškelis, P. Agro-Industrial Tomato by-Products and Extraction of Functional Food Ingredients. Zemdirb.-Agric. 2018, 105, 63–70. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Extraction of Anthocyanins and Other Phenolics from Black Currants with Sulfured Water. J. Agric. Food Chem. 2002, 50, 5939–5946. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Talib, R.A.; Law, C.L. Optimization of Total Monomeric Anthocyanin (TMA) and Total Phenolic Content (TPC) Extractions from Mangosteen (Garcinia mangostana Linn.) Hull Using Ultrasonic Treatments. Ind. Crops Prod. 2013, 50, 1–7. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical Solvent Extraction of Anthocyanins from Dried Red Grape Pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef]
- Pataro, G.; Bobinaitė, R.; Bobinas, Č.; Šatkauskas, S.; Raudonis, R.; Visockis, M.; Ferrari, G.; Viškelis, P. Improving the Extraction of Juice and Anthocyanins from Blueberry Fruits and Their By-Products by Application of Pulsed Electric Fields. Food Bioprocess Technol. 2017, 10, 1595–1605. [Google Scholar] [CrossRef]
- Lamanauskas, N.; Pataro, G.; Bobinas, Č.; Šatkauskas, S.; Viškelis, P.; Bobinaitė, R.; Ferrari, G. Impact of Pulsed Electric Field Treatment on Juice Yield and Recovery of Bioactive Compounds from Raspberries and Their By-Products. Zemdirb.-Agric. 2016, 103, 83–90. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of Pulsed Electric Field in the Production of Juice and Extraction of Bioactive Compounds from Blueberry Fruits and Their By-Products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef]
| Family | Species | Total Anthocyanin Content, mg/100 g FW | References |
|---|---|---|---|
| Ericaceae | Vaccinium myrtillus | 159–1000 | [13,14,26,27,28,29] |
| Vaccinium corymbosum | 88–430 | [13,30,31,32] | |
| Vaccinium macrocarpon | 31–102 | [32,33,34] | |
| Vaccinium vitis-idaea | 30–50 | [27,32,35] | |
| Caprifoliaceae | Lonicera caerulea | 90–709 | [36,37,38,39] |
| Sambucus nigra | 560–924 | [7,26,40,41,42] | |
| Sambucus caerulea | 149 | [41] | |
| Sambucus ebulus | 353–400 | [41,43] | |
| Grossulariaceae | Ribes rubrum | 10–62 | [44,45,46,47] |
| Ribes nigrum | 150–626 | [45,46,48,49,50] | |
| Rosaceae | Rubus idaeus | 22–130 | [30,45,51,52] |
| Rubus fruticosus | 70–180 | [45,53,54] | |
| Prunus avium | 50–680 | [26,55,56,57] | |
| Prunus cerasus | 30–150 | [26,58] | |
| Aronia melanocarpa | 280–690 | [32,59,60,61] |
| Anthocyanin | V. myrtillus (mg/100 g FW) | V. corymbosum (mg/100 g FW) | V. macrocarpon (mg/100 g FW) | V. vitis-idaea (mg/100 g FW) |
|---|---|---|---|---|
| Delphinidn-3-O-galactoside | 43.7 [14] 85.7 [64] 127.1 [29] 167.1 [31] | 18.74 [32] 23.4 [31] | 39.3 [35] | |
| Delphinidin-3-O-glucoside | 47.7 [14] 76.6 [64] 123.8 [29] 169.1 [31] | 11.23 [32] 15.4 [31] | ||
| Cyanidin-3-O-galactoside | 46.5 [14] 53.3 [64] 56.3 [29] 122.6 [31] | 4.2 [31] 7.49 [32] | 8.89 [32] 24.0 [33] | 39.3 [35] 48.6 [32] 53.6 [64] |
| Delphinidin-3-O-arabinoside | 85.9 [64] 97.4 [29] 152.3 [31] | 9.4 [32] 24.6 [31] | ||
| Cyanidin-3-O-glucoside | 47.7 [14] 48.8 [64] 58.1 [29] 130.4 [31] | 2.6 [31] 3.04 [32] | 0.74 [32] 19.1 [33] | 1.42 [32] 2.2 [64] 5.2 [35] |
| Petunidin-3-O-galactoside | 14.3 [14] 18.6 [64] 50.0 [31] | 11.7 [31] 14.43 [32] | ||
| Cyanidin-3-O-arabinoside | 110.6 [31] 31.5 [14] 68.9 [64] | 3.5 [31] | 4.80 [32] 6.85 [33] | 6.27 [32] 7.2 [35] 12.4 [64] |
| Petunidin-3-O-glucoside | 14.0 [14] 38.7 [29] 43.7 [53] 101.9 [31] | 7,03 [32] 12.4 [31] | ||
| Cyanidin-3-O-arabinoside | 31.5 [14] 37.1 [29] 68.9 [64] 110.6 [31] | 3.5 [31] | 4.80 [32] 6.85 [33] | 6.27 [32] 7.2 [35] 12.4 [64] |
| Petunidin-3-O-glucoside | 14.0 [14] 43.7 [53] 82.8 [29] 101.9 [31] | 7,03 [32] 12.4 [31] | ||
| Peonidin-3-O-galactoside | 3.3 [53] 13.3 [31] | 1.8 [31] | 21.36 [32] 44.5 [33] | |
| Petunidin-3-O-arabinoside | 14.7 [53] 23.1 [29] 23.9 [31] | 9.3 [31] 12.89 [32] | ||
| Peonidin-3-O-glucoside | 16.0 [53] 36.4 [29] 56.7 [31] | 2.1 [31] | 4.04 [32] 7.67 [33] | 4.13 [32] |
| Malvidin-3-O-galactoside | 18.1 [53] 27.5 [31] 26.8 [33] | 15.01 [32] 34.9 [31] | ||
| Peonidin-3-O-arabinoside | 3.3 [53] 4.5 [31] | 1.0 [31] | 9.97 [32] | |
| Malvidin-3-O-glucoside | 34.3 [14] 49.2 [53] 67.7 [31] 92.4 [33] | 11.46 [32] 31.2 [31] | ||
| Malvidin-3-O-arabinoside | 7.8 [14] 12.8 [31] 13.6 [64] 17.6 [29] | 11.45 [32] 34.7 [31] |
| Anthocyanin | Amount, Percent of Total Anthocyanins (%) |
|---|---|
| Dephinidin-3-O-glucoside | 13.7–14.0 |
| Cyanidin-3-O-galactoside | 9.0–9.2 |
| Cyanidin-3-O-glucoside | 8.5–10.1 |
| Cyanidin-3-O-arabinoside | 7.7–13.6 |
| Petunidin-3-O-glucoside | 6.0–8.8 |
| Peonidin-3-O-galactoside | 0.6–1.1 |
| Peonidin-3-O-arabinoside | 0.5–1.0 |
| Malvidin-3-O-glucoside | 7.9–8.4 |
| Delphinidin-3-O-galactoside | 14.3–14.9 |
| Delphinidin-3-O-arabinoside | 12.1–15.3 |
| Petunidin-3-O-galactoside | 2.1–4.0 |
| Petunidin-3-O-arabinoside | 1.3–2.6 |
| Peonidin-3-O-glucoside | 0.1–3.7 |
| Malvidin-3-O-galactoside | 2.5–3.1 |
| Malvidin-3-O-arabinoside | 1.5–2.4 |
| Anthocyanin | S. nigra (mg/100 g FW) | S. caerulea (mg/100 g FW) | S. ebulus (mg/100 g FW) | L. caerulea (mg/100 g FW) |
|---|---|---|---|---|
| Cyanidin-3-O-galactoside | 0.32 [41] | 0.05–0.83 [39] | ||
| Cyanidin-3-O-glucoside | 376.2 [12] 449 [41]; 739.8 [47] | 2.85 [41] | 0.49 [41] | 33.97–56.93 [76] 342–649 [38] 3.3–230 [39] |
| Cyanidin-3,5-O-diglucoside | 5.46 [47] 5.91 [41]; 14.34 [12] | 1.39–2.38 [76] 15–31 [38] 0.4–17.6 [39] | ||
| Cyanidin-3-O-sambubioside | 344.44 [41] 438.8 [12] 545.9 [47] | 63.43 [41] | 6.56 [41] | |
| Cyanidin-3-O-sambubiosil-5-O-glucoside | 30.77 [12] 42.19 [41] 82.6 [47] | |||
| Cyanidin-pentoside-hexoside | 1.08 [41] | 78.99 [41] | 345.82 [41] | |
| Cyanidin-3-O-rutinoside | 9.36 [41] | 5.66–10.21 [76] 10.0–37.0 [38] 0.21–10.04 [39] | ||
| Pelargonidin-3-O-glucoside | 1.80 [47] | 2.30–5.90 [76] 5–12 [38] | ||
| Pelargonidin-dihexoside | 0.82–6.29 [76] | |||
| Peonidin-3,5-O-dihexoside | 13.88–18.94 [76] | |||
| Peonidin-3-O-glucoside | 6.55–14.53 [76] 25.0 [38] 0.15–16.39 [39] |
| Anthocyanin | Amount, Percent of Total Anthocyanins (%) |
|---|---|
| Cyanidin-3-O-glucoside | 71–89 |
| Cyanidin-3-O-rutinoside | 7–23 |
| Cyanidin-3,5-O-diglucoside | 2.2–0.4 |
| Peonidin-3-O-glucoside | >0.5 |
| Pelargonidin-3-O-rutinoside | >0.1 |
| Anthocyanin | Ribes nigrum (mg/100 g FW) | Ribes rubrum (mg/100 g FW) |
|---|---|---|
| Delphinidin-3-O-glucoside | 39.64 [48] 37.2 [64] 44.33 [83] 52.88 [84] 113.21 [47] | |
| Delphinidin-3-O-rutinoside | 91.6 [53] 109.93 [83]; 157.58 [84] 194.17 [48] 311.42 [47] | |
| Cyanidin-3-O-glucoside | 8.52 [83] 16.5 [64] 16.68 [48] 28.56 [47] 39.42 [84] | 0.16 [47] 2.52 [85] |
| Cyanidin-3-O-rutinoside | 20.78 [83] 89.0 [64] 133.71 [48] 138.83 [47] 138.81 [84] | 1.61 [47] 3.68 [85] |
| Cyanidin-3-O-sambubioside | 3.39 [47] | |
| Delphinidin-3-O-sambubioside | 0.10 [47] | |
| Cyanidin-3-O-sophoroside | 0.11 [47] | |
| Cyanidin-3-O-glucosylrutinoside | 0.49 [47] | |
| Cyanidin-3-O-xylosylrutinoside | 6.93 [47] | |
| Petunidin-3-O-rutinoside | 0.1 [64] 4.15 [47] 5.67 [84] | |
| Petunidin-3-O-glucoside | 3.97 [84] T [47] | |
| Pelargonidin-3-O-rutinoside | 1.20 [84] 2.22 [47] | |
| Peonidin-3-O-glucoside | 2.64 [84] T [47] | |
| Peonidin-3-O-rutinoside | 1.2 [64] 2.94 [84] 0.77 [47] |
| Anthocyanin | Rubus idaeus (mg/100 g FW) | Rubus fruticosus (mg/100 g FW) | Prunus avium (mg/100 g FW) | Prunus cerasus (mg/100 g FW) | Aronia melanocarpa (mg/100 g FW) |
|---|---|---|---|---|---|
| Pelargonidin-3-O-sophoroside | 8.77 [90] | ||||
| Cyanidin-3-O-rutinoside | 10.53 [90] 4.1 [52] | 4.66 [91] 31.9 [54] | 13.5 [55] 52 [92] 64.16 [93] 66.81 [94] | 4.9 [58] 7.3 [95] 17.11 [93] | |
| Cyanidin-3-2-glucosylrutinoside | 11.9 [52] | 19.3 [54] | |||
| Pelargonidin-3-O-glucoside | 4.23 [90] | N.D. [93] | |||
| Cyanidin-3-O-glucoside | 11.5 [52] 25.12 [90] 43.61 [96] | 111.3 [54] 122.54 [91] | 2.3 [55] 5.26 [93] 8.02 [92] 8.85 [94] | 2.91 [93] 4.5 [97] 5.3 [95] | 1.69 [32] 5.62 [98] 37.6 [47] |
| Delphinidin-3-O-glucoside | 2.88 [90] | ||||
| Pelargonidin-3-O-rutinoside | 0.4 [92] 1.08 [55] 1.48 [93] 3.40 [94] | N.D. [93] | |||
| Peonidin-3-O-rutinoside | 0.9 [92] 2.89 [94] 3.84 [93] 16.2 [55] | 2.47 [93] 68.1 [95] | |||
| Cyanidin-3-O-galactoside | 100.68 [98] 125.63 [32] 989.7 [47] | ||||
| Cyanidin-3-O-glucosylrutinsoide | N.D. [93] | 16.5 [58] 43.82 [93] 235.1 [95] | |||
| Cyanidin-3-O-arabinoside | 0.41 [91] | 46.58 [98] 142.43 [32] 399.3 [47] | |||
| Cyanidin-3-O-xyloside | 6.37 [91] | 4.69 [32] 5.14 [98] 51.5 [47] | |||
| Malvidin-3-O-glucoside | 3.64 [90] | ||||
| Cyanidin-3-O-xylosyl-6-rutinoside | 3.6 [54] | ||||
| Cyanidin-3-O-glucorutinoside | 11.58 [90] | ||||
| Cyanidin-3-O-sambubioside | 1.8 [54] | ||||
| Cyanidin-3-O-sophoroside | 11.46 [96] 44.7 [52] 63.86 [90] | 35.3 [54] | N.D. [93] | 4.91 [93] 39.2 [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. https://doi.org/10.3390/horticulturae9020288
Petruskevicius A, Viskelis J, Urbonaviciene D, Viskelis P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae. 2023; 9(2):288. https://doi.org/10.3390/horticulturae9020288
Chicago/Turabian StylePetruskevicius, Aistis, Jonas Viskelis, Dalia Urbonaviciene, and Pranas Viskelis. 2023. "Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview" Horticulturae 9, no. 2: 288. https://doi.org/10.3390/horticulturae9020288
APA StylePetruskevicius, A., Viskelis, J., Urbonaviciene, D., & Viskelis, P. (2023). Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae, 9(2), 288. https://doi.org/10.3390/horticulturae9020288









